Abstract
Poly(L-lactic acid) (PLLA) fiber mats containing a crude extract of Garcinia cowa Roxb. (GC) were prepared by electrospinning. The extract was introduced at a level of either at 30 or 50 % with respect to the weight of PLLA. The fibers of both the neat and the GC-loaded PLLA fibers were smooth, with average diameters of 0.80–1.13 μm. The characteristics of the release of GC from the GC-loaded PLLA fiber mats were assessed by a total immersion method in acetate or phosphate buffer solution that contained 0.5 % v/v Tween 80 and 3 % v/v methanol (hereafter, A/T/M or P/T/M medium) at either 32 or 37 °C, respectively. The maximum cumulative amount of GC released from the GC-loaded PLLA fiber mats in the P/T/M medium was greater than that released in the A/T/M medium. The antioxidant activity of the GC-loaded PLLA fiber mats, assessed using a 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay, remained even after they had been exposed to a high electrical potential. The antimicrobial activity of the GC-loaded mats was greatest against Straphylococcus aureus ATCC 25923 and Straphylococcus aureus DMST 20654. Lastly, almost all of the GC-loaded PLLA fiber mats, except for those that contained 50 % GC, were found to be nontoxic to normal human dermal fibroblasts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last couple of decades, electrospinning has been well received as a simple and efficient method for the production of ultrafine fibers with sizes ranging from few micrometers down to tens of nanometers [1]. The outstanding characteristics of these fibers are, for example, their high surface area to mass or volume ratios, the small pore sizes of the resulting fibrous matrices, and the myriad possibilities for surface functionalization that they permit [1]. These characteristics make electrospun fibers ideal for various applications. In the field of biomedicine, they can be used as wound dressings [2–6], substrates for tissue/cell cultures [7, 8], and carriers for drug delivery [9–13]. This fiber fabrication technique requires simple tooling; i.e., a power supply that is capable of generating a 10–30 kV DC current that can be used to charge up a spinnable liquid (either a melt or solution) between the opening of the container and a collection device [1]. Beyond a critical electric field, the liquid is discharged from the opening as a continuous stream that solidifies to form a nonwoven fabric of ultrafine fibers on the collector [1, 14]. The size of the fibers varies from one system to the next, but it can generally be controlled by varying the process parameters appropriately (e.g., shear viscosity, electric field, flow rate, etc.) [15].
Poly(L-lactic acid) (PLLA), a biodegradable and biocompatible linear aliphatic thermoplastic polyester, is commonly produced from renewable resources. Due to its good processability, inherent biocompatibility and biodegradability, and useful mechanical properties, it has been widely used in biomedical applications as carriers for drug delivery, scaffolding materials, and prosthetic devices [16]. PLLA can easily be fabricated into ultrafine fibers by electrospinning. Several solvent systems are used in the fabrication of the electrospun PLLA fibers, such as 7:3 v/v dichloromethane (DCM)/dimethylformamide (DMF) [17], 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) [18], chloroform [19], 3:1 v/v chloroform/DMF [20], HFIP [21], and 2:1 v/v chloroform/acetone [22]. Moreover, various studies on the use of electrospun PLLA fibers as carriers for drug delivery systems have been reported. Zong et al. [23] studied the release of an antibiotic drug, Mefoxin, from electrospun poly(D,L-lactic acid) (PDLA) fiber mats. A burst release of the drug was observed within the first 3 h, and it took 48 h for the maximal amount of the drug to be released. Kenawy et al. [24] studied the characteristics of the release of tetracycline hydrochloride from electrospun fibrous matrices of poly(ethylene-co-vinyl acetate), poly(lactic acid), and their blends, and found that drug release from all of these materials was high for the first 10–12 h. Additionally, Chuysinuan et al. [25] reported that as-loaded gallic acid within electrospun PLLA fiber mats was initially released rather rapidly into different media (acetate buffer, citrate–phosphate buffer, and normal saline) upon initial submersion, and the amount of released gallic acid then gradually increased as the submersion time increased, before finally reaching a plateau level at long submersion times.
Garcinia cowa Roxb. (Guttferae) (GC) is indigenously known in Thailand as Cha-muang. It has been used in Thai folk medicine for several purposes. Its bark has been used as an antipyretic and antimicrobial agent, while the latex has found use as a fever suppressant [26]. Its fruits and leaves are used to treat cough and indigestion, while the roots are also employed for fever relief [27]. It has been found that crude extracts from the leaves of the plant exhibit antitumor activity [28, 29]. Upon isolating the compounds in the extracts, xanthones and their derivatives were found to be the major components. These compounds are well known for their wide range of biological and pharmacological activities, which include (but are not limited to) antimicrobial, antioxidant, anti-inflammatory, and antimalarial activities [30–35].
