Abstract
During aniline chemical oxidative polymerization which accompanies a falling pH, the initial acidity as well as the early stage pH profile actually plays an important role in the formation of polyaniline (PANI) micro/nanostructures. By utilizing an interfacial polymerization system, transfer and dosage of the reactants were controlled to change acidity profile during the early stage of reaction, without introducing any extra electrolytes. The results showed that, even for the polymerization systems with identical conditions, significant increase on the early stage pH profile induced by accelerated reactants’ transfer had led to PANI exhibiting molecular structures and morphologies typically belonging to those synthesized with weak acidity. Effects of the early stage acidity profile on the final PANI products can be attributed to the initially formed oligomeric products, which can direct the self-assembly of subsequently produced polymers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a typical intrinsic conducting polymer, polyaniline (PANI) especially its micro/nanostructures with well-defined morphologies are supposed to have excellent performance as novel electronic materials in comparison with their bulk counterparts [1]. The controllable construction of desired PANI micro/nanostructures through aniline chemical oxidative polymerization is thus of fundamental importance from the viewpoint of both science and technology. The work of Stejskal et al. has demonstrated that chain initiation pathways during aniline polymerization closely depend on the acidity of the reaction medium; and self-assembly of phenazine-containing units, which are easily produced in a mildly acidic reaction medium, determines the formation of various PANI supramolecular structures [2, 3]. These findings are very important for understanding the formation mechanism of PANI micro/nanostructures, as more and more experimental results have shown that acidity rather than other synthetic conditions actually affects the morphology as well as molecular structure of PANI products [4–6]. The aniline chemical oxidative polymerization is always accompanied a falling pH profile no matter the reaction is carried out in a highly/mildly acidic, neutral or even alkaline medium [2], due to continuously released protons. The falling pH thus results in increasing protonation degree of the products and larger ion strength in the system, which make it very difficult to control the non-covalent interactions among PANI molecules during their self-assembly. In order to obtain PANI nanostructures with higher homogeneity and uncover their formation mechanism with improved precision, a temporarily buffered reaction medium [5] as well as a pH-stat synthetic system [7] has been employed. Nevertheless, in our opinion, a buffer solution or constant pH maintained by continuously adding alkaline solution may, more or less, bring in foreign ions to the pristine aniline polymerization system, which mainly contains hydrogen ions, ammonium ions, aniliniums, sulfate ions and chloride ions. According to our recent study (unpublished results), when PANI molecules were introduced into an aqueous solution going to precipitate (just like what happened after the polymers were formed in the aniline polymerization system), the ion composition, especially molar ratio of hydrogen ions to other cations, can affect the morphology of precipitates, which are composed of collapsed PANI molecules. Moreover, it should be noted that, though pH profiles during aniline polymerization systems starting in a mildly acidic, neutral or alkaline medium will gradually fall and finally reach a low level as the reaction proceeds, their corresponding products actually have different molecular structures and morphologies [8]. We think that attention paid to investigating the effects of acidity should be never limited to roles played by the pre-added dopant acids, as lots of novel PANI micro/nanostructures were obtained under special synthetic conditions which are not directly correlated with the medium acidity. For example, the carefully adjusted reactants diffusion [9–11], excess or insufficient oxidant [12–14] and the hydrothermal polymerization approach [15–17] have been utilized to produce PANI with versatile morphologies. Despite the widely concerned effects of acidity, we address the questions and ask: how does the acidity profile affect the formation of PANI micro/nanostructures through the whole polymerization course? And what can we do to carry out the controllable synthesis of PANI micro/nanostructures effectively by controlling self-assembly behaviors of the polymerization products with specific acidity?
In order to answer these questions, the aniline interfacial polymerization was employed to investigate the formation process of PANI micro/nanostructures by examining effects of the acidity profiles during the early stage of reaction. In an interfacial polymerization system, the aniline monomers are slowly supplied to “meet” the oxidant by diffusing through the interface between the two immiscible liquids, and the products are kept away from the residual monomers as well as oligomeric intermediates, which are soluble in the organic phase. These features make it possible to vary the acidity profile by controlling the diffusion or dosage of the reactants instead of introducing extra electrolytes, as aniline and ammonium persulfate (APS) are actually a weak base and a weak bronsted acid, respectively. Moreover, in the interfacial polymerization system, structural evolution of the desired products could be monitored with fewer disturbances as compared with a conventional polymerization system, in which all the reactants, oligomeric intermediates and polymeric products present in the same reaction location. To our knowledge, the aniline interfacial polymerization has been extensively reported for preparing PANI nanofibers or their nanocomposites [18–27], whereas little effort has been made to gain more insight on the formation details of PANI micro/nanostructures in this approach. Herein, with an aim to elucidate the effects of acidity profile, acidity variation facilitated by the interfacial polymerization system was employed. Besides this, oligomeric aniline polymerization products pre-synthesized in a neutral medium were introduced into a typical aniline polymerization system to further verify the roles of acidity profile during the early stage of reaction.
Experimental
All the chemicals with analytic purity were from The Xi’an Chemical Reagents Factory. Aniline was distilled in the presence of zinc powder before use and the others were used as received.
