Abstract
A series of functional metallo-supramolecular materials based on polyhedral oligomeric silsesquioxane (POSS) and phenanthroline ligand were prepared using a two-step approach. Firstly, using a phenanthroline ligand, an amino-functionalized transition metal complex was prepared by tin(II) chloride. In the second step, this metal complex was subsequently reacted with the octakis(3-chloropropyl)octasilsesquioxane, resulting for the metallosupramolecular polymers bearing POSS structure. All the synthesized compounds were fully characterized by spectroscopic analysis, thermal and electron microscopy techniques. Stimuli responsible properties of metallo-supramolecular materials were also investigated the reversibility upon external factors, such as electrochemical or the addition of competitive complexing ligands by electroanalytic techniques and UV–Vis spectroscopy. The electro- and chemo-responsive properties of the metallo-supramolecular materials were also improved. As a result, prepared phenanthroline-functionalized polyhedral silsesquioxane are good candidates for electronic, opto-electronic, and photovoltaic applications as a smart stimuli-responsive material.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of novel materials with unique properties is critical to advances in industry, medicine, energy systems, microelectronics, aeronautics and many other fields [1–3]. Furthermore, in the last decades, metal containing polymers have grown from an unusual topic to one of the foremost research fields in material chemistry. Especially, stimuli-responsive supramolecular polymers have attracted great interest in the field of functional materials because they display enhanced properties as intelligent or smart materials [1–7]. Metallopolymers are organic–inorganic hybrid materials that feature metal complexes within a polymer matrix. These materials are attractive because they combine the advantages of polymers–low cost, ease of processing, good mechanical properties with the unique properties of metal complexes (e.g., optical, magnetic, electronic, catalytic) [8–10]. One important subset of these materials is the class of metallosupramolecular materials, where the metal-ligand interaction is dynamic in nature and thus acts as the supramolecular motif [11–13]. The use of metal ion-induced polymerizations of a di- or poli- topic ligand offers a facile route to the preparation of organic/inorganic hybrid materials. Such metallo-supramolecular polymers potentially offer the functionality of the metal ion along with the processibility of a polymer [14–18].
In the field of novel material development, another important highly interesting topic is polyhedral oligomeric silsesquioxanes (POSS) [19–22]. POSS is nano-sized inorganic materials with a silica core and reactive functional groups on the surface. The silsesquioxane [23–26] has an empiric formula (RSiO1.5)n, where R may be a hydrogen or some organic group such as alkyl, methyl, aryl, vinyl, phenyl or any organofunctional derivative from these organic groups. Each silicon atom is, on average, connected to 1.5 oxygen atoms and to an R group (hydrocarbon). When n = 4, 6, 8, 10, the resulting compounds are called polyhedral oligomeric silsesquioxanes [27–30] (POSS). The strong intermolecular forces between their constituent molecules and neighbours, as well as their strong framework with their shorter bond lengths make these silica nanocomposites even more resistant to chemical degradation.
In this study, we report, a novel series of supramolecular polymers based on phenanthroline ligand and POSS structure were prepared via ligand-metal coordination. These polymers were characterized by FT-IR, NMR and elemental analysis. All the polymers were readily soluble in common organic solvents and had substantially good thermal properties. Such materials show dramatic reversible responses to a variety of stimuli, including thermal, mechanical, chemical and electrochemical. The nature of the response can be controlled by the nature of the combination of transition metal ion used. The electro-optical properties of all materials have been studied in solution and in the solid state.
