Abstract
The development of photoluminescent materials based on the incorporation of quantum dots (QDs) into polymeric latexes has gained importance due to the multiple potential applications. Through the process of miniemulsion polymerization it is possible to encapsulate quantum particles into polymer matrix to provide both chemical stability and to maintain emission properties. The polymerization process was achieved with continuous magnetic stirring and nitrogen atmosphere. Cetyl trimethylammonium bromide (CTAB) was used as surfactant at concentration levels of 1.3 × 10-3, 3.2 × 10-3 and 5.4 × 10-3 M. The initiator 2,2-azobisisobutyronitrile (AIBN) was used at 0.50 and 0.75 wt% and CdSe QDS were used at 0.075, 0.15 and 0.225 wt%, both in relation to monomer content. A STEM study on the composite latexes and later a statistical study on the measurement of polymer particle diameter let us corroborate that the increment in surfactant concentration produces a decrement in polymer particle size. The obtained composite latexes were stable and showed fluorescence by excitation with UV light. The spectrofluorometry studies indicated that in composite latexes fluorescent emission was a function of polymer particle size, showing higher intensity those formulations with smaller surfactant concentrations and bigger polymer particle size.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quantum dots (QDs) are semiconductor nanocrystals with zero-dimensional quantum confinement and a size between 1 and 10 nm. Cadmium selenide (CdSe) QDs belongs to group II-VI nanocrystals. On one hand, by tailoring their surface, it is possible to make them dispersible in organic solvents. On the other hand, by changing their crystal size, color emission and physical properties can be manipulated [1]. Additionally, high quantum yield and multi-colored emissions can be excited by a single light source [2]. Such emissions are characterized by extensive absorption spectra, but with narrow, symmetric emission bands that can be located form the ultraviolet to the infrared region of the electromagnetic spectrum [3].
Several factors must be considered to preserve QDs photoluminescence during the incorporation into a polymer host. Examples of those factors are the relationship of surface to volume of QDs and the sensitivity of the QDs to the environment in which they are contained [4, 5].
In the last years research groups have demonstrated that the encapsulation of QDs in polymeric hosts increases photostability and improves optical characteristics [6–9]. The incorporation of fluorescent semiconducting nanocrystals into polymer particles, via polymerization in dispersed medium, has caused the development of new methods that allow the elaboration of submicrometric fluorescent particles with narrow particle size distribution, conferring them high photostability and making them adaptable to many processes. The importance of the incorporation of QDs nanoparticles into a polymer matrix is based on the resistance of the optical properties of the nanoparticles with those of the polymer, allowing an isolation that provides the QDs of improved photostability [10]. The polymer host not only stabilizes the QDs in a solid matrix, but it also provides particular characteristics to the resultant material; that is, the combination of the specific properties of the solid material and the polymer matrix allows new perspectives to studies and applications [10–12].
In the task of obtaining nanoparticles embedded in polymers, different methods have been used. Those methods offer important variations which should be considered before selecting the most suitable. Miniemulsion polymerization displays diverse advantages in contrast with other methods [8, 13].
Miniemulsion polymerization is based on the creation of an emulsion in which small droplets of monomer maintain their stability, avoiding both the diffusion (known as Ostwald ripening phenomenon) and fusion process due to particle collision. The addition of an appropriate surfactant allows retaining emulsion stability and its concentration determines some important aspects of the miniemulsion polymerization, like particle size and rate of polymerization [14, 15].
This research was focused on the evaluation of the effect of miniemulsión polymerization variables on the fluorescence of CdSe QDs / polystyrene composites. It was considered that variations in the concentrations of surfactant and initiator changes the kinetic process and the structural characteristics of the polymer particles (particle size and particle size distribution), which could affect, in turn, QDs structure and emission. Thus, changes in surfactant, initiator and CdSe QDs concentration were evaluated. The final purpose was to determine a set of conditions, where stable fluorescence composites could be obtained.
Experimental
Materials
Cadmium selenide (CdSe) quantum dots (QDs) ~4 nm in diameter and stabilized with trioctylphosphine oxide and surface-functionalized with napthylamine were synthesized according to literature [16]. Styrene monomer (Aldrich Co.) was distilled under vacuum and kept at 5 °C until polymerization. Initiator 2,2-azobisisobutyronitrile (Akzo Nobel) was purified from a saturated aqueous solution followed by precipitation at 5 °C. Cetyl trimethylammonium bromide (Acros, 99 + %), hexadecylamine (Aldrich Co.), and hexadecane (Aldrich Co.) were used as received.