Syntheses of PANI samples
-
(1)
Tuning acidity profile in an interfacial polymerization system
Based on the typical interfacial polymerization procedure described by Huang et al. [28], acidity profile in the aqueous phase was tuned in three ways. (a) Neutralizing the aqueous phase (APS solution in deionized water with a concentration of ca. 0.05 M) by adding a spot of sulfuric acid or concentrated ammonia water while maintaining the volume of aqueous phase as ca. 20 ml for all the parallel syntheses. Aniline solution (0.05 M) in ca. 20 ml carbon tetrachloride (CCl4) was used as the organic phase and the reaction system was kept static. In this case, carbon tetrachloride which has a much larger density than water was used for the safety consideration (the upper water layer can seal the organic vapor) as well as convenience of pH monitoring. (b) Aniline polymerization in the systems with different interfacial areas or in the partially stirred systems was carried out. For example, aniline solution in xylene (0.05 M) of ca. 20 ml was carefully transferred to the top of APS solution in deionized water of ca. 40 ml (0.025 M). Three polymerization systems with the same reactants’ concentration were performed in glass vessels with cross-section areas of ca. 2.5, 12.5 and 50.2 cm2 (as schematically shown in Fig. 3) simultaneously. Xylene instead of CCl4 was used as the organic phase in this case as the former is much easier to spread into a thin liquid layer on the top of water, due to its larger smaller spreading coefficient as compared with CCl4 (Supporting information). The experimental settings for the partially stirred polymerization system were shown in Fig. 6(c). In this case, aniline solution (0.05 M) of ca. 50 ml and APS solution (0.05 M) of ca. 50 ml were put in a conical flask (100 ml) to perform the polymerization, in order to ensure a larger bottom space for the rotating magnetic stirrer bar. Carbon tetrachloride was selected as the organic solvent phase when the organic phase was stirred and xylene was used when the aqueous phase was stirred, respectively, by considering their density differences as compared with water. (c) Tuning pH profile at the early stage of reaction by changing the reactants’ dosage. Aniline and APS with molar ratios of 3:1 or 1:3 were used, and the reaction systems were kept static. Similarly, carbon tetrachloride was used as the solvent of organic phase.
All the syntheses were carried out at 20 ± 1°C and the aqueous phase was separated using a separatory funnel from the polymerization system after certain reaction duration. In order to obtain the product with a high yield, the reaction time was maintained as 6 h for the polymerizations with higher acidity (initial pH < 3) and the reaction time was maintained as 12 h for the polymerizations with lower acidity (initial pH > 3). The solid products in the aqueous phase were then collected and washed with deionized water for several times through centrifugation until the supernatant liquid became colorless, after that the solid products were dried in vacuum (45°C) for 4–5 h.
-
(2)
Introducing pre-synthesized aniline oligomers
To the typical aniline polymerization conducted in a highly acidic system, aniline oligomers pre-synthesized in a neutral medium [14] were added. A small amount of the oligomers (with weight-average molecular weight of ca. 5062) of 20–30 mg were dissolved in 10 ml tetrahydrofuran (THF) and treated with ultrasonic irradiation for 2 h. After that, the insoluble compounds were removed by centrifugation (10000 rpm, 10 min) and the transparent reddish brown solution (ca. 0.9 mg/ml) was collected. Three parallel experiments were carried out to investigate the effects of pre-synthesized oligomers. (a) Aniline polymerization in the presence of pre-synthesized oligomers. About 5 ml of the oligomers solution in THF was first mixed with 10 ml APS hydrochloric solution (0.01 M) by vigorously shaking and then the mixture was well mixed with 5 ml aniline hydrochloric solution (0.01 M); (b) Aniline polymerization without pre-synthesized oligomers. Ammonium persulfate hydrochloric solution of ca. 10 ml was first mixed with ca. 5 ml THF and then with ca. 5 ml aniline hydrochloric solution; (c) Aniline oligomers oxidized by APS. The same oligomers solution of ca. 5 ml was mixed with ca. 10 ml APS hydrochloric solution and ca. 5 ml hydrochloric acid solution. Each of the three polymerization systems has a total volume of ca. 20 ml and initial pH of 0.5–0.6. All the polymerization systems were kept static to proceed at 20 ± 1°C for 10 h. The resulted solid products were separated and washed for 3–4 times with deionized water through centrifugation, and after that they were dried in vacuum (45°C) for 4–5 h.
Measurement and characterization
A pH meter (PHSJ-4A, Shanghai Precision & Scientific Instruments Co., Ltd., China) was used to in situ record the pH of the upper aqueous phase. Morphologies of the products were examined on a FE-SEM 6700 F (JEOL) field emission scanning electron microscope (SEM). The dried powder of products (in their doped state, showing a dark-green color) obtained from the systems with higher initial acidity were directly taken for SEM examination. The brown-yellow products obtained from the systems with lower initial acidity were coated with carbon through sputtering before SEM examination.
