Abstract
The paper presents a new method to prepare waterborne anionic alkoxysilane-terminated polyurethane dispersion (SPUD) which is substantially free from organic solvent: the NCO-terminated polyurethane (PU) containing carboxylic ions in backbone is synthesized by melt polycondensation with toluene 2,4-diisocyanate (TDI), polypropylene oxide (PPO) and 2, 2-dimethylolbutanoic acid (DMBA), the terminal NCO groups are reacted with 3-aminopropyltriethoxylsilane (APTES) to give alkoxysilane-terminated PU (SPU), and the waterborne SPUD with higher aminosilane content (10 wt% in SPU) is obtained by phase-inversion emulsification with the aid of protective colloid (polyethermethylsiloxanes, PEMS) and external emulsifier (sodium dodecyl sulfate, SDS). The anionic PU and SPU are characterized by FT-IR, gel permeation chromatography (GPC), differential scanning calorimeter (DSC) and thermo-gravimetric analysis (TGA). The thermal studies show that SPU has lower T g than anionic PU as well as thermal stability. The effects of emulsifier, protective colloid, solid content and pH value on the storage stability of waterborne SPUD are investigated, respectively. The results indicate that the storage stability decreases with increasing viscosity and dispersing ratio, whereas particle size and polydispersity index (PDI) are hardly affected by the solid content, the amount of emulsifier and protective colloid. The SPUD prepared with about 2 wt% SDS, 5 wt% PEMS, 31 wt% solid content and pH value of 7 is stable for more than 6 months at 50 °C and 8 months at room temperature, while exhibiting excellent freeze-thaw stability. The corresponding cured films exhibit excellent mechanical properties with 19.2 MPa tensile strength and 320 % elongation at break.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyurethane (PU) is proven high-performance engineering materials with excellent abrasion resistance, flexibility, hardness, light stability, chemical and solvent resistance, which makes them suitable for many useful products [1, 2]. One of the major applications of PU is in coating. Conventionally, PU coating formulations are diluted with volatile organic solvent and some also contain free isocyanate monomers. Because of the rapidly developing environmental regulations controlling air, water and land pollution, PU coating industries have changed many of their manufacturing process and coating formulations as a step toward minimizing the impact on the environment [3].
One of the products having little effects on environment is waterborne PU dispersion (PUD). In PUD, the molecular weight of the polymer is independent of the dispersion viscosity [4]. Because of the high molecular weight and solid content of the dispersions with low viscosity, the properties of PUD are superior to those of other coating analogues. However, it is difficult to emulsify or disperse because of the hydrophobic backbone of PU. A unique method of dispersing PU in aqueous media is by structural modification, i.e., modifying hydrophobic PU backbone with built-in hydrophilic segments [5]. The introduction of hydrophilic segments into the PU backbone is achieved by the incorporation of nonionic hydrophilic polyether [6, 7], anionic [8], cationic [9, 10], or zwitterionic [11] groups. Anionic PUDs, which are more stable than others, are commercially predominant [12]. Nevertheless, due to the presence of polar groups in the backbone of PU which are necessary to stabilize the PUD, the properties of PUD such as mechanical strength, solvent and chemical resistance, thermal resistance, etc., are reduced considerably [13, 14]. This decrease is restricting their utility for high performance applications.
According to the papers reported [13, 15], waterborne crosslinking alkoxysilane -terminated PUD (SPUD) can improve the chemical resistance, mechanical and thermal properties. A hydrophilically modified PU prepolymer with terminal NCO groups by the prepolymerionomer process was prepared, and aminosilane was incorporated quantitatively into the polymeric chains by its reaction with the terminal NCO groups of the PU prepolymer dissolved in acetone. After the phase inversion provoked, the acetone was evaporated and a self-curable hybrid waterborne SPUD was obtained. The alkoxysilane-terminated PU (SPU) and its dispersions unlike standard PU are isocyanate-free and are free from the toxicological problems associated with free isocyanate groups. Also, the alkoxysilane end groups undergo crosslinking reaction at room temperature, during the water evaporation process, to form a stable siloxane linked films which have improved properties, such as solvent and water resistance. There are a few papers reported which deal with the preparation of SPUD, but most of SPUDs are prepared with “acetone process” [16–21] and the utility of SPUD is limited by the residual acetone in the system.