Experimental
Materials
All chemicals, used for the synthesis of the octa(chloropropyl) octasilsesquioxane (POSS-Cl), were obtained from Sigma-Aldrich (Gillingham, U.K.). 3-chloropropyltrimethoxysilane (CLS) (Aldrich) was dried in molecular sieve before carrying out the experiment. Toluene and tetrahydrofuran from Fisher Chemical (Loughborough, U.K.) were dried by using suitable procedures. HEDTA-Na3 (Trisodium hydroxyethylenediaminetriacetate liquid), 5-nitro-1,10-phenanthroline, methanol and PtCl4 were obtained from Sigma-Aldrich. Acetonitryle and dimethyl sulfoxide (DMSO) used as received from Merck (Darmstad, Germany). MeOH, KBr, SnCl2 and HCl were used as received from Fluka (Madrid, Spain).
Instrumentation
Infrared spectra were recorded as KBr pellets in the range 4000–400 cm−1 on an ATI UNICAM systems 2000 Fourier transform spectrometer. Differential thermal analysis (DTA) and thermogravimetry (TG) were performed with Shimadzu DTA-50 and TGA-50 thermal analyzers, respectively. All of the thermal analysis studies were performed at a heating rate of 10 °C/min in an air atmosphere. 1H-NMR spectra (300 MHz) was obtained on a Bruker AM 300 WB FT spectrometer with δ referenced to the solvent DMSO. The chemical shifts were calibrated to the residual solvent peaks or TMS. The UV–Vis spectrums were measured on a Shimadzu 1601 UV/VIS spectrophotometer.
Chemical composition analysis of the sample was performed using Rönteck xflash detector analyzer associated to a scanning electron microscope (SEM, Leo-Evo 40xVP) with energy dispersive X-ray spectrometry (EDAX). Incident electron beam energies from 3 to 30 keV had been used. In all cases, the beam was at normal incidence to the sample surface and the measurement time was 100 s. All the EDAX spectra were corrected by using the ZAF correction, which takes into account the influence of the matrix material on the obtained spectra.
Inherent viscosities (ηinh = lnηr/c at polymer concentration of 0.5 g/dL) were measured with an Ubbelohde suspended-level viscometer at 30 °C using DMSO as the solvent. All the electrochemical operations such as cyclic voltammetry (CV) were performed by a BAS (Bioanalytical Systems, Inc.) 100 W electrochemical analyzer. The standard three-electrode system consisting of a Pt working electrode (CHI, 2 mm diameter), an Ag/AgCl reference electrode (CHI) and a Pt wire coil auxiliary electrode was used. All the experiments were carried out at room temperature. The pH measurement was performed with a Jenway 3010 pH meter.
Synthesis of the octakis(3-chloropropyl)octasilsesquioxane (POSS-Cl)
In a sol–gel polymerization (Fig. 1), the monomer solutions and the monomer and catalyst solutions (200 ppm by weight) were sealed in polypropylene bottles, the product was washed with H2O (3 × 100 mL) and ether (2 × 50 mL), and dried under vacuum for 24 h at 100 °C. A solution of 3-chloropropyltrimethoxysilane (CLS) (45 mL) was added to a solution of dry methanol. To this mixture was added 28 mL of concentrated HCl, and the reaction mixture was kept at room temperature for 2 days. PtCl4 was added to this solution as catalyst in an argon atmosphere. The reaction mixture was transferred to the Schlenk tube and heated to 50 °C. A crystalline precipitate formed after a day at 50 °C, which was collected and treated as described above. The product has a melting point of 207 °C and the chlorine content was found to be 27.6 %. The product, obtained in 45 % yield, was filtered, and washed with cold methanol and dried in a vacuum oven at 30 °C.
The 1H-NMR (DMSO-d6): 1.98 (m, SiCH2CH 2CH2, 16H), 0.98 (m, SiCH2CH2CH 2, 16H), 3.78 (m, SiCH 2CH2CH2, 16H), 13C-NMR (DMSO-d6): 51.2 (s, SiCH2CH2CH2), 19.4 (s, SiCH2CH2CH2) 14.2 (s, SiCH2CH2CH2). Elemental analysis: (C24H48O8Si8Cl8) (972.79 g/mol) Calc.: C, 29.60; H, 4.93; N, 0.00. Found: C, 29.89; H, 4.92; N,0.01.