The in situ ultraviolet–visible (UV–vis) spectra of products settled in the aqueous phase were recorded by carrying out the polymerization in the sample cell of the UV–vis spectrometer (U2001 UV–vis Spectrophotometer, Hitachi). The height of the interface between the two immiscible liquid phases in the sample cell was carefully adjusted to let the testing light penetrate through the bottom aqueous phase [29]. Before transferring the organic phase (as the upper layer) into the sample cell, the aqueous phase was first detected as the blank. The UV–vis spectra of the final product were recorded by dissolving the dedoped sample into N, N-dimethylformamide (DMF). A spot of the dried sample (doped) was well mixed with KBr powder and the mixture was pressed into a thin pellet to detect their Fourier transform infrared (FTIR) spectra on a spectrophotometer (Bruker FT-IR Tensor 27 Spectrometer). The molecular weight of PANI synthesized from the system with an initial pH = 3 in the aqueous phase was measured on a gel permeation chromatography (GPC), with THF as the flowing phase, polystyrene as the standard and the eluent flow rate of 1.0 ml/min. The GPC instrument (Waters-Breeze USA) was constituted by a Waters 717 plus Autosampler, a Waters 2414 Refractive Index Detector and a Waters 1515 Isocratic HPLC pump.
Results & discussion
Dependence of PANI micro/nanostructures on the initial acidity
In spite of an interfacial polymerization approach, PANI micro/nanostructures with versatile morphologies were obtained by tuning the initial acidity in the aqueous phase. Our study showed that probably due to PANI’s specific self-assembly behaviors related to the initial acidity, the interfacial polymerization could also yield other types of PANI micro/nanostructures besides the most often encountered nanofibers, which once have been considered as the common products resulted in this synthetic approach. Though acidity at the interface was said to be slightly lower than that of the bulk aqueous phase due to the presence of organic phase, they probably exhibit the same profile during the polymerization. So, pH value detected in the bulk aqueous phase can be considered as an approximation of pH at the interface, which is the main reaction site for the reactants. As a result, with the initial acidity of aqueous phase adjusted, aniline interfacial polymerization systems possessing nearly identical conditions have yielded PANI micro/nanostructures with versatile morphologies. For instance, when initial pH in the aqueous phase (APS solution in deionized water) was decreased to 1.2 by adding sulfuric acid solution, PANI nanofibers with much larger aspect ratios (Fig. 1a), which were similar to those usually obtained in the typical interfacial polymerization system [30] presented. And PANI obtained in the system with pristine APS solution in deionized water as the aqueous phase (pH = 2.0) were irregular nanoparticles mixed with a small portion of short nanofibers (Fig. 1b). Moreover, rod-like PANI with smooth surfaces were resulted from the systems with aqueous phase pH around 2.5 (Fig. 1c); PANI microspheres with hairy surface and diameters of 2–3 μm were obtained in the system with higher initial pH (pH = 3, 4 and 6 for Fig. 1d–f), respectively. It is rather interesting to find that these urchin-like PANI microspheres are successfully synthesized through the interfacial polymerization, which once has been well-known as “a general chemical route to PANI nanofibers” [30], without introducing special dopant [31] or using monomers with certain substituent groups [32].
PANI prepared in the interfacial polymerization systems with different initial pH values in their aqueous phases. (a)the typical nanofibers; (b) irregular nanoparticles mixed with a small amount of short nanofibers; (c) nanorods bearing much smoother surfaces; (d)-(f): urchin-like microspheres with hairy surfaces. The insets of (d)-(f) were SEM images with a larger magnification for the corresponding samples (Supporting information, S-Figure 6–9). The initial aqueous phase pH values were adjusted approximately to 1.2(a), 2.0(b), 2.5(c), 3.0(d), 4.0(e) and 6.0(f), respectively.Other synthetic conditions: [aniline] = [APS] = 0.05 M, the organic phase was CCl4
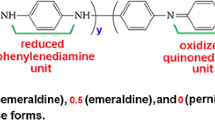
Molecular structures of these PANI products can be categorized into two types with respect to the initial acidity (pH > 2.5 or pH < 2.5) in their synthetic systems. The one-dimensional products and those irregular nanoparticles (synthesized with higher acidity, initial pH < 2.5) exhibited molecular structural features resembling that of the “standard” PANI (Fig. 2). For example, there are two absorbance bands with their maxima at 334 nm and 620 nm in the UV–vis spectra, which can be assigned to the π-π* excitation of the para-substituted benzenoid segment and excitation of the quinoid segment of the emeraldine base form PANI, respectively. In the FTIR spectra, peaks around 1580 cm−1, 1481cm−1, 1298 cm−1 and 1136 cm−1 correspond to the stretching deformation of quinoid structure, benzenoid structure, the C-N stretching of secondary aromatic and the aromatic C-H in-plane bending, respectively. As a comparison, PANI microspheres synthesized in the system with initial pH above 3 probably have branched structures resulted from ortho-coupling of the monomers, which are easily formed in the mildly acidic polymerization system [7]. These structural features are supported by the samples’ spectra (Fig. 2), e.g., the absence of quinoid segment excitation band (at 600–660 nm) in the UV–vis spectra and a much obvious band at 1444 cm−1 (assigned to the branched structures) in the FTIR spectra. It is probable that, formation of PANI micro/nanostructures is fundamentally directed by the self-assembly of PANI molecules at a certain acidity level, and the interfacial polymerization approach actually is not a prerequisite technique.