Critical micellar concentration
The critical micelle concentration (cmc) of CTAB was determined by electrical conductimetry technique. The procedure was as follows: First, a 0.01 M aqueous solution of CTAB was prepared. Next, 50 mL of distilled water, with electrical conductivity of 3.7 μS/cm, was loaded in a glass vessel and tempered at 20 °C. The electrode of the conductivity meter (Corning conductivity meter 441) was placed 1 cm above the vessel bottom. Progressive additions of 1 mL each time of the solution were achieved. Gentle magnetic stirring was used to homogenize the solution. The evaluation consisted in measuring the increase of electrical conductivity as function of salt concentration. From this data, a plot of electrical conductivity versus surfactant concentration was built.
The amount of CTAB to saturate a water / styrene miniemulsion system was also determined. The purpose was to estimate the amount of surfactant to stabilize a miniemulsion. First, a system consisting of 80 part in weight of water and 20 parts in weight of styrene was prepared. Next, 2 min of sonication at 40% amplitude (Sonic dismembrator FB-505) were applied on the system, followed by tempering at 25 °C. The analogous procedure as the used in CTAB cmc determination in water was applied.
Miniemulsion polymerization
Miniemulsion polymerization was achieved as follows: first, a surfactant solution of CTAB and distilled water (20 g) was prepared and loaded into a three-neck-round bottomed flask (the reactor). Next, a hydrophobe mixture containing CdSe QDs, styrene monomer (5 g), hexadecylamine (0.02 g), hexadecane (0.7 g) and initiator 2,2 azobisisobutyronitrile (AIBN) was charged into the reactor. Then, the mixture was sonicated for 2 min at 40% amplitude (Sonic dismembrator FB-505) to obtain the miniemulsion. The miniemulsion was polymerized at 70 °C with magnetic stirring in nitrogen atmosphere. The formulations where designed varying initiator, surfactant and QDs concentrations, Table 1.
Characterization
Final monomer conversions were determined by gravimetry. The procedure was as follows: A sample of latex was weighted and dried at 80 °C for 24 h. The weight of the dried sample was registered. According to the initial formulation the weight fraction for every solid component was calculated. The difference between the final solid mass (W 1 ) and the expected initial solid content (W 2 ) corresponds to the polymer, Eq. 1. W Mon stands for the monomer mass in the sample at t = 0.
Average particle size, particle size distribution and polidipersity index of the polystyrene latex (blank) and the CdSe QDs / polystyrene latex composites were determined by statistical study on micrographs obtained by scanning electron microscopy in transmission mode (STEM), using a field emission electron microscope Jeol JSM-7401 F. The measurement and counting of polymer particles were achieved using the software Image-Pro plus version 4.1.
Photoluminescence (PL) spectra of CdSe-polystyrene latexes were acquired using a Varian Eclipse spectrofluorometer at 360 nm excitation. The samples were prepared by placing dilute dispersions of the latexes in distilled water.
Results and discussion
Critical micellar concentration determination
The cmc is considered as the threshold concentration where micellar aggregates start to form [17]. The cmc is determined as the concentration of surfactant where the change in slop in a plot of k versus [S] is observed. In the case of the system CTAB / water, the change in slop (cmc) occurred at 8.8 × 10-4 M (see Fig. 1). This result is very close to 9.2 × 10-4 M reported in literature by Ravera et al. [18]. In the case of the system CTAB / water / styrene, the curve of conductivity presented two major changes in slope, one at 8.8 × 10-4 M, which was related to the cmc of CTAB and another at 1.68 × 10-3 M. The second transition was related to the saturation of the interface water / oil. The increment in the concentration of surfactant in the system CTAB / water / styrene, with respect to the CTAB / water, indicates the amount of surfactant required to saturate the interface water oil is approximately 0.8 × 10-3 M. It is worth mentioning that attempts to evaluate the CTAB / water / styrene system at 70 °C (temperature of polymerization) were performed, however, no stable measurements were obtained.
The importance of cmc determination in the CTAB / water / styrene system lies in the understanding of the reorganization of dynamics between the phases of the solution and the process to maintain the system in balance. Organic materials like styrene, with very low water solubility, can be intimately dispersed in a water system by means of the surfactant action. The presence in the solution of others solutes or additives affects the process of micelle formation through specific interactions with surfactant molecules. The addition of these compounds produces changes in the hydrophobic interactions of the hydrocarbon chains due to the insertion of new molecules in the surfactant and an increase in volume of the micellar phase modifies the capacity of the surfactant. These factors make necessary increments in surfactant concentration to form micelles [19].
Effect of surfactant and initiator concentration on particle size and rate of polymerization
CTAB concentration of 1.6 × 10-3 M, which is slightly below saturation concentration of the interface water / monomer, was selected as the lowest surfactant concentration. From this concentration stable miniemulsion latexes were obtained. Systems prepared at lower concentrations of CTAB presented phase separation before 2 h of polymerization, with very low conversion. Surfactant concentrations evaluated (1.6 × 10-3, 3.2 × 10-3 and 5.4 × 10-3 M) are in agreement with the value of 0.3 wt.% with respect to monomer content, reported by Antonietti and Landfester [20] as the required concentration to obtain a stable colloidal system.
The average particle size (Dn) is directly influenced by variations in surfactant concentration. A statistical analysis on the measurement of at least 500 polymer particles, from SEM images, was performed. This study indicated that the increment in surfactant concentration decreased polymeric particle diameter (Table 2). According to literature, the size of miniemulsión droplets is sensitive to the surfactant concentration [21]. Antonietti and Landfester [20] reported that surfactant surface coverage is related to particle size. An increase of the surfactant concentration increases the coverage of the particles while decreasing particle size.
Variations in surfactant concentration affected also the rate of polymerization (Rp) (Figs. 2 and 3). In the case of R p , the increment was related to the greater concentration of monomer droplets, since the increment in global surface area increases available sites for the generation of free radicals and consequent growth of polymeric chains. Therefore, at the very beginning of the polymerization the maximum polymerization rate is reached and immediately after a depletion of the polymerization rate is observed, as the monomer present in the drop is progressively consumed. Landfester [15] described the use of cationic surfactants in miniemulsion polymerization, finding that at higher surfactant concentration the size of monomer droplet reduces, increasing consequently polymerization rate.
It is important to mention that AIBN initiator was selected based on the fact that a monomer with very low water solubility is not in enough concentration in the water phase to create oligoradicals able to enter in monomer droplets [20]. The effect of increasing initiator concentration, on one hand, caused the rise in Rp, due to the increasing concentration of active free radicals per polymer particle, which implicated a faster monomer conversion (Fig. 3). On the other hand, no significant affect on Dn was observed, indicating that processes of either monomer diffusion or new polymer particle formation were not important [21].
As observed, variations in the amount of surfactant and initiator not only affect Dn and Rp but also the number of polymer particles (Np) and polydispersity index (Ip). Table 2 summarizes the results obtained in the different synthesis treatments. As expected, a higher concentration of surfactant induced a higher rate of polymerization, which directly influenced the final number of polymer particles per unit volume, and consequently the average particle size. Also the variations in the surfactant and initiator levels are reflected in the final conversion reached, as seen in Fig. 4.
Photoluminescence
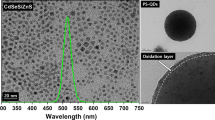
Photoluminescence spectra of CdSe-polystyrene latexes obtained by miniemulsion synthesis are shown in Fig. 5. Stable emission at 580 nm was observed at 360 nm excitation wavelength for all treatments. As it can be seen, emission intensity presented dependence with respect to synthesis parameters; i.e., the higher the QDs concentration, the higher the photoluminescence response. In relation to the prior facts Joumaa et al. [6] reported that emission intensity increases with the amount of dots. Nevertheless, in this research we observed, even more important variations in emission intensity strongly related to changes in polymer particle size; that is, latexes with larger polymer particle size displayed higher fluorescence intensity. For example, if the latexes with 0.225 wt% dots are compared, it is observed that Latexes 3 and 12, with the greatest average particle size, presented also the highest fluorescent emission intensities. Also, it was observed that narrow particle size distribution influenced fluorescence emission. For instance, Latex 3, with polydispersity index of 1.01, displayed an increase in the emission intensity of ~38% compared with Latex 12, with 1.02 polydispersity index.
Dots optical properties retention was attributed to the protection of polystyrene matrix, since the dots showed high stability in the latex, but not in the monomer. Dispersions of dots in styrene monomer were prepared and monitored. The emission peak was observed at 576 nm at 360 nm excitation wavelength. It was found that in a lapse of 48 h the dots experimented quenching, whereas in the polystyrene matrix, the optical properties remained stable after months. Similar results were previously reported by Joumaa et al. [22], whom attributed dots quenching to a partial aggregation of dots caused by the damage in dots surface caused by the monomer solution.