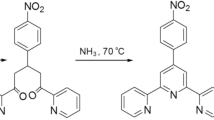
Synthesis of Bis(5-aminophenantroline)dichlorotin(II)
A solution of SnCl2 in dry dimethylacetamide (DMAc, 20 mL) was added drop-wise to a solution of 5-nitro-1,10-phenantroline (2.00 g, 8.88 mol) in anhydrous DMAc at 0 °C (Fig. 2). The mixture was stirred overnight at room temperature. The solvent was then reduced to about 10 mL under vacuum. Hexane was added, and an orange solid precipitated. The solid was washed with hexane several times and stirred in HCl (5 % in water) overnight. The final product was purified by column chromatography on silica gel (CH2Cl2/hexane, 2:1) to afford an orange crystalline solid (1.73 g, 86 %).
For 5-nitro-1,10-phenantroline: FT-IR: 1517 and 1345 cm−1 (NO2), 1H NMR (400 MHz, DMSO-d6, TMS) (δ ppm): 8.06 (m, Hphen3 and Hphen8, 2H), 8.88 (dd, J = 8.4 Hz, Hphen7, 1H), 8.99 (dd, J = 8.4 Hz, Hphen4, 1H), 9.14 (s, Hphen6, 1H), 9.35 (dd, J = 4.0 Hz, Hphen9, 1H), 9.39 (dd, J = 4.4 Hz, Hphen2, 1H), Elemental analysis: (C12H7N3O2) (225.00 g/mol) Calc.: C, 64.00; H, 3.11; N, 18.66. Found: C, 63.89; H, 3.22; N, 18.71.
For bis(5-aminophenantroline)dichlorotin(II): IR: 3326 and 3229 cm−1 (NH2), 1510 cm−1 (N = C). 1H NMR (400 MHz, DMSO-d6, TMS) (δ ppm): 6.26 (br, 4H, NH2), 7.18 (s, Hphen6, 2H), 7.62 (dd, J = 4.4 Hz, Hphen8, 2H), 7.85 (dd, J = 4.0 Hz, Hphen3, 2H), 8.16 (dd, J = 8.0 Hz, Hphen7, 2H), 8.79 (dd, J = 4.0 Hz, Hphen2, 2H), 8.11 (dd, J = 8.2 Hz, Hphen4, 2H), 9.17 (dd, J = 4.0 Hz, Hphen9, 2H), Elemental analysis: (C24H18N6Cl2Sn) (579.70 g/mol): Calc.: C, 49.69; H, 3.10; N, 14.49. Found: C, 49.61; H, 3.22; N, 14.51.
Synthesis of metallo-supramolecular polymers based on phenantroline and POSS structure
The synthesis was carried out following a standard procedure (Fig. 3). In a typical experiment, 1.94 g POSS-Cl was dissolved in 10 mL dry dimethyl sulfoxide (DMSO) and cooled to 0 °C. Bis(5-aminophenantroline)dichlorotin(II) (the ratio of amine: POSS-Cl was varied as shown in Table 1) and 2.24 g (40 mmol) KOH were added to the octakis(3-chloropropyl)octasilsesquioxane solution for a period of 15 min. and stirred overnight to give a viscous solution. The mixture was heated to 90 °C, xylene (5 mL) was added, and the mixture was refluxed for 5 h. After removing xylene by distillation under reduced pressure, the reaction mixture was the reaction mixture was cooled at room temperature and stirred overnight. The solvent was evaporated by rotary evaporation, and the crude product was purified by washing with water (2 × 10 mL) and diethyl ether (35 ml), yielded 2.38 g of as a powder. The obtained compound was characterized by 1H NMR, elemental analysis, and IR spectroscopy. In brief, metallo-supramolecular polymers based on phenantroline and POSS, MSP-1, MSP-2 and MSP-3 were prepared by changing the POSS-Cl:amine ratio from 1:1 to 1:4, respectively.