UV–vis (a) and FTIR (b) spectra of PANI obtained in the polymerization systems with different initial pH values in their aqueous phases (corresponding to the samples shown in Fig. 1)
Initial acidity profile tuned by reactants’ transfer
In corresponding to the different initial acidities, variation of early stage acidity which was tuned by controlling the reactants’ transfer also manifested its effects on the molecular structure and morphology of aniline polymerization products. Here, the polymerization systems with different interfacial areas (Fig. 3) and the ones which are partially magnetic-stirred (Fig. 6c) were employed. Observation on the three polymerization systems (with all the conditions except for the interfacial area kept identical) found that color change of the reaction mixture, i.e., from colorless transparent to light yellow, first took place in the reaction vessel with the largest cross section area (S3), and then appeared in S2 and S1, indicating the highest polymerization rate in S3. As the reaction continued, the aqueous phase in S1 gradually turned to black green, whereas that in S2 showed black brown and the aqueous phase in S3 looked orange red till the late stage of polymerization. After the same reaction duration, consequently, PANI nanofibers (Fig. 3a), urchin-like microspheres (Fig. 3b) and microscale tube/rod-like products mixed with nanoparticles (Fig. 3c) were produced in S1, S2 and S3, respectively. In the products from S2 and S3, there were still a small amount of rod-like products with smooth surface and lengths up to several-micrometers. Structural analyses (Fig. 4) showed that S2 and S3 systems yielded PANI with much obvious features like those obtained from the mildly acidic systems. For example, in the FTIR spectra of these products (obtained from S2 and S3), additional bands at 1446 cm−1 and 1041 cm−1 (as compared with the standard PANI) can be assigned to ortho-coupling induced units and sulfonic \( {\text{SO}}_{{3}}^{{{2 - }}} \) groups [7], respectively. As a comparison, the S1 system resulted in PANI bearing molecular structure in the typical emeraldine salt form, which is usually encountered in a highly acidic system.
The schematic aniline polymerization systems with different cross section areas and typical SEM images of the corresponding products. (a) Interacted nanofiber-like products obtained from S1; (b) urchin-like microspheres obtained from S2; (c) microscale tube/rod-like products mixed with nanoparticles obtained from S3. The cross section areas of S1, S2 and S3 were approximately 2.5, 12.5 and 50.2 cm2, respectively. For the three polymerization systems: [aniline] =0.05 M, [APS] =0.025 M. The molar ratio of aniline and APS was kept at 1:1 by setting the volume of APS solution two times as that of the aniline solution
FTIR spectra of PANI prepared in the polymerization systems which are carried out in the reaction vessels with different cross section areas (corresponding to the samples shown in Fig. 3)
We think that the reactants’ specific diffusion behaviors in the three systems during the early stage of reaction have induced different acidity profiles and consequently led to molecular structural and morphological differences among those PANI samples. Take the cases of S1 and S2 as examples, pH at the interface of S2 where a much larger number of aniline monomers presented, will increase more quickly than that in S1 at the early stage of reaction. And polymerization in S2 will proceed faster than that in S1, as these neutral aniline molecules presenting at a much higher pH level are more oxidizable than anilinium cations (existing at lower pH level) [2]. In comparison with S2 and S1, due to the highest initial pH level, the S3 system probably has yielded the majority of polymerization products at the early stage of reaction, during which a rapid proton releasing also occurred. The pH profile in S3 thus is supposed to increase rapidly at first and then drastically drop, as schematically shown in Fig. 5. The short belt-like and rod-like microstructures are likely formed through self-assembly of the polymerization products directed by non-covalent interactions, e.g., hydrogen bondings, which are indicated by the vibrational band (at 3236 cm−1) in the sample’s FTIR spectrum (Fig. 4). At the late stage of reaction in S3, subsequent polymerization proceeding with much strong acidity may lead to irregular PANI nanoparticles, as reported by Travas-Sejdic et al. [33]. Due to modest monomer diffusion and slow monomer diffusion in the S2 and S1 systems, respectively, their pH profiles can also be sketched as what shown in Fig. 5. In the S1 system, a much lower pH level through the whole polymerization duration can be used to explain the presence of interacted fiber-like products (Fig. 3a), and these urchin-like microspheres (Fig. 3b) are probably formed due to a moderate acidity level at the early stage of reaction in S2.
Sketched pH profiles in the polymerization systems with different interfacial areas (corresponding to those shown in Fig. 3)
Variation of the early stage acidity as well as its effects on the molecular structure and morphology of PANI was further elucidated by employing a partially-stirred interfacial polymerization system. In this case, slow stirring applied to the bottom phase was utilized to change the acidity profile by accelerating reactants’ transfer while maintaining a complete interface between the two immiscible phases (Fig. 6c). As protons are not fast released at the beginning of reaction, acidity of the aqueous phase then is dominated by the diffusion of aniline. The in-situ monitored pH profile in the upper aqueous phase showed that there was indeed a significant increase of pH during the early stage of reaction due to accelerated aniline diffusion, as compared with the static system (Fig. 7). With this increasing pH, the stirred system first showed a light yellow color at about 45 min after the reactants were put together, whereas its static counterpart first showed a light pink color at about 90 min and then gradually turned to yellow brown. After the same reaction duration, irregular nanoparticles were obtained in the static system (Fig. 6a), and micro-sized urchin-like spheres (Fig. 6b) were produced in the partially stirred system, respectively. These PANI microspheres have molecular structures (Supporting information, S-Figure 3) and morphologies very similar to those synthesized in the system with initial pH of 3 or higher than 3 (Fig. 1d–f). However, in the partially stirred system, PANI microspheres were obtained with pristine APS solution as the aqueous phase, which had an initial pH value around 2 at the reaction temperature and seemed not to result in PANI microspheres in a normal system (Fig. 1b; Supporting information, S-Figure 1, S-Figure 2). It can be said that a much significant pH increase induced by accelerated reactants’ diffusion during the early stage of polymerization, has actually played a similar role as the pre-lowered initial acidity. The controllable synthesis of PANI, therefore, is possible to carry out by adjusting the transfer of reactants with a consideration on the pH profile during the early stage of reaction.