Here, a crude DCM extract from the leaves of GC was loaded into a 10 % w/v PLLA solution in 7:3 v/v DCM/DMF at a level of either 30 or 50 % (based on the weight of PLLA), and the resulting solutions were then fabricated into ultrafine fibers by electrospinning. Various properties (i.e., morphological, water retention, mass loss, and cytotoxicity) of both the neat and the GC-loaded PLLA fiber mats were investigated. The characteristics of the release of GC from the PLLA fiber mats into A/T/M or P/T/M medium were investigated by the total immersion method. Finally, the GC and the GC-loaded PLLA fiber mats were tested for their antioxidant and antimicrobial activities against some common pathogenic microorganisms found on burn wounds.
Experimental details
Materials
PLLA (M n = 200,000 g mol−1 and intrinsic viscosity = 0.332 dL g−1) was obtained from NatureWorks (Minnetonka, MN, USA). Dichloromethane (DCM) and dimethylformamide (DMF) were purchased from Labscan (Asia) (Bangkok, Thailand). Sodium acetate, sodium chloride, anhydrous disodium hydrogen orthophosphate, sodium dihydrogen orthophosphate (Ajax Chemicals, Auburn, Australia), glacial acetic acid (Carlo Erba, Milan, Italy), and all other chemicals were of analytical reagent grade and used without further purification.
Extraction of Garcinia cowa (GC)
The leaves of GC were collected from Trang Province in the southern part of Thailand. The dried leaves (∼1.3 kg) were ground and extracted with 5.4 L of dichloromethane for three weeks. The extracts were filtered through Whatman no. 4 filter papers and concentrated with an evaporator to remove the solvent, yielding a brown, viscous liquid extract. The crude extract (∼72 g) was further separated by silica gel quick column chromatography and eluted with hexane and dichloromethane in a polarity-gradient system. The eluted fractions were combined into 18 fractions on the basis of their chromatographic characteristics. Finally, the eighteenth fraction (8.3757 g), eluted with 100 % dichloromethane, was used in this study.
Preparation and electrospinning of neat and GC-containing PLLA solutions
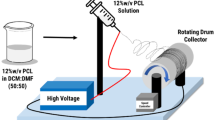
The base PLLA solution in 7:3 v/v DCM/DMF was first prepared at a fixed concentration of 10 % w/v. The GC-containing PLLA solutions were prepared by dissolving the same amount of PLLA powder and GC in the DCM/DMF mixture at either 30 or 50 % (based on the weight of PLLA powder). Prior to electrospinning, the solutions were characterized for their viscosities and conductivities at room temperature (26 ± 1 °C) using a Brookfield (Middleboro, MA, USA) RVDV-II + P viscometer and a CyberScan (Nijkerk, Netherlands) Con100 conductivity meter, respectively. The solutions were then electrospun under a fixed electric field of 20 kV/18 cm. The collection time was ∼12 h, resulting in fiber mats of thickness 70 ± 10 μm Note that Chuysinuan et al. [25] reported that the thickness of their gallic acid-loaded PLLA fiber mats was ∼400 μm (collection time ∼12 h). We achieved thinner GC-loaded PLLA fiber mats due to clogging of the spinning solution at the tip of the nozzle, which led to inconsistent solution flow. In order to obtain neat PLLA fiber mats of a similar thickness to that of their GC-loaded counterparts, less PLLA solution was used.
Characterization of the neat and GC-loaded PLLA fiber mats
The morphological appearances of both the neat and the GC-loaded PLLA fiber mats were observed using a LEO (Cambridge, UK) 1450 VP scanning electron microscope (SEM). Prior to being observed under the SEM, each sample was coated with a thin layer of gold using a Polaron SC-7620 sputtering device (Quorum Technologies, Newhaven, UK). Fiber diameters were measured directly from SEM images using the SemAphore 4.0 software package.
The water retention and mass loss behaviors of both the neat and the GC-loaded PLLA fiber mats were measured in an acetate or a phosphate buffer solution containing 0.5 % v/v Tween 80 and 3 % v/v methanol (i.e., the A/T/M medium and the P/T/M medium, respectively; see below for media preparation) at skin temperature or the physiological temperature (32 and 37 °C, respectively) for 24 and 48 h according to the following equations:
And
where M is the mass of each sample after submersion in a buffer solution for a certain period of time (24 or 48 h), M d is the mass of the sample after submersion in the buffer solution for a certain period of time (24 or 48 h) in its dry state, and M i is the initial mass of the sample in its dry state.
Release of GC from GC-loaded PLLA fiber mats
Preparation of the media into which the GC was released
An acetate buffer solution was chosen to simulate the human skin pH condition of 5.5. To prepare 1,000 mL of this solution, 150 g of sodium acetate were dissolved in 250 mL of distilled water. Exactly 15 mL of glacial acetic acid were then added very slowly, and distilled water was finally added to achieve the required volume. On the other hand, to prepare 1,000 mL of phosphate buffer solution, 6.177 g of anhydrous disodium hydrogen orthophosphate and 1.014 g of sodium dihydrogen orthophosphate were dissolved in 100 mL of distilled water. Exactly 8.7 g of sodium chloride were then added to 20 mL of this solution. Distilled water was finally added to achieve the required volume and, if necessary, the pH was adjusted to 7.4. Due to the insolubility of GC in aqueous media, slight modifications to both types of buffer media were necessary. Thus, 0.5 % v/v of Tween 80 and 3 % v/v of methanol were added to the media, respectively.