POSS-Phen-Sn (MSP-1): 1H NMR (400 MHz, DMSO-d6, TMS): (δ ppm) 1.22 (m, SiCH 2 CH2CH2, 16H), 2.31 (m, SiCH2CH 2 CH2, 16H), 2.53 (m, SiCH2CH2CH 2 , 16H), 4.72 (s, NH, 8H), 7.19 (s, H phen7, 2H), 7.32 (t, J = 4.4 Hz, H phen5, 2H), 7.65 (t, J = 4.0 Hz, H phen2, 2H), 7.91 (dd, J = 8.0 Hz, H phen4, 2H), 8.11 (dd, J = 4.0 Hz, H phen1, 2H), 8.43 (dd, J = 8.2 Hz, H phen3, 2H),8.57 (dd, J = 4.0 Hz, H phen6, 2H). Anal. Calcd. for C48H64N8O8Si8SnCl2: C, 44.49; H, 4.94; N, 8.65. Found: C, 44.71; H, 4.99; N, 8.21.
Stimuli responsive properties of supramolecular POSS-Phen-Sn polymers and Ligand Exchange Experiments with HEDTA-Na3
For stimuli responsive properties of the supramolecular POSS-Phen-Sn polymers, 1 ml of HEDTA-Na3 ((Trisodium hydroxyethylenediaminetriacetate, Trilon D liquid, 39.0–41.0 wt.-%) was added in the solution of 25 mg of supramolecular POSS-Phen-Sn (MSP) polymer in 5 ml DMSO. The solution was stirred for 1 h at 40 °C. The colourless precipitate was removed by filtration and dried. The free monomer could be isolated in 90 % yield (18.3 mg). Upon addition of a diluted solution of SnCl2 in DMSO into the NMR tube an immediate colour change could be observed and the signals of the complexed phenantroline ligands reappeared.
0.00124 mmol of POSS-Phen-Sn was dissolved in 25 mL of DMSO and 0.0043 mmol of SnCl2 solution was dissolved in 100 mL DMSO. This solution was added to the ligand in steps of 50 mL and the formation of the tin(II) complex monitored by UV–Vis spectroscopy after each addition.
Results and Discussion
The functional material that can respond to different external stimuli is the basis for developing a new generation of intelligent device [31]. Stimuli-responsive materials have attracted significant attention from both fundamental research as well as industry during the last few years, because of their remarkable and sensitive response signal. Metallo-supramolecular polymers offer the possibility of designing novel materials that can combine the properties of polymers with the characteristic sensitive responsive properties offered by the metal-ligand coordination [32–34]. In metallo-polycondensation type polymerization reactions, each chelating monomer is linked by transition metal ions via coordinative bonds. [35]. In order to perform metallo-polycondensation reactions, first the monomer bearing phenantroline unit has to be synthesized.
Molecular recognition induced from poly-association of complementary molecular components through covalent forces via a metal complex has been shown to yield polymeric species of the metallo-polymer type II in which the metal complex is linked to the polymer molecule by coordination. The use of a metal allows the creation of materials comprised of rigid blocks as shown in Fig. 4. The assembly of such species would require the design of molecular components and owed with the ability to spontaneously assembly such species through molecular recognition-directed self-assembly.
The concept that two complementary units may be combined in the presence of an organic solvent to lead to the self-assembly of a polymeric rigid rod is seen throughout the literature. In the present study metallosupramolecular polymers were chosen as the rigid rod core component and 5-aminophenantroline monomer (Phen) as the complimentary unit to be used for coordination of the metal.
Characterization of octakis(3-chloropropyl)octasilsesquioxane (POSS-Cl)
The first step was to attempt to synthesize octakis(3-chloropropyl)octasilsesquioxane (POSS-Cl) by acid-catalyzed condensation of 3-chloropropyltrimethoxysilane in MeOH. Then obtained POSS-Cl structure was investigated using FTIR, 1H-NMR and 13C-NMR spectra.