(a) PANI nanoparticles obtained in the static interfacial polymerization system; (b) urchin-like microspheres obtained in the interfacial polymerization system with its organic phase magnetically stirred; (c) photograph of the experimental settings used for the mildly stirred interfacial polymerization system; (d) microrods and nanospheres obtained in the system with its aqueous phase magnetically stirred. Other synthetic conditions: [aniline] = [APS] =0.05 M. The stirring speeds for (b) and (d) were 240 rpm and 150 rpm, respectively. APS solutions in distilled water were used as the aqueous phases for all the three systems. The organic phase was aniline solution in xylene (or CCl4) for the system with its aqueous phase (or its organic phase) magnetically stirred.)
When the aqueous phase was magnetically stirred, PANI spherical nanoparticles as well as thick-rods with lengths up to several micrometers presented as the major and minor products, respectively (Fig. 6d). In this case, pH in the aqueous phase may start to increase at the beginning of reaction, as what happened in the system with a stirred organic phase. However, due to accelerated diffusion in the aqueous phase, APS enriched at the interface could afford a higher polymerization rate which will lead to fast pH drop once the polymerization begins. Probably, formation of these PANI nanospheres (Fig. 6d) occurred during the early reaction stage at a higher pH level, and the rod-like products were produced in the subsequent reaction phase at a lower pH level. FTIR spectra of PANI produced in the system with a stirred aqueous phase also exhibited characteristics which are usually assigned to those synthesized in a mildly acidic medium (Supporting information, S-Figure 3), further indicating the PANI’s structural dependence on the early stage acidity profile.
Initial acidity profiles affected by the reactants’ dosage
Besides a larger interfacial area or stirring applied to the system, excess aniline was also found to induce acidity variation during the early stage of polymerization, and consequently bring in PANI with molecular structural and morphological features which are typical for those obtained in the mildly acidic medium. In the interfacial polymerization system with excess aniline, pH in the aqueous phase kept increasing for ca. 150 min once the reactants were mixed (Fig. 7c). A rather long pH increasing period (nearly 15 h) was detected in the system with a much lower APS concentration (e.g., 8.3 × 10−3 M, Supporting information. S-Figure 4). As a result, in the presence of excess aniline, microscale rod-like PANI with smooth surfaces and pseudo PANI microspheres with rough surfaces again presented (Fig. 8a); and these spherical products became dominant when the reactants’ concentrations were lowered (Fig. 8b). This can be explained as, the APS solution with a concentration of 8.3 × 10−3 M gives pH of ca. 2.7 at the reaction temperature, and when this APS solution serves as the aqueous phase, a long pH increasing period beginning with mild initial acidity (Supporting information, S-Figure 4) could easily induce PANI microspheres, as we have shown in the former sections.
(a) Microscale pseudo spheres as well as microrods obtained with excess aniline; (b) nearly pure microspheres obtained with excess aniline but at a lower concentration as compared to that shown in (a); (c) irregular nanoparticles obtained with excess APS; (d) a TEM image of the microsphere from the sample shown in (b). Other synthetic conditions: (a): [aniline] =3[APS] =0.05 M; (b): [aniline] =3[APS] =0.025 M; (c) [APS] =3[aniline] =0.15 M
These PANI micro-spheres (Fig. 8b) obtained with excess aniline are undoubtedly constructed through self-assembly of PANI molecules with structural features induced by specific pH profiles. They exhibit a brown yellow color which is common for the insulating PANI samples obtained from a mildly acidic system. Their FTIR and UV–vis spectra (Fig. 9) also indicated structural features typically belonging to PANI synthesized in this case, e.g., an additional band at 1446 cm−1 in the FTIR spectrum and the absence of π→polaron absorbance band in the UV–vis spectrum, indicating the presence of branched units [34]. With structural defects as compared with the “standard” PANI which bears a well-developed para-linked conjugation structure, these PANI molecules may exhibit less hydrophilicity due to deficiency of doping. TEM image shows that the microsphere is solid and has numbers of nanofibrils on its surface (Fig. 8d). Based on these characterization results, a possible formation process of these PANI microspheres, including the ones shown in Figs. 1 and 3, can be given as follows. At the early stage of reaction under a higher pH level, PANI molecules bearing dual features, i.e., composed of hydrophilic and hydrophobic units [2] formed at the interface between the two immiscible phases will self-assemble into microspheres. Afterwards, the hydrophilic PANI molecules formed with stronger acidity (probably bearing structures as the “standard” PANI) may constitute nanofibrils on the surface of those early formed microspheres.