Figure 5 also indicates no change in the position of emission peak. This feature can be attributed to high monodispersity in dots particle size distribution, since, as it is known, dots emission wavelength depends on the dot diameter [23]. In addition, the quantum confinement and narrow size distribution of CdSe dots were maintained during the synthesis. This conclusion can be emitted from the point of view that there was no difference between the positions of maximum excitation energy among the different treatments [24]. An emission at 580 nm corresponds to ~4 nm diameter quantum dots; thus, we consider that CdSe dots maintained original dimensions after encapsulation. Therefore, from this study can be concluded that miniemulsion polymerization method did not affect optical properties of the dots; nevertheless, process variations induced changes in the particle size, which influenced emission intensity, as summarized in Table 2.
Composite microstructure
Previous to the polymerization process, CdSe dots were analyzed by HRTEM, finding that dots have an average particle size of ~3.7 nm with 1.06 polydispersity index (Fig. 6). Figure 7 illustrates HRTEM micrographs of CdSe / PS composite. The images allowed noting that the particles did not present significant change in either morphology or size. Nevertheless, in the latex it was not possible to observe neither the dots nor its location.
It is also important to consider that the average number of dots encapsulated per polymer particles (NQD) depends directly on the particle size. That is, at a certain dots concentration, the larger the diameter of polymer particles, the larger the number of dots per polymer particle. Consequently, in the case of a low concentration of dots per polymer particle luminescence decrement is significant. If we consider that CdSe theoretical density is 15.985 g/cm3 with 3.7 nm of diameter, in a concentration of 0.15 wt% in the polymerization solution, we have 3.5391 × 1015 particles of CdSe/cm3. So, in Latex 11, with average particle size of 144.5 nm and 9.17 × 1015 latex particles/cm3, it contains 0.3859 CdSe dots/polymer particle, whereas Latex 8, whose polymer particles diameter is 97.9 nm and 4.1 × 1016 latex particles/cm3, contains only 0.086 CdSe dots/polymer particle. These results indicated that the dots density per polymer particle influences directly in the fluorescent emission intensity such as Joumaa et al. [6] reported that with the increase of the incorporated dots, the fluorescence signal increases. This calculation assumed that the CdSe quantum dots were uniformly distributed among the latex particles, if this condition occurred the relationship of the number of dots per polymer particle would be directly proportional to the fluorescence emission intensity; nevertheless, this was not the case, since the experimental data showed no linearity of fluorescence with respect to polymer particle size. This let us consider that there was no a homogeneous encapsulation of dots by polymer particles and that there were dots which were not encapsulated by polymer particles. These results allowed us to emphasize two facts: a) that the dots, which were not encapsulated into polymer particles, underwent degradation during polymerization because they were in direct interaction with the water, and the presence of solved oxygen produces fluorescence quenching by oxidation [13]. And b) dots agglomeration, which favors intimate particle contact causes quenching due to electronic coupling [25].
Conclusion
The miniemulsion polymerization process proved to be a useful technique for obtaining fluorescent polymer latex due to the incorporation of CdSe QDs. The latexes showed a stable fluorescent emission in the different treatments. Variations in the levels of surfactant and initiator influenced the polymer particle size obtained. An increase in surfactant concentration minimized the size of the monomer drop, increased in number and increased the probability of droplet nucleation, consequently the rate of polymerization and the polymer conversion rate increased. The variation in the level of initiator was mainly changed in the kinetics of polymerization without affecting the photoluminescent emission. Changes in the variables of miniemulsion polymerization process were reflected in the nanocomposite photoluminescent emission that was a function of polymer particle size whose diameter was modified mainly by the amount of surfactant used in the process.
The amount of encapsulated dots per polymer particle directly affects de fluorescent emission. When increasing the dots/polymer particle relationship, the intensity emission increases. These results open the possibility to control the latex nanocomposite emission by tailoring the synthesis conditions.
References
Galian RE, De la Guardia M (2009) The use of quantum dots in organic chemistry. Trends in Analytical Chemistry 28:3
Bailey RE, Smith AM, Nie S (2004) Quantum dots in biology and medicine. Physica E: Low-dimensional Systems and Nanostructures 25:1
Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM (2007) Biological applications of quantum dots. Biomaterials 28:4717
Dridi C, Haouari M, Ouada HB, Legrand A-P, Davenas J, Bernard M, André J-J, Sai AH, Mattoussi F (2006) Spectroscopic investigations on hybrid nanocomposites: CdS: Mn nanocrystals in a conjugated polymer. Materials Science and Engineering: C 26:415
Zhou C, Shen H, Gou Y, Xu L, Niu J, Zhang Z, Du Z, Chen J, Li LS (2010) A versatile method for the preparation of water-soluble amphiphilic oligomer-coated semiconductor quantum dots with high fluorescence and stability. Journal of Colloid and Interface Science 344:279–285
Joumaa N, Elaissari A, Lansalot M, Sukhanova A, Artemyev M, Nabiev I, Cohen J (2005) Synthesis of fluorescent latexes by miniemulsión polymerization. Proceeding 8th Polymers Advanced Technologies International Symposium. Budapest, Hungary
Yang X, Zhang Y (2004) Encapsulation of Quantum nanodots in polystyrene and silica micro-/nanoparticles. Langmuir 20:6071
Yang Y, Wen Z, Dong Y, Gao M (2006) Incorporating CdTe nanocrystals into polystyrene microspheres: towards robust fluorescent beads. Small 2(7):898
Sheng W, Kim S, Lee J, Kim S-W, Jensen K, Bawendi MG (2006) In-situ encapsulation of quantum dots into polymer microspheres. Langmuir 22:3782
Sharma H, Sharma SN, Singh G, Shivaprasad SM (2007) Studies of optical and structural properties of CdSe/polymer nanocomposites: evidence of charge transfer and photostability. Colloid & Polymer Science 285:213
Kietzke T, Neher D, Landfester K, Montenegro R, Gutner R, Scerf U (2003) Novel approaches to polymer blends based on polymer nanoparticles. Nature Materials 2:408–412
Mohanta D, Mishra NC, Choudhury A (2004) SHI- induced grain growth and grain fragmentation effects in polymer-embedded CdS quantum dot systems. Materials Letters 58:3694
Hofstraat JW, Verhey HJ, Verhoeven JW, Kumke MU, Li G, Hemmingsen SL, McGown LB (1997) Fluorescence lifetime studies of labeled polystyrene lattices. Polymer 38(12):2899–2906
Schork FJ, Luo Y, Smulders W, Russum JP, Butté A, Fontenot K (2005) Miniemulsion Polymerization. Advances in Polymer Science 175:129
Landfester K (2001) Polyreactions in miniemulsions. Macromolecular Rapid Communications 22:12
Vassiltsova OV, Zhao Z, Petrukhina MA, Carpenter MA (2007) Surface-functionalized CdSe quantum dots for the detection of hydrocarbons. Sensors and Actuators B: Chemical 123:522
Odian G (1991) Principles of polymerization, 3rd edn. Wiley, New York
Ravera F, Ferrari M, Liggieri L, Loglio G, Santini E, Zanobini A (2008) Liquid-liquid interfacial properties of mixed nanoparticle-surfactant systems. Colloids and Surfaces A 323:99
Myers D (1992) Surfactant science and technology, 2nd edn. VCH, New York
Antonietti M, Landfester K (2002) Polyreactions in miniemulsions. Progress in Polymer Science 27:689
Miller CM, Sudol ED, Silebi CA, El-Aasser MS (1995) Capillary hydrodynamic fractionation (CHDF) as a tool for monitoring the evolution of the particle size distribution during miniemulsión polymerization. Journal of Colloid and Interface Science 172:249
Joumma N, Lansalot M, Théretz A, Elaissari A, Sukhanova A, Artemyev M, Nabiev I, Cohen JHM (2006) Synthesis of quantum dot-tagged submicrometer polystyrene particles by miniemulsion polymerization. Langmuir 22:1810
Costa-Fernandez J, Pereiro R, Sanz-Medel A (2006) The use of luminescent quantum dots for optical sensing. Trends in Analytical Chemistry 25:3
Khanna PK, Singh N (2007) Light emitting CdSe quantum dots in PMMA: synthesis and optical studies. Journal of Luminescence 127:474
Noh M, Kim T, Lee H, Kim C, Joo S, Lee K (2010) Fluorescence quenching caused by aggregation of water-soluble CdSe quantum dots. Colloids and Surfaces A 359:39
Acknowledgement
Authors wish to thank the National Council for Science and Technology of Mexico (CONACyT) for the grand awarded to Anilú Rubio Ríos.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rubio-Ríos, A., Aguilar-Castillo, B.A., Flores-Gallardo, S. et al. Effect of synthesis variables on the fluorescence properties of CdSe-polystyrene latexes. J Polym Res 19, 9833 (2012). https://doi.org/10.1007/s10965-012-9833-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-012-9833-5