The FT-IR assignments for the spectra of the POSS-Cl (Fig. 5) were as follows: 1165 cm−1 (asymmetrical) υ as (Si-O-Si), 1050 cm−1 υ (Si-O-), 938 and 785 cm−1 (symmetrical) υs(Si-O-Si) and 620 and 500 cm − 1 υs (Si-O-Si). Polysiloxanes made up tetrahedral (T) units, [RSiO1:5]x, and showed a broad, structure less absorption covering the entire region of 1160–1000 cm−1. The 1H-NMR and 13C-NMR spectra were measured to examine the structure of the octakis(3-chloropropyl)octasilsesquioxane as shown in Fig. 5. The typical resonances of POSS-Cl are observed at 3.33 (t, CH 2N, 16H), 1.93 (m, SiCH2CH 2, 16H) and 0.91 (t, SiCH 2, 16H) [22, 23]. The elemental analysis data are found to be in good agreement with the proposed.
Characterization of aminophenantroline Sn(II) complex
Bis(5-aminophenantroline)dichlorotin(II) was synthesized from SnCl2 and phenantroline ligand. The chemical structure of the synthesized compound was characterized by FT-IR, NMR and elemental analysis techniques.
FTIR spectrum of the 5-nitrophenantroline (b) and bis(5-aminophenantroline)dichlorotin(II) showed Fig. 6. FTIR spectrum of the 5-nitrophenantroline showed a shift for peaks at 1650 cm−1 and 1435 cm−1 for free phenanthroline. When this was compared by IR spectrum of the complex, a new peak which appeared at 750 cm−1 was assigned to (υSn) stretching, as shown in Fig. 6. two peaks in the spectrum of bis(5-aminophenantroline)dichlorotin(II) complex showed at 3326 and 3229 cm−1 (υ = NH2). The bands at 1435, 1385 and 1028 cm−1 were assigned to the (C = C) alkene moiety in ligant. The movement of peaks for complex at 1510 cm−1 and 1624 cm−1 in comparing with free phenanthroline to lower region accrued.
The molecular structure of the bis(5-aminophenantroline)dichlorotin(II) complex was also confirmed by 1H-NMR. The typical resonances of this complex are observed at 6.26 (br, NH 2, 4H), 7.18 (s, Hphen6, 2H), 7.62 (dd, J = 4.4 Hz, Hphen8, 2H), 7.85 (dd, J = 4.0 Hz, Hphen3, 2H), 8.16 (dd, J = 8.0 Hz, Hphen7, 2H), 8.79 (dd, J = 4.0 Hz, Hphen2, 2H), 8.11 (dd, J = 8.2 Hz, Hphen4, 2H), 9.17 (dd, J = 4.0 Hz, Hphen9, 2H). The 1H-NMR, mass spectra, and elemental analysis data of the compounds are in agreement with the proposed structures.
Characterization of supramolecular POSS-Phen-Sn structures (MSP)
Phenantroline-functionalized polyhedral silsesquioxanes as a metallo-supramolecular hybrid material were synthesized from the condensation of bis(5-aminophenantroline)dichlorotin(II) and chloro- group functionalized polyhedral silsesquioxane. The structures of the synthesized compounds were characterized by FT-IR, UV–Vis, NMR and elemental analysis techniques. The construction of supramolecular POSS-Phen-Sn polymers is established by FTIR and 1H-NMR spectrum.