UV–vis(a) and FTIR(b) spectra of the PANI samples as shown in Fig. 8
When excess APS was used, electrical insulating PANI with a brown color was obtained. These products were shown to be irregular aggregates mixed with very short nanofibers (Fig. 8c), and their spectral characteristics are analogous to those synthesized by Chiou et al. [12]. In the presence of excess APS, probably the majority of monomers were consumed to produce aniline oligomers during the early stage of reaction [12]. However, these oligomers formed at the interface can be easily dissolved into the organic phase and less oligomers will be left to direct the self-assembly of subsequently formed polymers [12], resulting in irregular aggregates. Therefore, by virtue of the interfacial polymerization system, PANI’s molecular structural and morphological dependence on the dosage of the reactants was again attributed to the acidity change, especially acidity profile during the early stage of reaction.
Effects of the reactants’ dosage on the acidity profile discussed with our findings are still shown to be valid by referring the work of Zhou et al. [14]. In their study, the polymerization system containing aniline and APS with a molar ratio of 5:1 started with an initial pH nearly in the neutral range. We think that, probably due to excess aniline which can neutralize the released protons, pH level in this reaction system was still above 5.5 after 1 h since the beginning of the reaction. As a comparison, pH in the polymerization system containing aniline and APS with a molar ratio of ca. 1.7:1 had dropped below 4.75 at the same reaction duration. With the specific pH profiles in the two systems especially during the early stage of reaction, it is then reasonable to obtain nearly pure PANI nanoplates in the former case and nanofibers on flower-like superstructures in the latter case, respectively.
Specific structures of polymerization products determined by early stage acidity level
In order to gain more insight on the roles played by the early stage pH profile, aniline polymerizations carried out in the highly acidic system and mildly acidic system, i.e., with initial pH of 0.4 and 4.0, were in-situ monitored, respectively. It was found that both of the two systems showed continuously decreased pH with nearly the same falling trend, except that pH level in the system with initial pH of 4.0 still stayed above 3 almost 300 min after the reactants were put together (Supporting information, S-Figure 5). Much obvious UV–vis absorbance bands corresponding to the polymerization products have been detected at about 17 min and 25 min reaction duration in the two systems, respectively (Fig. 10). The in-situ detected UV–vis spectra are similar to those recorded in a typical acidic system and a natural system obtained by Fu et al., respectively [35]. For example, the spectrum recorded from the highly acidic system shows an absorbance band at ca. 425 nm and a “tail” above 660 nm, which can be attributed to the typical characteristics of conventionally synthesized PANI in the emeraldine salt form (Fig. 10a). The spectrum recorded from the mildly acidic system just exhibits a significant absorbance band at ca. 407 nm, and the lack of a peak around 600 nm may probably due to presence of O=Q=N- [36], where both O and N could be preferred sites for hydrogen bonding [7]. It can be said that though pH in most of the aniline polymerization systems will drop to a low level at the end of reaction, the specific pH profile during the early reaction stage (which in some cases may last for a considerable long period) has accompanied formation of a certain amount of polymerization products.
A closer look at the in-situ detected UV–vis spectra indicates that with different initial acidities, aniline polymerization products have exhibited significant structural differences since the beginning of the reaction. For example, an absorbance band at 288 nm was first detected from the polymerization system with initial aqueous phase pH of 0.4, whereas its counterparts with a lower initial acidity (pH = 4.0) first gave an absorbance band located at 278 nm. These two kinds of spectral features can be attributed to the presence of aniline dimers (p-aminodiphenylamine) and substituted quinines, respectively. As the former were found to have an absorbance band at 290 nm in the in-situ detected UV–vis spectrum [37], and the latter were usually obtained from the aniline polymerization system with mild acidity, showing a strong π-π* peak centered at 250–314 nm in their UV–vis spectra [38]. Moreover, the absorbance band at 288 nm quickly became weak and nearly diminished as the reaction continued, whereas the absorbance band at 278 nm exhibited strong intensity for a long period (Fig. 10). Evolution of these UV–vis spectra indicates that polymeric products with increasing conjugation length are gradually formed in the highly acidic system [37], whereas the amount of oligomeric products containing branched units increases in the mildly acidic system, respectively. This also agrees with the GPC result that PANI exhibiting microscale sphere-like morphology (synthesized in the polymerization system with an initial pH of 3) had weight-average molecular weight of ca. 4063, which is much lower than that of the “standard” PANI obtained in the medium with higher acidity [14].
Self-assembly of early stage products
Dependence of PANI’s molecular structure and morphology on the acidity profile is probably originated from the early stage polymerization products formed at a certain acidity level. This is further illustrated by introducing aniline oligomers pre-synthesized in a neutral medium into the typical polymerization system conducted in a highly acidic medium. Aniline oligomers exhibiting branched units (as shown in the FTIR spectrum, Fig. 11, down-right panel) induced by ortho-substitution were chose because their self-assembled supramolecular structures are much different from those commonly constructed in a highly acidic system. In this case, it is expected that effects of the self-assembled oligomers on the final products could be effectively investigated. These oligomers were supposed to quickly precipitate and probably serve as nucleates for the subsequent formed polymers, when their solution in THF was introduced into the aqueous polymerization system. In the presence of oligomers pre-synthesized in a neutral medium, aniline polymerization proceeded in a highly acidic medium then can be studied as the one conducted in a “falling pH” system with a much higher initial pH. During the experiment, it was observed that, brown yellow products firstly precipitated in the system with the addition of oligomers solution and dark green color showed up after a certain period (ca. 3 h). In the system where the oligomers’ solution was merely mixed with APS hydrochloric solution, brown yellow solid products precipitated quickly and kept their color till the late stage of the reaction. As a comparison, the aniline polymerization system without pre-synthesized oligomers showed a blue color at first and later became dark green, which is usually occurred in the conventional aniline polymerization system. After the same reaction duration, the polymerization system containing pre-synthesized oligomers yielded mainly microscale belt-like products (Fig. 11a), and nanofibers (Fig. 11b) exhibiting spectral features belonging to the “standard” PANI were produced in the counterpart system which is free of pre-synthesized oligomers.