Actual GC content
The actual amounts of GC in the GC-loaded PLLA fiber mats were first determined. Each sample (a circular disc ∼2.8 cm in diameter) was dissolved in 10 mL of 7:3 v/v DCM/DMF. After that, 1.0 mL of the solution was measured using a PerkinElmer (Waltham, MA, USA) Lambda 35 UV-vis spectrophotometer at the wavelength of 412 nm. The actual amounts of GC in the GC-loaded PLLA fiber mats were then back-calculated from the resulting data against a predetermined calibration curve.
GC release assay
The release characteristics of GC from the GC-loaded PLLA fiber mats were investigated by the total immersion method in the A/T/M or the P/T/M media. Each sample (a circular disc ∼2.8 cm in diameter) was immersed in 20 mL of the A/T/M medium at 32 °C or 20 mL of the P/T/M medium at 37 °C. After a specified immersion time ranging between 0 and 48 h (2880 min), 1.0 mL of the medium (i.e., the sample solution) was withdrawn, and an equal amount of fresh medium was added. The amount of GC in each sample solution was determined spectrophotometrically at the wavelength of 412 nm. The resulting data were then carefully analyzed to determine the cumulative amount of GC released.
Antioxidant activity
The antioxidant activities of the as-extracted and the as-loaded GC were determined using the DPPH assay. For the as-extracted GC, the stock solution of GC in DCM was serially diluted to obtain GC solutions with final concentrations of 2.5, 1.25, 0.625, 0.3125, 0.156, 0.078, 0.039, and 0.0195 mg mL−1. Exactly 1.0 mL of a methanolic solution of DPPH (100 μM) was added to 1.0 mL of each GC dilution, and the obtained mixtures were incubated for 30 min at 37 °C. The free-radical scavenging activity of the as-extracted GC was determined spectrophotometrically at the wavelength of 517 nm. As for the as-loaded GC, the method was a slightly modified version of that utilized by Robert et al. [36]. Specifically, each sample (a circular disc ∼2.8 cm in diameter) was first dissolved in 10 mL of 7:3 v/v DCM/DMF and then treated with a methanolic solution of DPPH (100 μM) for 30 min (i.e., 1.0 mL of the as-loaded GC solution against 1.0 mL of the DPPH solution) at 37 °C. The free-radical scavenging activity of the as-loaded GC was determined spectrophotometrically at the wavelength of 517 nm. The antioxidant activity (%AA) of either the as-extracted or the as-loaded GC was expressed as the percentage decrease in DPPH compared with the level in the control solution (i.e., the test solution without either type of GC), according to the following equation:
where A control and A sample are the absorbance values of the test solution without and with either type of GC.
Antimicrobial evaluation
The antimicrobial activities of both the neat and the GC-loaded PLLA fiber mats were tested against some common pathogenic microorganisms by the disc diffusion method: Acinetobacter calcoaceticus, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus ATTC 25923, Staphylococcus aureus DMST 20654, Staphylococcus epidermidis, Streptococcus agalactiae, Streptococcus pyogenes, and Candida albican. Suspension of microorganisms in the CriterionTM nutrient broth (NB) was spread as a thin layer on CriterionTM Mueller–Hinton (MH) agar in Petri dishes. After that, each neat and GC-loaded PLLA fiber mat specimen (13 mm in diameter) was placed on top of the smeared agar, and the plate was incubated at 37 °C for 24 h. The neat PLLA fiber mats were used as controls. Upon reaching the inhibitory concentration, microbe growth ceases, which can be seen as clear or inhibition zones around the disc specimens. These were photographed for further evaluation.
Indirect cytotoxicity evaluation
The indirect cytotoxicity evaluations of both the neat and the GC-loaded PLLA fiber mats were adapted from the ISO 10993-5 standard test method performed in a 96-well tissue-culture polystyrene plate (TCPS; Costar®, Corning, NY, USA) using normal human dermal fibroblasts (NHDF; 24th passage) as a reference. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma–Aldrich, St. Louis, MO, USA) supplemented with 10 % fetal bovine serum (FBS; Invitrogen Corp., Carlsbad, CA, USA), 1 % L-glutamine (Invitrogen Corp.) and a 1 % antibiotic and antimycotic formulation [containing penicillin G sodium, streptomycin sulfate, and amphotericin B (Invitrogen Corp.)]. The samples cut from both the neat and the GC-loaded PLLA fiber mats were first sterilized by UV radiation for ∼1 h and then immersed in serum-free medium (SFM; containing DMEM, 1 % L-glutamine, and 1 % antibiotic and antimycotic formulation) for 24 h of incubation to produce extraction media at various extraction ratios (i.e., 10, 5, and 0.5 mg mL−1). NHDF cells were separately cultured in wells of TCPS at 8,000 cells/well in serum-containing DMEM for 24 h to allow cell attachment. The cells were then starved with SFM for 12 h. After that, the medium was replaced with extraction medium, and the cells were re-incubated for 24 h. The viability of the cells cultured by each extraction medium was finally determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The viability of the cells cultured with the fresh SFM was used as a control.
The MTT assay is based on the reduction of the yellow tetrazolium salt to purple formazan crystals by dehydrogenase enzymes secreted from the mitochondria of metabolically active cells. The amount of purple formazan crystals is proportional to the number of viable cells. First, the culture medium in each plate was aspirated and replaced with 25 μL/well of MTT solution at 5 mg mL−1. The plate was incubated further for 4 h at 37 °C. The solution was then aspirated and 100 μL/well of dimethylsulfoxide (DMSO; Sigma–Aldrich) was added to dissolve the formazan crystals. After 3 min of rotary agitation, the absorbance at the wavelength of 570 nm, representing the viability of the cells, was measured using a SpectraMax M2 microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Statistical analysis
Data are presented as means ± standard errors of means. Statistical analysis was carried out by one-way analysis of variance (one-way ANOVA) and Scheffe’s post hoc test in SPSS (SPSS, IBM, Armonk, NY, USA). The statistical significance was considered to be p < 0.5.
Results and discussion
Electrospinning of neat and GC-containing PLLA solutions
Prior to the electrospinning, both the neat and the GC-containing PLLA solutions were characterized for shear viscosity and electrical conductivity, and the results are summarized in Table 1. While the presence of GC in the neat PLLA solution did not affect the shear viscosity of the resulting solutions, it caused the electrical conductivity to increase marginally. A dramatic increase in the electrical conductivity of the PLLA solution was reported when gallic acid (present at a level of 40 % based on the weight of PLLA) was incorporated into the neat PLLA solution [25], which was hypothesized to be due to the dissociation of gallic acid into ionic species when it was subjected to a high electric field. Here, the dissociation of certain compounds in GC into ionic species was also expected, but to a much lesser extent.
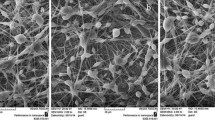
Electrospinning of both the neat and the GC-containing PLLA solutions was carried out at a fixed electric field of 20 kV/18 cm. Representative SEM images of the neat and the GC-loaded PLLA fiber mats are also shown in Table 1. Cross-sectionally round fibers with smooth surfaces were common features of the obtained electrospun products. Since no evidence of any kind of aggregate was observed on the surfaces of the fibers from the GC-containing PLLA solutions, we can hypothesize that GC was incorporated well within the mass of the fibers. The diameters of these fibers were measured, and the results are also summarized in Table 1. While the diameters of the neat PLLA fibers were 1.13 ± 0.02 μm, the marginally higher electrical conductivity of the GC-containing PLLA solutions compared to that of the neat PLLA solution is though to have led to the observed decrease in fiber diameter for the GC-loaded fiber mats (i.e., 0.80 ± 0.16 and 1.04 ± 0.24 μm for the PLLA solutions containing 30 and 50 % of GC, respectively). The increased electrical conductivity of the GC-containing PLLA solutions was a direct result of the increase in the amount of charge present within the GC-containing PLLA solutions due to the incorporation of GC, which led to an increase in the number of charge carriers within a jet segment. This increase in the number of charge carriers caused both the electrostatic and the Coulombic repulsive forces to increase, leading to further thinning of the resulting GC-loaded PLLA fibers compared to their neat PLLA counterparts.
Physicochemical properties of neat and GC-loaded PLLA fiber mats
The physicochemical properties (water retention and mass loss) of both the neat and the GC-loaded PLLA fiber mats after submersion in the A/T/M or the P/T/M medium for 24 h or 48 h were characterized, and the results are shown in Fig. 1. It should be noted that the fiber mats from the PLLA solutions that contained 30 and 50 % GC are hereafter denoted as 30 % and 50% GC-loaded PLLA fiber mats, respectively. At 24 h after submersion in the A/T/M medium, the water retentions of the neat, the 30% GC-loaded, and the 50% GC-loaded PLLA fiber mats were 398, 441, and 474 % on average, respectively (see Fig. 1a). At 48 h, the values increased to 481, 482, and 504 % on average, respectively. In the P/T/M medium, the values at 24 h after submersion were 486, 496, and 527 % on average, respectively, while they were 487, 502, and 592 % on average, respectively, at 48 h. Thus, the water retentions of both the neat and the GC-loaded PLLA fiber mats in both types of releasing medium increased with increasing submersion time, except for the neat and the 30% GC-loaded PLLA fiber mats in the P/T/M medium, which showed equivalent values at both time points. Between the two media, the water retentions of both the neat and the GC-loaded PLLA fiber mats in the P/T/M medium, at any given time point, were greater than the water retentions of these materials in the A/T/M medium.
a Water retentions and b mass loss behavior of neat and GC-loaded PLLA fiber mats in two types of medium: acetate or phosphate buffer solution containing 3 % v/v methanol and 0.5 % v/v Tween 80 (i.e., A/T/M or P/T/M medium) (n = 3). *p < 0.05 for the comparison between A/T/M and P/T/M for any given type of sample and submersion time point, and # p < 0.05 compared with PLLA fiber mats for any given submersion time point and type of medium
The mass losses of the neat and the GC-loaded PLLA fiber mats upon submersion in either medium is shown in Fig. 1b. At 24 h after submersion in the A/T/M medium, the mass losses of the neat, the 30% GC-loaded, and the 50% GC-loaded PLLA fiber mats were 8, 12, and 20 % on average, respectively, while the values were 8, 18, and 22 % on average, respectively, at 48 h. In the P/T/M medium, the values at 24 h after submersion were 16, 17, and 23 % on average, respectively. Slightly greater values of 21, 21, and 27 % on average, respectively, were obtained at 48 h after submersion. Similar to the water retention behavior, the mass losses of both the neat and the GC-loaded PLLA fiber mats in both types of releasing medium increased with increasing submersion time, except for the neat PLLA fiber mats in the A/T/M medium, which showed equivalent values at both time points. Also similar to the water retention behavior, the mass losses of all of the fibrous matrices at any given time point were greater in the P/T/M medium than in the A/T/M medium.
The mass losses for the neat PLLA fiber mats in the A/T/M medium were equivalent at both time points. The mass losses of the neat PLLA fiber mats were greater in the P/T/M medium, and they also increased with increasing the submersion time. Partial hydrolysis of the ester bonds on the surfaces of the fibers occurred more readily in the more basic pH of the P/T/M medium as opposed to its A/T/M counterpart. This partial hydrolysis resulted in not only the observed increase in mass loss, but also in increased hydrophillicity and thus a slight increase in water retention for the neat PLLA fiber mats upon their submersion in the P/T/M medium. When GC was incorporated within the fibers, a marginal increase in the mass loss of the GC-loaded PLLA fiber mats was observed upon their submersion in a given medium, which is expected to be the result of the release of GC from the fibers. The release of GC from the fibers led to increased accessibility of the fibers to water molecules, resulting in the observed increase in the water retention of the GC-loaded PLLA fiber mats.
Release of GC from GC-loaded PLLA fiber mats
The actual amounts of GC in the GC-loaded PLLA fiber mats were determined prior to investigating the release characteristics of GC from these samples, and it was found that the actual amounts of GC in the 30% GC- and the 50% GC-loaded PLLA fiber mats were 90.3 ± 5.7 and 98.9 ± 2.7 % (based on the amounts of GC initially present in the spinning solutions), respectively. These values were later used to calculate the cumulative amounts of GC released from these GC-loaded materials.
The release characteristics of GC from the GC-loaded PLLA fiber mats were investigated by the total immersion method over a period of 2880 min in the A/T/M or the P/T/M medium. The cumulative release profiles of GC from all of the GC-loaded PLLA fiber mats were determined as the percentage corresponding to the weight of GC released divided by the actual weight of GC in the sample (see Fig. 2 and Fig. S1 in the “Electronic supplementary material,” ESM). In all cases, the cumulative amounts of GC released into either medium initially increased quite rapidly with increasing submersion time (i.e., within the first 200 min), and increased more gradually afterwards. In the A/T/M medium, the cumulative amounts of GC released seemed to plateau towards the end of the observational time period. Such trends were, however, not observed (within the time frame investigated) in the P/T/M medium. The susceptibility of PLLA to partial hydrolysis in the more basic conditions of the P/T/M medium was hypothesized to be the main reason for the observed release behavior.
Cumulative release profiles of GC from GC-loaded PLLA fiber mats, reported as the percentage corresponding to the weight of GC released divided by the actual weight of GC in the sample, as measured by the total immersion method in a A/T/M medium at the skin temperature of 32 °C or b P/T/M medium at the physiological temperature of 37 °C
In the A/T/M medium, the cumulative amounts of GC released from both the 30% GC- and the 50% GC-loaded PLLA fiber mats were equivalent within the first 60 min of submersion, after which the values from the 50% GC-loaded PLLA fiber mats were greater. In the P/T/M medium, on the other hand, the cumulative amounts of GC released from the 50% GC-loaded PLLA fiber mats were slightly greater than that from those from the 30% GC-loaded counterparts at all time points investigated. Additionally, the cumulative amounts of GC released from the 30% GC-loaded PLLA fiber mats into the P/T/M medium at submersion time points of >120 min were greater than those released into the A/T/M medium. On the other hand, the values from the 50% GC-loaded PLLA fiber mats in the P/T/M medium at most time points investigated were slightly lower than those in the A/T/M medium. Specifically, the maximum cumulative amounts of GC released from the 30% GC- and the 50% GC-loaded PLLA fiber mats upon submersion in the A/T/M medium were ∼34 and ∼59 %, respectively, on average. On the other hand, average values of ∼57 and ∼64 % were observed in the P/T/M medium.
Release kinetics of GC from GC-loaded PLLA fiber mats
The release kinetics of a therapeutic agent from a carrier can be characterized using an equation of the following form [37, 38]:
where M t is the cumulative amount of the drug released at an arbitrary time t, M ∞ is the cumulative amount of the drug released after an infinite amount of time, n is an exponent characterizing the mechanism that can be used to describe the release kinetics, and k is the rate of release of the drug, which incorporates the physical characteristics of the matrix/drug system as well as some physical contributions from the measurement method.
When n equals 0.5, the release mechanism can be `described as Fickian diffusion [39]. For this, a straight line is expected when the fractional, cumulative amounts of the drug released (i.e., M t/M ∞) are plotted versus the square root of the submersion time (i.e., t 0.5). Here, the release characteristics of GC from all of the GC-loaded PLLA fiber mats were analyzed according to the Fickian diffusion mechanism. The average values of k (along with the values of r 2, signifying the goodness of the curve fit, reported in parentheses) associated with the release kinetics of GC from the 30% GC-loaded PLLA fiber mats in the A/T/M and the P/T/M media were found to be 0.0039 (0.94) and 0.0035 (0.99) s−0.5, respectively, while those associated with the release kinetics of GC from the 50% GC-loaded PLLA fiber counterparts were 0.0064 (0.98) and 0.0033 (1.00) s−0.5, respectively.
Antioxidant activity of as-extracted and as-loaded GC
Mahabusarakum et al. [35] reported that GC is rich in phenolic compounds. Many phenolic compounds are known to be potent antioxidants (i.e., they exhibit good hydrogen and/or electron donor ability). DPPH• is a stable free radical, and is capable of accepting an electron or a hydrogen radical to revert to a stable molecule. When a DPPH solution is mixed with a substance that acts as a proton donor, a stable non-radical form of DPPH is obtained. This is coupled with a change of color from violet to pale yellow. Here, the DPPH radical scavenging assay was used to quantify the antioxidant activities of both of the as-extracted and the as-loaded GC. Table 2 shows the antioxidant activity of the as-extracted GC as a function of GC concentration, using DCM as the solvent. As expected, the antioxidant activity of the as-extract GC increased with GC concentration. Specifically, as the concentration decreased from 2.5 to 0.0195 mg mL−1, the antioxidant activity decreased from ∼66 to ∼0.5 %, and the half-maximal inhibitory concentration (IC50) of the as-extracted GC was interpolated to be about 1.4 mg mL−1. Similarly, the antioxidant activity of the as-loaded GC was also determined, and it was found to be 8.7 ± 0.4 and 11.8 ± 0.5 % for the GC that had been loaded into the 30% GC- and the 50% GC-loaded PLLA fiber mats, respectively. Upon the dissolution of the respective GC-loaded PLLA fiber mats in 10 mL of 7:3 v/v DCM/DMF, given the actual amounts of GC loaded within these fibers, the concentrations of the as-loaded GC in the DCM/DMF mixture should be ∼0.183 and ∼0.374 mg mL−1, respectively. Based on this information, the observed antioxidant activity of the as-loaded GC corresponded well to the antioxidant activity of the as-extracted GC shown in Table 2. These results confirm that the antioxidant activity of the as-loaded GC was retained even after it had been subjected to a high electrical potential during electrospinning.
Antimicrobial activities of GC-loaded PLLA fiber mats
The antimicrobial activities of the GC-loaded PLLA fiber mats against some common pathogenic microorganisms were evaluated, and the results are summarized in Table 3, while photographic images showing the activities of some mats against E. coli, P. aeruginosa, S. aureus DMST 20654, and C. albican are shown in Fig. 3. The activities of the neat PLLA fiber mats against these microbes were used as a control. The initial diameter of each sample was fixed at 1.3 cm. As expected, the neat PLLA fiber mats showed no activity against the tested microbes. After 24 h of incubation, inhibition zones against almost all of the tested microbes were observed around the edges of the 30% GC- and the 50% GC-loaded PLLA fiber mat specimens. The only exception was the 30% GC-loaded PLLA fiber mat sample, which showed no activity towards A. calcoaceticus. The 50% GC-loaded PLLA fiber mats showed high potential for antimicrobial activity against both of the tested S. aureus strains (i.e., ATTC 25923 and DMST 20654); these mats presented inhibition zones in the range of 1.76–2.15 cm. Furthermore, the 50% GC-loaded PLLA fiber mats showed moderate activity against the growth of S. epidermidis, S. agalactiae, and S. pyogenes, with the corresponding inhibition zones being in the range of 1.51–1.75 cm. On the other hand, the 30% GC-loaded PLLA fiber mats exhibited moderate activity against both of the tested S. aureus strains (i.e., ATTC 25923 and DMST 20654), with their inhibition zones being in the range of 1.51–1.75 cm. While the antimicrobial activities of both types of GC-loaded PLLA fiber mats were low against E. coli, P. aeruginosa, and C. albican, those of the 30% GC-loaded PLLA fiber mats were also low against the growth of S. epidermidis, S. agalactiae, and S. pyogenes.