Figure 7 shows FTIR spectrum of supramolecular POSS-Phen-Sn structures with different ratio of monomers. This figure shows the presence of NH stretching frequencies at 3250–3400 cm−1 in FTIR spectrum of POSS-Phen-Sn structures (MSP). These stretching frequencies were not observed indicating the POSS-Cl. Moreover, new peaks at 1618 cm−1 (C = C stretching of aromatic ring), 1447–1390 cm−1 (C–N and C–H of aromatic ring), 1398 cm−1 (C–H deformation of aromatic CH2), and corresponding to the POSS-Cl group, and simultaneously another peak at 1000–1090 cm−1 (the Si-O–Si stretching peak) were observed. The results of FTIR measurements reveal clearly the phenantroline-functionalized polyhedral silsesquioxanes structure and show that the good connection between bis(5-aminophenantroline)dichlorotin(II) and chloro group functionalized polyhedral silsesquioxane structure.
Figure 8 displays 1H-NMR spectrum of POSS-Phen-Sn structures (MSP-1). The 1H-NMR spectra of DMSO-d6 solutions of complex at room temperature show two triplets, a singlet and two doublet for the aromatic protons of phenantroline ligand. In the 1H-NMR spectrum of POSS-phen, appearances of the three peaks at 1.22, 2.31 and 2.53 ppm related to three different C–H groups in the POSS structure. The absorption of aromatic protons appeared in the range of 7.19–8.57 ppm. The absorption of the one N-H proton group of POSS structure appeared as a singlet peak at 4.72 ppm. The elemental analyses of the resulting POSS-Tpy were in good agreement with the calculated values for the proposed structure.
Figure 9 showed EDAX spectrum of the POSS-Cl and POSS-Phen-Sn (MSP-1). EDAX analysis was carried out to identify the Sn atoms present in the POSS-Phen-Sn (MSP-1). The spectrum shows strong peaks of Sn at 0.69 and 3.44 keV. These peaks indicate the presence of Sn(II) ion in the POSS-Phen-Sn. These peaks show the supramolecular assembly generated from Sn ions and the phenantroline ligand. Figure 10 show C, N, Si and Sn image mapping of the POSS-Phen-Sn (MSP-1). This figure confirmed homogeneous dispersion of Sn ion on the MSP-1.
Stimuli-responsive properties of supramolecular POSS-Phen-Sn structures
The aim of this study is the formation of supramolecular polymers from the octa-functional POSS-phenantroline ligand and Sn(II) ion interaction. These POSS containing supramolecular polymers are soluble and sufficiently stable to be processed and characterized in solution. They can gel with increasing metal ion concentration and shows excellent gelation properties with both low critical gelation concentration and short gelation time. The supramolecular gels and polymers show reversible propetries.
The reversibility of the complex formation was investigated by applying HEDTA. HEDTA-Na3 (Trisodium hydroxyethylenediaminetriacetate, Trilon D) is known for its very strong association with metal ions and was therefore chosen as metal-extracting reagent. The addition of an aqueous solution of HEDTA to solution of POSS-Phen-Sn resulted in a decoloration of the solution of the coordination polymer within about 1 min, a result of the decomplexation of the phenantroline moieties. Figure 11 shows the colour changing of supramolecular polymers with increasing HEDTA concentration. The decomplexation occurred immediately after the respective HEDTA solution in DMSO was added to a 30 mg/mL solution of the in DMSO. Addition of a strong competitive ligand HEDTA-Na3 resulted in an efficient decomplexation. The reversibility of the complex formation was investigated by applying HEDTA. HEDTA represents a very strong chelating ligand for transition-metal ions with the ability to open phenantroline metal complexes through ligand exchange and the characteristic colour of complex disappeared. By the choice of the appropriate transition-metal ion, the optical properties of the resulting complex material could be varied from colorless to brown for Sn(II) complexes.
The synthesized and characterized metallosupramolecular polymer exhibited reversible complexation above room temperature with a series metal salts on addition of HEDTA. Figure 12 showed the reversible complexation properties of POSS-Phen with Sn(II) ions and reversible decomplexation properties of supramolecular polymers with the competing ligand HEDTA. The emission colour changes from brown to colourless on the MSP-1 with addition of HEDTA, and the colour change is also visible to the naked eye as shown in the Fig. 12. When the Sn(II) ions was cooled to same solution of MSP-1, the colour of solution can recover its original brown colour completely. The reversible change in colour can also be repeated (Fig. 12.). The response time was investigated by these colour changes of the phenantroline-based coordination polymer. All the supramolecular polymers show a low response time (< 1 s) and good stimuli responsive properties.