(a) Microscale belt-like PANI obtained in a highly acidic reaction medium with pre-synthesized oligomers introduced; (b) PANI nanofibers obtained with nearly the same synthetic conditions as that for (a) but in the absence of pre-synthesized oligomers; (c) Bundle-like products formed by mixing the pre-synthesized oligomers’ solution in THF with APS hydrochloric solution. The down-right panel shows the FTIR spectra of the samples shown in (a), (b) and (c). Other synthetic conditions: (a) [aniline] = [APS] =0.01 M, [oligomers] =0.9 mg/ml; (b) [aniline] = [APS] =0.01 M, [oligomers] =0 M; (c) [APS] =0.01 M, [aniline] =0 M, [oligomers] =0.9 mg/ml
Formation of the belt-like products can be attributed to self-assembly of PANI molecules directed by the early precipitated oligomeric aggregates. As these pre-synthesized oligomers with branched units are hardly to dope even in a highly acidic reaction medium [2], they could be more easily oxidized by APS than anilinium cations. In the reaction system containing pre-synthesized oligomers, it was observed that brown yellow solid products first precipitated prior to the dark green products. And in the parallel reaction system, short belt-like products stacking into bundles were produced when the pre-synthesized oligomers were mixed with APS (Fig. 11c). Therefore, we suppose that in the polymerization system with pre-synthesized oligomers introduced, the oligomers can be first oxidized by APS and simultaneously self-assemble into micro/nanoscale aggregates (e.g., bundles composed of short nanorods) with well-defined morphology. The later produced PANI molecules then will probably deposit on these early formed oligomeric precipitates, due to the autocatalytic effects of aniline polymerization and the strong tendency for heterogonous nucleation. Consequently, microscale belt-like products instead of nanofibers were obtained in the presence of pre-synthesized oligomers. The specific effects of oligomers’ concentration, aniline’s concentration and the organic solvent, etc., on the morphology of PANI obtained with pre-synthesized oligomers may need further investigation, which is beyond the scope of this paper. However, the present results show that aniline oligomers pre-synthesized in a neutral medium have, indeed, affected the self-assembly behavior of PANI molecules formed at a high acidity level. In a common aniline polymerization system, the early formed products thus probably play important roles during the construction of PANI micro/nanostructures, regardless of the whole polymerization course. The pH profile during the early reaction phase, which can induce oligomeric products with certain molecular structures and self-assembly behaviors, is rather important to concern. It is easily understood that the exact duration of the “early stage” of a specific polymerization system depends on the temperature, reactants’ concentrations and types of oxidants, etc. Nevertheless, the findings based on our investigation could help others to concentrate more on the self-assembly behaviors of PANI molecules when they were formed at a certain acidity level, other than merely correlating various synthetic conditions with the products’ morphologies.
Conclusions
Transfer and dosage of the reactants were employed to tune the aqueous phase pH profile in the aniline interfacial polymerization system, which is free of extra electrolytes. The accelerated monomer transfer was found to induce increase of pH at the early stage of reaction especially in the absence of pre-added dopants. Due to fast oxidation rate at a high pH level, pH exhibiting an increasing profile during the early stage of reaction was found to have similar effects as the pre-lowered initial acidity and consequently determine the molecular structures and morphologies of the final products. Acidity profiles in the early stage of reaction were shown to play their roles by affecting the molecular structures of early formed polymerization products, which probably direct the self-assembly of subsequently formed PANI. This was verified by introducing aniline oligomers pre-synthesized in a neutral medium into the aniline polymerization system performed in a highly acidic medium, where the most often encountered nanofibrillar products have been replaced by microbelts. Therefore, for the controllable synthesis of PANI micro/nanostructures, acidity profile during the early stage of reaction, rather than that through the whole polymerization course should be highly concerned.