Indirect cytotoxicity evaluation
The potential use of the GC-loaded PLLA fiber mats as wound dressings was assessed by investigating the cytotoxicity of these samples, using the neat PLLA fiber mats as an internal control group. A comparison of the viability of the NHDF that had been cultured with the extraction media from these samples with the viability of the cells that had been cultured with the fresh culture medium is shown in Fig. 4. The extraction media were prepared at three different extraction ratios (i.e., 10, 5, and 0.5 mg mL−1). The relative viability of the cells that had been cultured with all of the extraction media from the neat PLLA fiber mats ranged between ∼98 and ∼102 %, indicating that the neat PLLA fiber mats did not release any substance at levels that were harmful to the cells. At the lowest extraction ratio investigated (i.e., 0.5 mg mL−1), none of the GC-loaded PLLA fiber mats released any substance at levels that were harmful to the cells. At 5 and 10 mg mL−1, the 30% GC-loaded PLLA fiber mats still posed no threat to the cells, as the relative viability of the cells still exceeded the threshold value of at least ∼80 %. On the other hand, the relative viability of the cells that had been cultured with the extraction media from the 50% GC-loaded PLLA fiber mats was lower than the threshold value, indicating that there must be some substances in the crude extract that had been leached from the fibers in the levels that were present at levels that were detrimental to the cells.
Indirect cytotoxicity evaluation of neat and GC-loaded PLLA fiber mats: the viability of normal human dermal fibroblasts (NHDF) that had been cultured with the extraction media from the fibrous materials was compared with the viability of the cells that had been cultured with fresh culture medium (n = 3). *p < 0.05 compared with fresh culture medium at any given extraction ratio
Conclusions
In this study, a DCM extract of the plant Garcinia cowa Roxb. (Guttiferae) (GC) was added to a neat solution of PLLA (10 % w/v in 7:3 v/v DCM/DMF) in various amounts (i.e., 30 and 50 wt% based on the weight of the PLLA powder). Both the neat and the GC-containing PLLA solutions were successfully fabricated into fibers by electrospinning at a fixed electric field of 20 kV/18 cm. Adding the GC to the neat PLLA solution did not affect the morphology of the fibers. Cross-sectionally round fibers with smooth surfaces were obtained. The average diameters of the neat, the 30% GC- and the 50% GC-loaded PLLA fiber mats were ∼1.13, ∼0.80, and ∼1.04 μm, respectively. The water retention and the mass loss behavior of the GC-loaded PLLA fiber mats in both two types of releasing medium (i.e., the A/T/M or the P/T/M medium) increased with increasing submersion time. Moreover, the water retention and mass loss behavior of these samples after submersion in the P/T/M medium were greater than those in the A/T/M medium. The cumulative amounts of GC released from the GC-loaded PLLA fiber mats in the A/T/M medium gradually increased with increasing submersion time before reaching a plateau value at 1440 min, while those in the P/T/M medium increased more gradually over the testing period before reaching a plateau value at the longest submersion time investigated. The maximum amounts of GC released from the 30% GC- and the 50% GC-loaded PLLA fiber mats after submersion in the A/T/M medium were ∼34 % and ∼59 %, respectively, while those in the P/T/M medium were ∼57 % and ∼64 %, respectively. The fact that a greater cumulative amounts of GC released from the GC-loaded PLLA fibers into the P/T/M medium are likely due to the greater water retention and mass loss of these samples after submersion in the P/T/M medium. The IC50 of GC was ∼1.4 mg mL−1. Moreover, the antioxidant activity (obtained using the DPPH assay) of the GC-loaded PLLA fiber mats was retained even after they had been subjected to a high electrical potential during the electrospinning process. The antimicrobial activity of the GC-loaded PLLA fiber mats was strongest against Straphylococcus aureus ATCC 25923 and Straphylococcus aureus DMST 20654. Lastly, the potential use of the GC-loaded PLLA fiber mats as wound dressings was assessed using an indirect cytotoxicity test. The results showed that only the 50% GC-loaded PLLA fiber mats at extraction ratios of 5 and 10 mg mL−1 were toxic to the normal human dermal fibroblasts.