Figure 13 shows the characteristic UV–Vis spectrum of the supramolecular POSS-Phen-Sn polymers. UV–Vis spectra recorded in dichloromethane revealed the characteristic absorption bands for all analyzed compounds. Thus, bis phenantroline complexes displays a strong absorption at about 350 nm due to the presence of Sn(II) species [36, 37]. The absence of this absorption band at 350 nm in the final product indicates showed the success and effectiveness of metallo-polycondensation reaction and confirms the composition of the metal–phenantroline-POSS complexes.
The UV–Vis spectrum reveals the reversibility of the complexation. Figure 14 shows the characteristic UV–Vis titration spectrum of the supramolecular POSS-Phen-Sn polymers with increasing HEDTA concentration. The UV–Vis spectrum of HEDTA containing solutions showed significant differences. The decomplexation can be easily monitored by UV–vis spectroscopy. The red shift of the π-π* band at about 350 mm, which is typical for the formation of phenantroline transition metal complexes can be disappear. This UV–Vis titration experiment showed a linear decrease in the metal-to-ligand charge transfer (MLCT) band around 350 nm up to the addition of one equivalent HEDTA solution (addition of 4 × 0.1 eq.).
The redox behaviour of the synthesized supramolecular polymers and the 5-aminophenantroline Sn(II) complex has been studied by cyclic voltammetry (CV). Electrochemical measurement was carried out in a standard one compartment cell. Cyclic voltammograms for the prepared POSS-Phen-Sn polymers are shown in Fig. 15. The voltammograms were recorded in acetonitrile containing 0.1 M NH4PF6. The oxidation potentials are in agreement with the literature data for Sn(II) phenantroline complexes showing that the electrochemical properties were mainly ruled by the metal complex and not by the ligand itself. These voltammograms was observed almost completely reversible reaction for phenantroline Sn(II) ion complexs.
Conclusions
Using a two-pot procedure, we successfully synthesized supramolecular polymers based on phenanthroline ligand and POSS structure. phenanthroline-containing these polymers were characterized by FT-IR, UV–Vis, NMR and elemental analysis techniques. The characterization techniques showed the success and effectiveness of functionalization and confirm the composition of the metal–phenanthroline-POSS complexes. In addition, the stimuli responsible properties of these metallo-supramolecular polymers were determined by 1H NMR, UV spectroscopic techniques and electrochemical analyses. These measurements showed that prepared supramolecular polymers completely reversible for phenantroline metal ion complexes. Additionally, we show examples for the complexation of the phenanthroline ligand with Sn(II) ions leading to various types of supramolecular assemblies and potential applications in the fields of smart materials.
References
Lehn J-M (1995) Supramolecular Chemistry, Concepts and Perspectives. VCH, Weinheim
Lehn J-M, Mascal M, DeCian A, Fischer J (1992) J Chem Soc Perkin Trans 2:461–467
Yang H, Ma Q, Tan Y (2013) J Polym Res 20:100–106
Knof U, von Zelewsky A (1999) Angew Chem 111:312–333
Schubert US, Eschbaumer C, Hochwimmer G (1998) Tetrahedron Lett 39:8643–8644
Eisenbach CD, Schubert US (1993) Macromolecules 26:7372–7374
Blagodatskikh IV, Bezrodnykh EA, Abramchuk SS, Muranov AV, Sinitsyna OV, Khokhlov AR, Tikhonov VE (2013) J Polym Res 20:73–82
McKenzie BM, Miller AK, Wojtecki RJ, Johnson JC, Burke KA, Tzeng KA, Mather PT, Rowan SJ (2008) Tetrahedron 64:8488–8495
Hofmeier H, Hoogenboom R, Wouters MEL, Schubert US (2005) J Am Chem Soc 127:2913–2921
Huang WM, Zhao Y, Wang CC, Ding Z, Purnawali H, Tang C, Zhang JL (2012) J Polym Res 19:9952–9985
Hamers C, Kocian O, Raymo FM, Stoddart JF (1998) Adv Mater 10:1366–1369
Ciferri A (2000) Supramolecular Polymers. Marcel Dekker, New York
Kelch S, Rehahn M (1999) Macromolecules 32:5818–5828
Adeloye AO, Ajibade PA (2010) Molecules 15:7570–7581
Schubert US, Eschbaumer C (2002) Angew Chem 41:2892–2926
Arounaguırı S, Easwaramoorthy D, Ashokkumar A, Dattagupta A, Maıya BG (2000) Proc Indian Acad Sci (Chem Sci) 112(1):1–17
Kurth DG, Higuchi M (2006) Soft Matter 2:915–927
McKenzie BM, Rowan SJ (2007) Metallo-supramolecular polymers. In: Atwood JL, Steed JW (eds) The encyclopedia of supramolecular chemistry. Taylor and Francis, New York
Gravel M-C, Zhang C, Dinderman M, Laine RM (1999) Appl Organometal Chem 13:329–336
Kuo SW, Chang FC (2011) Prog Polym Sci 36:1649–1696
Leu CM, Chang YT, Wei KH (2003) Chemistry of Material 15(16):3721–3727
Leu CM, Chang YT, Wei KH (2003) Macromolecules 36(24):9122–9127
Wang W, Ding W, Yu J, Fei M, Tang J (2012) J Polym Res 19:9948–9953
Baney RH, Itoh M, Sakakibara A, Suzuki T (1995) Chem Rev 95:1409–1430
Lickiss PD, Rataboul F (2008) Adv Organomet Chem 57:1–116
Chou CH, Hsu SL, Dinakaran K, Chiu MY, Wei KH (2005) Macromolecules 38(3):745–751
Ayandele E, Sarkar B, Alexandridis P (2012) Nanomaterials 2:445–475
Wang J-H, Cheng C-C, Yen Y-C, Miao C-C, Chang F-C (2012) Soft Matter 8:3747–3750
Seçkin T, Köytepe S, Adigüzel HI (2008) Mater Chem Phys 112:1040–1046
Cui HW, Kuo SW (2013) J Polym Res 20:114–126
Li W, Yan D, Gao R, Lu J, Wei M, Duan X (2013) Journal of Nanomaterials Article ID 586462:1–14
Chiper M, Hoogenboom R, Schubert US (2008) e-Polymers 157:1–9
Mugemana C, Guillet P, Hoeppener S, Schubert US, Fustin C-A, Gohy J-F (2010) Chem Commun 46:1296–1298
Schubert US, Schmatloch S, Precup AA (2002) Des Monomers Polym 5:211–221
Chiper M, Hoogenboom R, Schubert US (2009) Macromol Rapid Commun 30:565–578
Archer SJ, Koch KR, Schmidt S (1987) Inorg Chim Acta 126(2):209–218
Dragica L, Branko S, Jelena P-Š, Slavica S, Ljubica V, Dragana B, Zoran O (2010) Chemical. Industry and Chemical Engineering Quarterly 16(2):193–198
Acknowledgments
Authors would like to thank TÜBİTAK - The Scientific and Technological Research Council of Turkey for the financial support with the project number MAG110M751.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demirel, M.H., Köytepe, S., Gültek, A. et al. Synthesis and stimuli-responsive properties of the phenanthroline based metallo-supramolecular polymers. J Polym Res 21, 345 (2014). https://doi.org/10.1007/s10965-013-0345-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-013-0345-8