This research article presents a new method to prepare stable waterborne SPUD which is substantially free from organic solvent. The NCO-terminated PU prepolymers containing carboxylic ions in backbone were synthesized by melt polycondensation, and the terminal NCO groups were reacted with 3-aminopropyltriethoxysilane (APTES) to give SPU. The stable waterborne SPUD with higher aminosilane content were obtained by phase-inversion emulsification with the aid of protective colloid and external emulsifier, and the effects of emulsifier, protective colloid, solid content and pH value on the storage stability of SPUD were investigated, respectively. The mechanical properties of the cured films prepared from SPUD were measured.
Experimental
Materials
The raw materials used in this work are presented in Table 1. The PPO1000 was dried in vacuum at 100 °C for 4 h before use. The TEA was distilled and stored over well-dried molecular sieve. The other raw materials and solvents were analytical reagent grade and were used as received.
Polyethermethylsiloxanes (PEMS) used as protective colloid was synthesized in our laboratory via typical procedure of hydrosilylation reaction at the presence of a Pt-catalyst [22, 23]. Poly(methylhydro)siloxane (Fluka, \( \overline{M} \) n = 2940 g/mol, active H wt% = 0.21) was charged into a flask with excess allyl polyethylene oxide (\( \overline{M} \) n = 350 g/mol, 1.05 Eq Si-H) and anhydrous toluene. The mixture was stirred at 110 °C until absorption peak of Si-H (FT-IR, around 2160 cm−1) disappeared. The toluene was removed under reduce pressure to give viscous pale-yellow PEMS.
Preparation of waterborne SPUD
The preparation process to prepare SPUD is outlined in Scheme 1.
Synthesis of anionic SPU
PPO1000 (15 mmol), TDI (26 mmol) and DMBA (5 mmol) were firstly charged into a flask equipped with a mechanical stir. The mixture was stirred at 60 °C for about 30 min under dry nitrogen atmosphere to get homogeneous melt. Two drops of DBTDL were introduced as catalyst. The change in isocyanate (NCO) content was determined by using a standard di-n-butylamine back titration until the theoretical end point was reached which was approximately 3 h of reaction. TEA (6 mmol) was added to neutralize carboxyl groups grafted on the chain of the prepared NCO-terminated anionic PU and the mixture was allowed to react for 40 min. APTES (12 mmol) was introduced to react with the terminal NCO groups in anionic PU to produce the SPU, and the reaction was carried out until the NCO peak (2270 cm−1) disappeared in the FT-IR spectrum.
Preparation of SPUD
PEMS (5.0 wt% of solid content) was introduced and mixed with SPU at 60 °C for about 30 min. After complete mixing, the water bath was removed and 20 mL SDS (2.0 wt% of solid content) aqueous solution was added into the system with moderate stirring. After the SPU was partially dispersed which needed about 15 min, the stirring rate was increased gradually to about 300 rpm. Additional 30 mL water was added dropwise during bout 40 min, the stirring rate was sped up continuously and up to about 700 rpm in the end. After the water was added, the system was stirred at 700 rpm for 20 min to accomplish the dispersion of SPU. At the end, the pH of the dispersion was adjusted to 7 with hydrochloric acid (1.0 mol/L) and the dispersion was filtrated with double-layer medical gauze to give SPUD.
Preparation of cured films
The cured films were obtained by casting the SPUD onto Teflon trough and water was allowed to evaporated under controlled conditions of temperature (25 ± 0.2 °C) and humidity (50 ± 2 %). The dispersions were self-cured for 4 days and the remaining moisture was removed at 40 °C under vacuum. The films were aged for an extra week before testing.
Instruments and characterization
FT-IR spectra were recorded on a Nicolet 560 infrared spectrometer (Nicolet, USA) in the range 400–4000 cm−1 at room temperature. The samples were prepared according to the conventional method.
The average molecular weights (\( \overline{M} \) w and \( \overline{M} \) n) and molecular weight distribution index (DI, \( \overline{M} \) w/\( \overline{M} \) n) were measured by gel permeation chromatography (GPC) using a analyzer (TriSEC302, Viscotec, USA) with chloroform as eluent at 25 °C. The GPC was calibrated with narrow polydispersity polystyrene as standards.
Thermal properties were studied by a differential scanning calorimeter (DSC) (DSC2910, Universal, USA) under nitrogen atmosphere (30 mL/min) at a heating rate of 10 °C/min from −80 to 100 °C.
Thermo-gravimetric analysis (TGA) was carried out with a TGA 2050 analyzer (Universal, USA). The samples were performed from 50 to 500 °C at a heating rate of 20 °C/min under nitrogen atmosphere with a gas flow rate of 30 mL/min.
The particle size and polydispersity index (PDI) of dispersion were measured using a Zetasizer 3000HS laser particle size analyzer (Marlven, England). The viscosity of dispersion was measured with a NDJ-8S rotating viscosimeter (Shanghai Precision & Scientific Instrument Co. Ltd, China).
Dispersion in a sealed bottle was kept in a biochemistry cultivating box to examine the storage stability at room temperature and 50 °C, respectively. The freeze-thaw stability was carried out by keeping a sealed bottle containing dispersion at −18 °C for 17 h and then at room temperature for 6 h, which concluded a typical testing cycle. At least three cycles were performed to observe whether any precipitation could be detected.
Mechanical properties of the films were measured using a single-column tensile test machine (Model HY939C, Hengyu Instruments, Ltd., Dongguan, China) at a cross-head speed of 50 mm•min−1. Dumbbell-shaped specimens were prepared from the films with a punching die of 12 mm width and 75 mm length, the neck width and length were 4.0 and 30 mm, respectively. Thickness of the film was 1.5-3.0 mm. Five tensile specimens were tested, and the results were averaged.
Results and discussion
SPU synthesis
Synthesis
SPU is synthesized via melt polycondensation, so the viscosity of the system is much higher than that of “acetone process”, which can seriously affect the extent of reaction and dispersion process. Suitable viscosity is important in the system. As reported by Kim JH [13], the viscosity was mainly due to the molecular weight of polyol and the contents of carboxylic ions. When PPO400 is used to substitute for PPO1000, the corresponding SPU has much high viscosity and approaches solid state which cannot be dispersed in water. This phenomenon should be due to the high hard segments content. Obviously, PPO with high molecular weight can decrease the viscosity, but the low hard segments content maybe affect the properties of corresponding cured film. The amount (mol) of -COOH is controlled to one-eighth of -OH in raw materials in order to decrease the content of carboxylic ions. Also, DMBA is applied as dimethylol acid to replace dimethylolpropionic acid (DMPA) which is often used to prepare waterborne PUD and SPUD [4, 14, 24]. Due to the longer side group in DMBA, the SPU with slightly lower viscosity is obtained. Obviously, increasing the ratio of NCO/OH can decrease the molecular weight of PU and obtain low-viscosity PU. While higher NCO/OH ratio results in more terminal NCO groups in PU, which need more APTES to react with the terminal NCO groups, and the dispersion with more trialkoxysilane groups has poor stability. The APTES wt% in solid content must be controlled less than 7 wt% in “acetone process” as the papers reported [4, 14]. Moreover, almost all terminal NCO groups in PU are reacted with APTES before dispersing in water unlike “acetone process” in which at least 20 % NCO groups seem to be consumed for the side reaction with H2O [25, 26]. In this study, PU is synthesized at 1.3 NCO/OH ratio with PPO1000 and DMBA, and APTES is reacted with all the residual NCO groups to give SPU. Thus, the system has a suitable viscosity and active aminosilane (APTES) content in SPU is controlled to about 10 wt% which is higher than that of previous SPU.
During the synthesis of PU, the reaction end point was determinated by measuring NCO groups content via di-n-butylamine back titration. After 3 h of reaction, the NCO groups content was measured to be 2.47 wt% which reached the theoretical value (2.49 wt%). The disappearance of NCO peak (2270 cm−1) in FT-IR means that all the residual NCO groups were reacted with APTES, which need about 1.5 h during the synthesis of SPU. The molecular weights of anionic PU and SPU were measured by GPC analysis. The values of anionic PU (\( \overline{M} \) n: 7341, \( \overline{M} \) w: 9941, DI: 1.35) and SPU (\( \overline{M} \) n: 11,800, \( \overline{M} \) w: 14,620, DI: 1.24) are higher than their theoretical values, which may be due to chain extension reaction of isocyanate and trialkoxysilane groups with trace water.
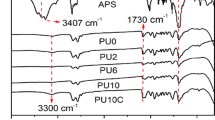
FT-IR analysis
FT-IR spectra of APTES, anionic PU and SPU are given in Fig. 1. The characteristic NCO absorption peak around 2270 cm−1 in anionic PU is attributed to the terminal NCO groups in PU. The peak disappears completely in spectrum of SPU, which indicates a complete reaction of NCO groups with aminosilane. Strong absorptions at 1730 cm−1 (C=O stretching of urethane and carboxylic groups), 2887–2970 cm−1 (CH2 stretching vibration of PPO and APTES), 1559 cm−1 (N+-C stretching vibration of ammonium salt), 1105 cm−1 (C-O-C stretching vibration of PPO, Si-O-C stretching and Si-O-Si asymmetric vibration of APTES), 769–790 cm−1 (Si-C stretching and Si-O-C deformational vibration of APTES), 1225 cm−1 (C=O and N-H stretching vibration of urea), 3306 cm−1 (N-H stretching vibration) and 1533 cm−1 (N-H bending vibration) confirm the formation of SPU.
Thermal transition
The DSC thermograms for anionic PU and SPU are shown in Fig. 2. The T g of SPU is observed at −39 °C which is lower than the T g (−28 °C) of anionic PU. This result can be explained based on the basis that the aminosilane incorporated in PU chains enhances the chain flexibility. Taking into account that the T g of pure PPO was about −70 °C [27], when this segments were incorporated in the PU chains, the T g shifts towards higher value, as a consequence of its chemical linkage with hard segments, restricting their movement and increasing the T g value. An endothermic peak at about −16 °C was observed in DSC thermograms of anionic PU and SPU, which attributed to the melting process of the soft segments of PPO units. In previous publication [15], the endothermic peak was observed at about 40 °C in self-cured films of PU and SPU. The endothermic peak moving to high value is consistent with the reducing chain mobility in the cross-linked system. The peak area of SPU is much smaller than that of anionic PU, which means that the SPU is more nearly amorphous. The reason is that the aminosilane incorporated in PU chains reduces the interassociation among the soft segments and limits the capacity of the soft segments to crystallize.
Thermal stability
In order to examine the effect of APTES on the thermal stability, TGA analysis (Fig. 3) for anionic PU and SPU were carried out. The degradation onset temperature of SPU (135 °C) is lower than that of anionic PU (250 °C), which may be attributed to the degradation of end capped aminosilane. The anionic PU showes a mass loss distributed in two steps. The first weight loss (25 wt%) occurs at around 240–340 °C which is the degradation of rigid segments. In the temperature region at around 340–440 °C, the soft segment decomposes almost completely, and the observed mass loss (72.5 wt%) is consistent with the PPO content in anionic PU (72 wt%). In TGA curve of SPU, three degradation stages are found. The weight loss occurred at around 135–225 °C (6 wt%), 225–320 °C (24 wt%) and 320–450 °C (65.5 wt%) are attributed to the degradation of end capped aminosilane, rigid segments and soft segments, respectively. Also, the weight loss of soft segments is consistent with the PPO content in SPU (64 wt%). The residue content (4.3 wt%) agrees with the content of the inorganic domains (SiO2, 3.4 wt%). The results show the thermal stability of SPU is lower than that of PU. However, it is reported in earlier paper that cured SPU exhibits a higher thermal resistance than cure PU because of the higher chemical bond energy of Si-O-Si chain segments [13].
Effect on the stability of SPUD
The waterborne SPUD with higher aminosilane content (10 wt%) were obtained by phase-inversion emulsification with the aid of protective colloid (PEMS) and external emulsifier (SDS). The method is different with the SPUD prepared with “acetone process”, which containing lower aminosilane content (2~7 wt%) and higher ions content could be prepared via self-emulsification at the aid of hydrophilic groups. The storage stability of SPUD, which is an important parameter, depended on many functions, such as the amount of emulsifier and protective colloid, pH value, solid content, particle size and viscosity. The effects of emulsifier, protective colloid, solid content and pH value on the stability of SPUD are researched, respectively. The dispersions are prepared according to the recipes of Preparation of waterborne SPUD.
pH value
During the synthesis process of anionic PU, excess TEA was added to react with carboxylic groups in order to increase the neutralization degree. Thus, the pH of dispersions obtained is weakly basic (pH ≈ 9.0) because of the residual TEA, which can affect the stability of SPUD. The SPUDs with different pH value are prepared, the storage stability and other properties are shown in Table 2. It can be seen from Table 2 that the storage stability of SPUD is closely related with the particle size and viscosity of the dispersion. The SPUDs at pH 8 and 7, especially at pH 7, have smaller particle size and lower viscosity, and thus own better storage stability. While the dispersions at pH 9.0 and 6.0 have higher viscosity and worse storage stability, which is attributed to hydrolysis and condensation of alkoxysilane groups at acid or alkaline condition. The much higher viscosity of A4 may be due to the more inorganic salt produced by the reaction of TEA and HCl. The results suggest that the SPUD must be adjusted to about pH 7 in order to improve the storage stability.
Emulsifier
The effect of the amount of emulsifier (SDS) on the storage stability and other physical properties is given in Table 3. The data show that dispersion (B1) prepared with 1.0 wt % SDS of solid content has larger particle size and worse storage stability. When the amount of SDS is higher than 1.5 wt%, the corresponding SPUDs have the similar particle size (200–220 nm) and PDI (0.1-0.2). This shows that the particle size and PDI are not affected by the amount of SDS. With the extra amount of SDS used, the viscosity increases slightly and the storage stability decreases sharply. This can be explained that SDS is a kind of organic salt and the excess SDS in dispersion produces salt-out effect, which can increase the viscosity and destroy the storage stability. The dispersing ratio is almost the same when the amount of SDS is higher than 2.0 wt%, and this shows that excess emulsifier has no obvious benefit to the dispersing ratio. From these data, it is known that the amount of SDS should be controlled about 2 wt% in order to get SPUD with excellent storage stability. In addition, the SPUDs prepared with 2 wt% or more SDS, exhibit satisfactory stability in the freeze-thaw stability test.
Protective colloid
As far as we know, the protective colloid (silicon surfactant) was not used during the preparation process of SPUD in previous papers. For the higher aminosilane content and lower ions content in SPU synthesized in this paper, the stable SPUD cannot be obtained by self-emulsification according to “acetone process”. The silicon surfactant, PEMS synthesized in our lab with polysiloxane as main chain and hydrophilic polyether as side chain, is used as protective colloid during the emulsification process. The protective mechanism of PEMS to alkoxysilane groups can be explained that the polysiloxane segments are adsorbed by the alkoxysilane groups of SPU on the surface of latex particle, and the hydrophilic polyether segments are distributed on the interface of water/latex particle and isolate the water and alkoxysilane groups [28].
The effects of the amount of protective colloid (PEMS) on the storage stability and other physical properties are summarized in Table 4. The dispersing ratio is also invariant, and this result shows that PEMS have no effect on the dispersing ratio. When the amount of PEMS is lower than 4.0 wt%, the corresponding SPUDs have much higher viscosity and worse storage stability. The phenomenon is due to the hydrolysis and condensation of part of alkoxysilane groups which are not protected by PEMS. With the amount of PEMS increasing, the particle size of SPUDs increases slightly. This result can be explained that the latex particles are encapsulated with PEMS and the hydrodynamic diameter of particle is increasing with more and more PEMS used. The data of PDI show that the SPUDs have narrow particle size distribution. Also, the SPUDs prepared with 5 wt% or more PEMS, own satisfactory storage stability and freeze-thaw stability. But excess PEMS results in higher viscosity and can decrease the storage stability of SPUD. All the results show that the suitable amount of PEMS is about 5 wt%.
Solid content
It is reported that the solid content of waterborne SPUD prepared by self-emulsification should be controlled less than 30 wt% in order to obtain the satisfactory storage stability. Lewandowski K reported the SPUD with solid content of 44 wt% by using the hydrophilic sulfonated diol, but the storage stability was not studied in his papers [29, 30]. According to the preparation method described in this paper, a series of SPUDs with actual solid content of 29~35 wt% are prepared by controlling the amount of water, and the physical properties of the SPUDs are presented in Table 5. As shown, the dispersing ratio, particle size and PDI are hardly affected by the solid content. When the solid content is higher than 31.4 wt%, the crosslinking is observed only after 2 days at 50 °C and the storage stability at room temperature is also unsatisfied. When the solid content increases from 32.4 to 35.3 wt%, the viscosity of corresponding dispersions increases sharply and the storage stability was worse. The higher viscosity should be due to the higher concentration of latex particles. The SPUDs with solid content at 29.2~31.4 wt% not only have better storage stability at 50 °C and at room temperature, but also exhibit satisfactory freeze-thaw stability. From the data, the solid content of SPUD should be controlled at approximately 31 wt%.
From the results of the effect of pH value, emulsifier, protective colloid and solid content on the properties of SPUD, it can be concluded that the dispersing ratio, particle size and PDI are hardly affected by the solid content, the amount of emulsifier and protective colloid. These properties maybe related with the content of hydrophilic carboxylic ions in SPU. The SPUD prepared with about 2 wt% emulsifier (SDS), 5 wt% protective colloid (PEMS), 31 wt% solid content and pH value of 7 is stable for more than 6 months at 50 °C and 8 months at room temperature, and in addition, exhibits excellent freeze-thaw stability.
Mechanical properties
The films are obtained from the SPUD prepared under the optimal conditions (2 wt% emulsifier, 5 wt% protective colloid, 31 wt% solid content and pH = 7) and having the best storage stability and freeze-thaw stability. The mechanical properties of the cured films are tested, the tensile strength and elongation at break are 19.2 MPa and 320 %, respectively. The films show higher tensile strength and lower percentage elongation at break, which is mainly due to higher content of triethoxylsilane at chain end in SPU and higher crosslinking density in films [13].
Conclusions
A new method is presented to prepare waterborne anionic SPUD without the use of organic solvent. The stable SPUD with higher aminosilane content (10 wt% in SPU) is obtained by phase-inversion emulsification with the aid of protective colloid (PEMS) and external emulsifier (SDS). The thermal studies show that SPU has lower T g than anionic PU as well as thermal stability. The storage stability of SPUD decreases with the increasing viscosity. The dispersing ratio, particle size and PDI of SPUD are hardly affected by the solid content, the amount of emulsifier and protective colloid. The SPUD, which is prepared with about 2 wt% SDS, 5 wt% PEMS, 31 wt% solid content and pH value of 7, is stable for at least 6 months at 50 °C and 8 months at room temperature. The freeze-thaw stability test shows that the SPUD exhibits satisfactory freeze-thaw stability. And the corresponding cured films exhibit excellent mechanical properties with 19.2 MPa tensile strength and 320 % elongation at break.
References
Chattopadhyay DK, Webster DC (2009) Prog Polym Sci 34:1068–1133
Nanda AK, Wicks DA (2006) Polymer 47:1805–1811
Wang X, Shen Y, Lai X, Liu G, Du Y (2014) J Polym Res 21:367–374
Subramani S, Lee JM, Cheong IW, Kim JH (2005) J Appl Polym Sci 98:620–631
Dieterich D (1981) Prog Org Coat 9:281–340
Li B, Peng D, Zhao N, Mu Q, Li J (2003) J Appl Polym Sci 127:1848–1852
Zhu G, Wang F, Gao Q (2012) Int J Polym Mater 61:532–543
Kim BK, Lee JC (1996) Polymer 37:469–475
Chan WC, Chen SA (1988) Polymer 29:1995–2001
Lee JS, Kim BK (1995) Prog Org Coat 25:311–318
Yang CZ, Hwang KKS, Cooper SL (1983) Macromol Chem 184:651–668
Kim BK (1996) Colloid Polym Sci 274:599–611
Subramani S, Lee JM, Lee JY, Kim JH (2007) Polym Adv Technol 18:601–609
Subramani S, Cheong IW, Kim JH (2004) Eur Polym J 40:2745–2755
Sardon H, Irusta L, Santamaría P, Fernández-Berridi MJ (2012) J Polym Res 19:9956–9964
Lai X, Li X, Wang L, Shen Y (2010) Polym Bull 65:45–57
Yeh JM, Yao CT, Hsieh CF, Yang HC, Wu CP (2008) Eur Polym J 44:2777–2783
Sardon H, Irusta L, Fernández-Berridi MJ, Luna J, Lansalot M, Bourgeat-Lami E (2011) J Appl Polym Sci 120:2054–2062
Barni A, Levi M (2003) J Appl Polym Sci 88:16–723
Wang L, Shen Y, Lai X, Li Z, Liu M (2011) J Polym Res 18:469–476
Lai X, Shen Y, Wang L (2011) J Polym Res 18:2425–2433
Powell DE, Carpenter JC (1997) The handbook of environmental chemistry. Springer-Verlag Press, Berlin Heidelberg
Li X, Guo F (2006) Spec Petrochem 23:11–14
Fu H, Wang L, Zhang H (2011) Int J Polym Mater 60:654–664
Jhon YK, Cheong IW, Kim JH (2001) Colloids Surf A 179:71–78
Cheong IW, Kong HC, Shin JS, Kim JH (2002) J Dispers Sci Technol 23:511–518
Mark JE (1999) Polymer data handbook. Oxford University Press, Oxford
Tadros TF (1982) The effect of polymers on dispersion properties. Academic, London
Lewandowski K, Krepski LR, Mickus DE (2002) J Polym Sci A Polym Chem 40:3037–3045
Lewandowski K, Krepski LR, Mickus DE (2004) J Appl Polym Sci 91:1443–1449
Acknowledgments
This work was financially supported by Shandong Provincial Natural Science Foundation, China (No. ZR2013EMM004) and Jinan City Universities and Institutes Independent Innovation Planning Project, China (No.201402044)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, Zs., Qu, Wq. & Kan, Cy. Synthesis and properties of triethoxysilane-terminated anionic polyurethane and its waterborne dispersions. J Polym Res 22, 111 (2015). https://doi.org/10.1007/s10965-015-0757-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-015-0757-8