References
Reneker DH, Yarin AL (2008) Polymer 49:2387–2425
Suwantong O, Ruktanonchai U, Supaphol P (2008) Polymer 49:4239–4247
Sikareepaisan P, Suksamrarn A, Supaphol P (2008) Nanotechnology 19:015102
Torres Vargas EA, do Vale Baracho NC, de Brito J, de Queiroz AAA (2010) Acta Biomater 6:1069–1078
Rujitanaroj P, Pimpha N, Supaphol P (2008) Polymer 49:4723–4732
del Valle LJ, Roa M, Díaz A, Casas MT, Puiggalí J, Rodríguez-Galán A (2012) J Polym Res 19:9792
Shalumon KT, Binulal NS, Selvamurugan N, Nair SV, Menon D, Furuike T, Tamura H, Jayakumar R (2009) Carbohyd Polym 77:863–869
Meng ZX, Wang YS, Ma C, Zheng W, Li L, Zheng YF (2010) Mater Sci Eng C 30:1204–1210
Wu X, Branford-White CJ, Zhu L, Chatterton NP, Yu D (2010) J Mater Sci Mater Med 21:2403–2411
Suwantong O, Opanasopit P, Ruktanonchai U, Supaphol P (2007) Polymer 48:7546–7557
Im JS, Yun J, Lim Y, Kim H, Lee Y (2010) Acta Biomater 6:102–109
Park J-Y, Lee I-H (2011) J Polym Res 18:1287–1291
Pornsopone V, Supaphol P, Rangkupan R, Tantayanon S (2007) J Polym Res 14:53–59
Baji A, Mai Y, Wong S, Abtahi M, Chen P (2010) Compos Sci Technol 70:703–718
Lyons J, Li C, Ko F (2004) Polymer 45:7597–7603
Renouf-Glauser AC, Rose J, Farrar DF, Cameron RE (2005) Biomaterials 26:5771–5782
Yang F, Murugan R, Wang S, Ramakrishna S (2005) Biomaterials 26:2603–2610
He L, Liao S, Quan D, Ma K, Chan C, Ramakrishna S, Lu J (2010) Acta Biomater 6:2960–2969
Maretschek S, Greiner A, Kissel T (2008) J Control Release 127:180–187
Kontogiannopoulos KN, Assimopoulou AN, Tsivintzelis I, Panayiotou C, Papageorgiou VP (2011) Int J Pharm 409:216–228
Kurpinski KT, Stephenson JT, Janairo RRR, Lee H, Li S (2010) Biomaterials 31:3536–3542
Zeng J, Yang L, Liang Q, Zhang X, Guan H, Xu X, Chen X, Jing X (2005) J Control Release 105:43–51
Zong X, Kim K, Fang D, Ran S, Hsiao BS, Chu B (2002) Polymer 43:4403–4412
Kenawy E, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, Wnek GE (2002) J Control Release 81:57–64
Chuysinuan P, Chimnoi N, Techasakul S, Supaphol P (2009) Macromol Chem Physic 210:814–822
Na Pattalung P, Thongtheeraparp W, Wiriyachitra P, Taylor WC (1994) Planta Med 60:365–368
Poomipamorn S, Kumkong A (1997) Edible multipurpose tree species. FuangFa, Bangkok, p 486 (in Thai)
Ilham M, Yaday M, Norhanom AW (1995) Nat Prod Sci 1:31–42
Murakami A, Jiwajiinda S, Koshimizu K, Ohigashi H (1995) Cancer Lett 95:137–146
Negi PS, Jayaprakasha GK, Jena BS (2008) LWT Food Sci Technol 41:1857–1861
Panthong K, Hutadilok-Towatana N, Panthong A (2009) Can J Chem 87:1636–1640
Xu G, Kan WLT, Zhou Y, Song J, Han Q, Qiao C, Cho C, Rudd JA, Lin G, Xu H (2010) J Nat Prod 73:104–108
Panthong K, Pongcharoen W, Phongpaichit S, Taylor WC (2006) Phytochemistry 67:999–1004
Likhitwitayawuid K, Phadungcharoen T, Krungkrai J (1998) Planta Med 64:70–72
Mahabusarakam W, Chairerk P, Taylor WC (2005) Phytochemistry 66:1148–1153
Robert RE, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Free Rad Bio Med 26:1231–1237
Philip LR, Peppas NA (1987) J Control Release 5:23–36
Peppas NA, Khare AR (1993) Adv Drug Delivery Rev 11:1–35
Verreck G, Chun I, Rosenblatt J, Peeters J, Dijck AV, Mensch J, Noppe M, Brewster ME (2003) J Control Release 92:349–360
Acknowledgments
This work was supported by the Thailand Research Fund (grant number: MRG5380120). We are grateful to Mae Fah Luang University for its partial financial support and allowing us to use its laboratory facilities. The authors acknowledge that they were partially financially supported by the “Integrated Innovation Academic Center: IIAC,” Chulalongkorn University Centenary Academic Development Project, Chulalongkorn University, which was used to procure the electrospinning apparatus used in this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 57 kb)
Rights and permissions
About this article
Cite this article
Suwantong, O., Pankongadisak, P., Deachathai, S. et al. Electrospun poly(L-lactic acid) fiber mats containing a crude Garcinia cowa extract for wound dressing applications. J Polym Res 19, 9896 (2012). https://doi.org/10.1007/s10965-012-9896-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-012-9896-3