References
Jang J (2006) Conducting polymer nanomaterials and their applications. Emissive Mater Nanomaterials 199:189–259. doi:10.1007/12_075
Sapurina I, Stejskal J (2008) The mechanism of the oxidative polymerization of aniline and the formation of supramolecular polyaniline structures. Polym Int 57(12):1295–1325. doi:10.1002/Pi.2476
Stejskal J, Sapurina I, Trchov M. Polyaniline nanostructures and the role of aniline oligomers in their formation. Prog Polym Sci 35 (12):1420–1481
Sedenkova I, Trchova M, Stejskal J, Bok J (2007) Polymerization of aniline in the solutions of strong and weak acids: the evolution of infrared spectra and their interpretation using factor analysis. Appl Spectrosc 61(11):1153–1162
Zhang LJ, Zujovic ZA, Peng H, Bowmaker GA, Kilmartin PA, Travas-Sejdic J (2008) Structural characteristics of polyaniline nanotubes synthesized from different buffer solutions. Macromolecules 41(22):8877–8884. doi:10.1021/Ma801728j
Konyushenko EN, Trchova M, Stejskal J, Sapurina I (2010) The role of acidity profile in the nanotubular growth of polyaniline. Chem Pap 64(1):56–64. doi:10.2478/s11696-009-0101-z
Laslau C, Zujovic ZD, Zhang LJ, Bowmaker GA, Travas-Sejdic J (2009) Morphological evolution of self-assembled polyaniline nanostuctures obtained by pH-stat chemical oxidation. Chem Mater 21(5):954–962. doi:10.1021/Cm803447a
Stejskal J, Sapurina I, Trchova M, Konyushenko EN (2008) Oxidation of aniline: polyaniline granules, nanotubes, and oligoaniline microspheres. Macromolecules 41(10):3530–3536. doi:10.1021/ma702601q
Ding H, Zhu C, Zhou Z, Wan M, Wei Y (2006) Monodispersed and oriented microspheres of polyaniline. Macromol Chem Phys 207(13):1159–1165. doi:10.1002/macp.200600158
King RCY, Roussel F (2009) Toward a simple method for the fabrication of 1D or 3D nanostructures of polyaniline. Synth Met 159(23–24):2512–2518. doi:10.1016/j.synthmet.2009.08.048
Wang JX, Wang JS, Zhang XY, Wang Z (2007) Assembly of polyaniline nanostructures. Macromol Rapid Commun 28(1):84–87. doi:10.1002/marc.200600557
Chiou NR, Lee LJ, Epstein AJ (2007) Self-assembled polyaniline nanofibers/nanotubes. Chem Mater 19(15):3589–3591. doi:10.1021/Cm070847v
Li GC, Zhang CQ, Peng HR (2008) Facile synthesis of self-assembled polyaniline nanodisks. Macromol Rapid Commun 29(1):63–67. doi:10.1002/marc.200700584
Zhou CQ, Han J, Guo R (2008) Controllable synthesis of polyaniline multidimensional architectures: From plate-like structures to flower-like superstructures. Macromolecules 41(17):6473–6479. doi:10.1021/Ma800500u
Pan LJ, Pu L, Shi Y, Sun T, Zhang R, Zheng YD (2006) Hydrothermal synthesis of polyaniline mesostructures. Adv Funct Mater 16(10):1279–1288. doi:10.1002/adfm.200500543
Song SY, Pan LJ, Li Y, Shi Y, Pu L, Zhang R, Zheng YD (2008) Self-assembly of polyaniline: mechanism study. Chin J Chem Phys 21(2):187–192. doi:10.1088/1674-0068/21/02/187-192
Zhang YS, Xu WH, Yao WT, Yu SH (2009) Oxidation-reduction reaction driven approach for hydrothermal synthesis of polyaniline hollow spheres with controllable size and shell thickness. J Phys Chem C 113(20):8588–8594. doi:10.1021/Jp810491u
He YJ (2005) Interfacial synthesis and characterization of polyaniline nanofibers. Mater Sci Eng B Solid State Mater Adv Technol 122(1):76–79. doi:10.1016/j.mseb.2005.04.014
Li W, Li L, Zhang QH, Chen DY, Donghua Univ SKLMCF, Polymer M (2005) Study on dendritic nanofibers of polyaniline via interfacial polymerization. In: Proceedings of 2005 International Conference on Advanced Fibers and Polymer Materials. Chemical Industry Press, Beijing, pp 861–865
Lai Y-q, Lu H, Zhang Z-a, Li J, Li J, Liu Y-x (2007) Preparation and capacitive performance of polyaniline nanofibers by interfacial polymerization. Journal of Central South University (Science and Technology):1110–1114
Li JB, Jia QM, Zhu JW, Zheng MS (2008) Interfacial polymerization of morphologically modified polyaniline: from hollow microspheres to nanowires. Polym Int 57(2):337–341. doi:10.1002/pi.2353
Shihai D, Hui M, Wanjin Z (2008) Fabrication of DBSA-doped polyaniline nanorods by interfacial polymerization. J Appl Polym Sci: 2842–2847. doi:10.1002/app.28355
Tong YC, Hu CL, Wang QY, Bai J, Lei ZQ, Su BT (2008) Preparation of PANI/TiO2 composite nanofiber materials by interfacial polymerization. Chem J Chin Universities Chin 29(2):415–418
Xing SX, Zhao GK, Yuan Y (2008) Preparation of polyaniline-polypyrrole composite sub-micro fibers via interfacial polymerization. Polym Compos 29(1):22–26. doi:10.1002/pc.20344
Xing SX, Zheng HW, Zhao GK (2008) Preparation of polyaniline nanofibers via a novel interfacial polymerization method. Synth Met 158(1–2):59–63. doi:10.1016/j.synthmet.2007.12.004
Bedre MD, Basavaraja S, Salwe BD, Shivakumar V, Arunkumar L, Venkataraman A (2009) Preparation and characterization of Pani and Pani-Ag nanocomposites via interfacial polymerization. Polym Compos 30(11):1668–1677. doi:10.1002/pc.20740
Georgakilas V, Dallas P, Niarchos D, Boukos N, Trapalis C (2009) Polypyrrole/MWNT nanocomposites synthesized through interfacial polymerization. