Abstract
Molecularly imprinted polymers on the surface of multiwalled carbon nanotubes (MWNTs) have been developed for selective recognition for Ribavirin. The composites (MWNTs/MIPs) were prepared by using Ribavirin as the template molecule, acrylamide (AAM) as the functional monomer, N, N-methylenebisacrylamide (NNMBA) as the cross-linker. MWNTs/MIPs obtained were characterized by fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and thermogravimetric analysis (TGA). The properties such as adsorption dynamics, special binding, and the selective recognition ability were evaluated. The adsorption equilibrium was arrived in about 4 h which indicated that the adsorption kinetic was comparatively fast. The results of binding and selectivity experiments showed that the MWNTs/MIPs could bind the template molecule selectively in aqueous media.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years considerable attentions have been paid to molecular imprinting technology (MIT) as an excellent and simple approach to producing artificial receptors with predetermined ligand selectivity [1, 2]. The technique is based on the copolymerization of a monomer with cross-linker in the presence of a template molecule, so that the positions and orientations of the functional residues of the monomer are immobilized in the polymer, which are complementary in size, shape and interaction patterns complementary to the template molecule. Thus, MIPs can rebind template molecule with very high affinity and specificity. Until now, MIPs have been widely used in many areas such as the chromatographic separation [3, 4], solid phase extraction [5–8], various sensor strategies [9–11], catalysis studies [12, 13], pharmaceutical analysis [14–16], etc. This technique is a conceptually simple and straightforward method of applying to a wide variety of target molecules, however, it still has some disadvantages, such as the heterogeneous distribution of the binding sites, embedding of most binding sites, and lower binding kinetic of MIPs towards the template molecule.
In recent years, multiwalled carbon nanotubes (MWNTs) have enjoyed widespread attention for their high electrical and thermal conductivity properties [17, 18]. MWNTs, with unique mechanical properties and extremely large surface areas, should be an excellent candidate, as the supported material, which would endow MIPs with large surface areas if the MIPs were prepared onto the surface of MWNTs. Thus, the binding sites in the outer layer of the composite would improve the accessibility of template molecule and reduce the binding time [19].
Ribavirin is an anti-viral drug indicating for severe RSV infection (individually), a hepatitis C infection and other viral infections. In this article, composites of multiwalled carbon nanotubes and molecularly imprinted polymers for Ribavirin were prepared by using MIT on the surface of MWNTs, Ribavirin as the template molecule, acrylamide (AAM) as the functional monomer, N,N-methylenebisacrylamide (NNMBA) as the cross-linker. The composite polymers (MWNTs/MIPs) obtained were characterized by FTIR, scanning electron microscopy (SEM), and thermogravimetric analysis (TGA). The properties such as adsorption dynamics, special binding, and selective recognition capacity were evaluated.
Materials and methods
Materials and instruments
Multiwall CNTs (diam. <10 nm, length 5–15 μm) were purchased from the Shenzhen Nanotech Port Co., Ltd. (Shenzhen, China). Ribavirin and Telbivudine were obtained from the national institute for the control of pharmaceutical and biological products (Beijing, China). S-Napoxen was purchased from Fluka, structures of the three chemicals were shown in Fig. 1. AAM and NNMBA were purchased from the Kermel Chemical Reagent Co., Ltd. (Tianjin, China). All other chemicals were of analytical grade and used as received.
UV–Vis was measured on a UV2550 spectrophotometer (Shimadzu, Japan). The morphology of MWNTs/MIPs was observed by JSM-6510LV scanning electron microscopy (JEOL, Japan). Thermal gravimetric analysis (TGA) was performed under nitrogen on a Diamond TG/DTA (Perkin Elmer, America) under nitrogen protection at a heating rate of 10.0 °C/min. FTIR spectra in KBr were recorded by using a VERTEX 70 spectrometer (Bruker, Germany).
Preparation of carboxylic acid-functionalized MWNTs (MWNTs-COOH)
Received MWNTs (0.5 g) were added to 60 mL of 68 % HNO3/ 98 % H2SO4 (1:3) under sonication for 30 min, followed by refluxing at 90 °C for 60 min. The reaction mixture was then diluted with water and allowed to stand overnight for precipitation. The supernatant was decanted, and the remains were filtered through a 0.22 um polytetrafluoroethylene (PTFE) membrane (China) and washed thoroughly with distilled water for several times until the pH value of the filtrate was neutral. The solid powders were dried at 80 °C under vacuum, obtaining carboxylic acid-functionalized MWNTs (MWNTs-COOH).
Synthesis of MWNTs /MIPs
0.1 g MWNTs-COOH was mixed with 5 mL pure water in a round bottomed flask, and ultrasonic dispersed adequately. 0.5 mmol Ribavirin, 2 mmol AAM, 2 mmol NNMBA and 0.5 mg ammonium persulfate [(NH4)2S2O8] were accurately weighed into 50 ml round bottomed flask , and 15 mL pure water was added to dissolve the mixture, then the solution was ultrasonicated for 20 min to ensure complete association of the imprint molecule with AAM. Finally, MWNTs-COOH which had been dispersed uniformly was added in. The round-bottomed flask was warmed at 75 °C to ensure complete cross-linking, and the reaction was allowed to proceed for 12 h in N2 atmosphere. The resulting product was collected by centrifugation and washing thoroughly with pure water until no Ribavirin was found in the solution after centrifugation. MWNTs/MIPs, i.e, the composites of multiwalled carbon nanotubes and molecularly imprinted polymers were obtained. As a control, MWNTs/NIPs were prepared in the similar manner described as above, except for the absence of the template.
Binding Experiments
Binding experiments were achieved according to the literature previously reported with some modification [16].
The concentration of Ribavirin in the binding experiments was detected by UV-vis spectrophotometer. Through the UV-vis scanning, Ribavirin in water had maximum absorption at 201 nm. So wavelength at 201 nm was chosen for detection. With concentrations of ribavirin(C) as horizontal ordinate, ultraviolet absorbance(A) as vertical coordinate, a standard curve was obtained and the linear regression equation was A = 0.01783 C + 0.03922(r = 0.99953), with good linear relationship in 2.0 ~ 20.0 ug/mL concentration range (Fig. 2).
In binding isotherm experiments, 20.0 mg of the polymer particles was equilibrated with adsorbate of varied initial concentration in each centrifuge tube. The centrifuge tube was shaken for 6 h at room temperature. Then, the saturated polymer was separated by centrifugation. The residual concentration of the adsorbate was measured by UV-vis spectrophotometer at 201 nm. The amount of Ribavirin bound (mg/g) to the MWNTs/MIPs (Q) was calculated by subtracting the amount of free Ribavirin in the supernate from the amount of Ribavirin initially added.
Similarly, the adsorption dynamics of the MWNTs/MIPs were investigated. In a centrifuge tube, 20 mg of MWNTs/MIPs was suspended in 2.0 mL of an initial Ribavirin concentration with 1.0 mg/mL. The tube was incubated at room temperature with shaking at different adsorption time intervals. The amount of Ribavirin adsorbed by MWNTs/MIPs was detected by UV-vis spectrophotometer at 201 nm.
According to the test methods mentioned before, the selectivity of MWNTs/MIPs was investigated by using Telbivudine and S-Napoxen as the structure related compounds. The residual concentration of the adsorbate was measured by UV-vis spectrophotometer at 265 nm for Telbivudine and at 330 nm for S-Napoxen.
Results and discussion
Preparation of MWNTs/MIPs
The reaction mixture must be dispersed fully before initiation reaction began. Different molar ratios of AAM to Ribarivin were explored during the polymerization. In Fig. 3, when the amount of the cross-linker was fixed, all the MWNTs/MIPs prepared in different molar ratios of template to monomer showed higher adsorption amount of Ribarivin than that of the corresponding MWNTs/NIPs did. When the molar ratio was 1: 4, MWNTs/MIPs had the best binding performance. The results showed that at a fixed amount of template to increase the amount of monomer for the polymerization could increase adsorbed amount of the MWNTs/MIPs towards Ribarivin. However, when the amount of monomer was further increased, agglomeration of the raw materials would happen and the binding capacity for Ribarivin decreased. In Table 1, the imprinted ratios of the MWNTs/MIPs prepared from different molar ratios were listed. The imprinted ratio was defined as the ratio of the binding capacity of the MWNTs/MIPs for Ribarivin to the binding capacity of the corresponding MWNTs/NIPs for Ribarivin. At the molar ratio of 1:4, the maximum imprinted ratio of 1.65 was obtained. As the molar ratio was changed, the imprinted effect declined accordingly. Hence, we may conclude that with 1:4 of molar ratio of template to monomer, best binding capacity as well as imprinted effect was achieved.
IR of MWNTs/MIPs
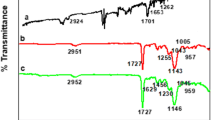
The preparation of MWNTs/MIPs was primarily followed by IR spectroscopy evaluation. The received MWNTs, MWNTs-COOH, MWNTs/MIPs, and pure MIPs were shown in Fig. 4. The received MWNTs(Fig. 4a), almost had not IR adsorption bands. Compared with the IR spectrum of the received MWNTs, the characteristic peaks of carbonyl and hydroxyl introduced by -COOH, obviously appeared in the spectrum of MWNTs-COOH (Fig. 4b). The peaks at 3420 and 1720 cm-1 belonged to stretching vibration of O-H and C = O respectively. The characteristic peaks of pure MIPs (Fig. 4d) also appeared in the spectrum of MWNTs/MIPs, as shown in Fig. 4c, the peaks at 3053 and 2935 cm-1 were contributed by -CH3 and -CH2 on the surface of the MWNTs, and the peak at 1114 cm-1 which was assigned to C-N or C-O stretching vibrations. All these adsorption bands suggested composites of multiwalled carbon nanotubes and molecularly imprinted polymers were formed.
TG of MWNTs-MIPs
On the basis of the different thermal stability between the imprinted polymer and MWNTs, the TGA measurements were used to provide evidence indicating that the polymer was formed on the surface of the MWNTs. Figure 5 showed TGA weight loss curves of the received MWNTs, MWNTs-COOH, MWNTs/MIPs, and pure MIPs respectively. As shown in Fig. 5a, the received MWNTs lost some weight under 300 °C, which may be caused by a few impurities. The acid-processed MWNTs showed a small weight loss of approximately 10.1 % in the whole process which was attributed primarily to the release of water molecules and the decomposition of –COOH (Fig. 5b). However, the TGA curve of MWNTs/MIPs had a dramatic weight loss similar to pure MIPs in the 300-450 °C region. At 300 °C, the weight of MWNTs/MIPs was 93.9 %, while at 450 °C, the weight of it was 38.0 %, and this dramatic loss of weight was caused by the decomposition of organic imprinted polymer coated on the surface of the acid-processed MWNTs. In accordance with the quality of MWNTs/MIPs obtained, the imprinted layer content coated on the MWNTs could be estimated by subtracting the quantity of MWNTs-COOH, and the imprinted layer accounted for about 70 % of the total weight.
SEM images of MWNTs/MIPs
The SEM images of received-MWNTs, MWNTs-COOH and MWNTs/MIPs were shown in Fig. 6, it could be seen that the acid-processed MWNTs, which were used as supports for the MWNTs/MIPs, showed a morphology similar to received-MWNTs, but gathering and winding degree of carbon nanotubes reduced. This kind of situation could be also confirmed by the phenomenon, i,e, MWNTs-COOH in the water could form stable suspended solids ,but received-MWNTs could not.
It can be seen that the average diameter of carbon nanotubes in MWNTs/MIPs (Fig. 6d) were obviously larger than that of MWNTs-COOH (Fig. 6b) obviously, indicating that MIPs were coated on the surface of the MWNTs. And the ablation phenomena of the imprinted polymer on the composites further supported the formation of the MWNTs/MIPs when SEM test was carried out.
Binding experiments
Binding experiments were carried out at room temperature in this paper. If the temperature was too low, mass transfer efficiency of MIPs would reduce. A high temperature would cause swell of imprinted polymers[12], and it affected the three-dimensional network structure of the MIPs which matched with the template molecule.
The curves of adsorption kinetics were shown in Fig. 7. This was the typical kinetic curve for most rebinding processes. The adsorption kinetics data showed that the adsorption rate was fast in the first 2 h and that equilibrium was achieved in 4 h. It could be clearly observed that the adsorbance Q of Ribavirin increased with time, and a higher amount was absorbed by MWNTs /MIPs compared with MWNTs /NIPs.
The binding isotherm was studied by changing Ribavirin concentration in the range of 0.2-1.2 mg.mL-1. The results were shown in Fig. 8. It could be seen that the amounts of Ribavirin bound to both MWNTs/MIPs and MWNTs/NIPs at equilibrium concentration increased along with the increase of the initial concentration of Ribavirin, but the binding amount of Ribavirin on MWNTs/MIPs was greater than that on MWNTs/NIPs in the whole concentration range. This result indicated that the MWNTs/MIPs had a specific binding capacity for the template molecule.
In order to verify the selectivity of MWNTs/MIPs for Ribavirin, the selectivity test of MWNTs/MIPs and MWNTs/NIPs were performed by using Telbivudine and S-Napoxen as the structure related compounds. The amounts bound to MWNTs/MIPs and MWNTs/NIPs were also determined by adsorption method mentioned above. The obtained Q of the substrates was shown in Fig. 9. It was obvious that the binding capacity of Telbivudine and S-Napoxen was also much lower than that of Ribavirin, which confirmed that the MWNTs/MIPs had a good selectivity for recognition, and the chemical structure of Telbivudine with Ribavirin was closer than that of S-Napoxen, so the adsorption capacity of Telbivudine was greater than that of S-Napoxen. As one of the artificial receipted materials, the key property of the molecularly imprinted polymers was its capability of special adsorption and selective recognition for template molecule, which was based on the imprinted cavities in complement to the size, shape, and functionality of the template molecule [20]. The selective recognition for Ribavirin of MWNTs/MIPs may be attributed to the shape selective fitting of Ribavirin into complementary cavities created in the MWNTs /MIPs during the imprinting procedure.
Conclusions
New MWNTs/MIPs composites for the specific binding of Ribavirin were prepared by using the molecular imprinting method. Molecularly imprinted polymer could be coated on the surface of MWNTs. The MIPs formed on the nanotube were about 70 % amount of the total weight of the MWNTs/MIPs. With 1:4 of molar ratio of template to monomer, best binding capacity as well as imprinted effect was obtained. The resulting MWNTs/MIPs possessed a faster adsorption dynamics, higher selectivity for Ribavirin. The present imprinting method is a potential method for the preparation of the receptors which can recognize water soluble compounds in aqueous media.
References
Yang Y, Long YY, Cao Q, Li K, Liu F (2008) Molecularly imprinted polymer using β-cyclodextrin as functional monomer for the efficient recognition of bilirubin. Anal Chim Acta 606:92–97
Mazzotta E, Malitesta C (2010) Electrochemical detection of the toxic organohalide 2,4-DB using a Co-porphyrin based electrosynthesized molecularly imprinted polymer. Sens Actuators B 148:186–194
Amut E, Fu Q, Fang Q, Liu R, Xiao AP, Zeng AG, Chang C (2010) In situ polymerization preparation of chiral molecular imprinting polymers monolithic column for amlodipine and its recognition properties study. J Polym Res 17:401–409
Kubo T, Nomachi M, Nemoto K, Sano T, Hosoya K, Tanaka N, Kaya K (2006) Chromatographic separation for domoic acid using a fragment imprinted polymer. Anal Chim Acta 577:1–7
Beltran A, Marce RM, Cormack PAG, Borrull F (2010) Synthetic approaches to parabens molecularly imprinted polymers and their applications to the solid-phase extraction of river water samples. Anal Chim Acta 677:72–78
Han DM, Fang GZ, Yan XP (2005) Preparation and evaluation of a molecularly imprinted sol–gel material for on-line solid-phase extraction coupled with high performance liquid chromatography for the determination of trace pentachlorophenol in water samples. J Chromatogr A 1100:131–136
Zhang ZH, Yang X, Zhang HB, Zhang ML, Luo LJ, Hu YF, Yao SZ (2011) Novel molecularly imprinted polymers based on multi-walled carbon nanotubes with binary functional monomer for the solid-phase extraction of erythromycin from chicken muscle. J Chromatogr B 879:1617–1624
Shen ZL, Yuan D, Su QD, Zhang H, Wang J, Zhu JH, Liu YM (2011) Selective solid-phase extraction using molecularly imprinted polymer for analysis of methamidophos in water and soil samples. Biosci Biotechnol Biochem 75:473–479
Kriz D, Mosbach K (1995) Competitive amperometric morphine sensor based on an agarose immobilised molecularly imprinted polymer. Anal Chim Acta 300:71–75
Andrea P, Miroslav S, Silvia S, Stanislav M (2001) A solid binding matrix/molecularly imprinted polymer-based sensor system for the determination of clenbuterol in bovine liver using differential-pulse voltammetry. Sens Actuators B 76:286–294
Kröger S, Turner APF, Mosbach K, Haupt K (1999) Imprinted polymer-based sensor system for herbicides using differential-pulse voltammetry on screen-printed electrodes. Anal Chem 71:3698–3702
Wulff G (2002) Enzyme-like catalysis by molecularly imprinted polymers. Chem Rev 102:1–28
Ramström O, Mosbach K (1999) Synthesis and catalysis by molecularly imprinted materials. Curr Opin Chem Biol 3:759–764
Gholivand MB, Khodadadian M (2011) Rationally designed molecularly imprinted polymers for selective extraction of methocarbamol from human plasma. Talanta 85:1680–1688
Sambe H, Hoshina K, Haginaka J (2007) Molecularly imprinted polymers for triazine herbicides prepared by multi-step swelling and polymerization method. Their application to the determination of methylthiotriazine herbicides in river water. J Chromatogr A 1152:130–137
Xu ZF, Xu L, Kuang DZ, Zhang FX, Wang JQ (2008) Exploiting β-cyclodextrin as functional monomer in molecular imprinting for achieving recognition in aqueous media. Mater Sci Eng C 28:1516–1521
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Tsai FC, Shu CM, Tsai LC, Ma N, Wen Y, Wen S, Yang YK, Zhou W, Xiao HW, Shu YC , Jiang T. Chapter 7: Carbon nanotube industrial applications, In: Jose Mauricio Marulanda (Ed.) Carbon nanotubes applications on electron devices, ISBN: 9789533074962, InTech, Aug 2011, pp. 387–404.
Kan XW, Zhao Y, Geng ZR, Wang ZL, Zhu JJ (2008) Composites of multiwalled carbon nanotubes and molecularly imprinted polymers for dopamine recognition. J Phys Chem 112:4849–4854
Lee SC, Chuang FL, Tsai YL, Chen H (2010) Studies on the preparation and properties of sol-gel molecularly imprinted polymer based on tetraethoxysilane for recognizing sulfonamides. J Polym Res 17:737–744
Acknowledgments
This study was partially supported by the National Natural Science Foundation of China (No.20202015) and the Key Academic Program of 211 Project of South China Agricultural University (2009B010100001). The support by the large-scale scientific instrumentation foundation of South China Agricultural University was also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, L., Xu, Zf. Molecularly imprinted polymer based on multiwalled carbon nanotubes for ribavirin recognition. J Polym Res 19, 9942 (2012). https://doi.org/10.1007/s10965-012-9942-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-012-9942-1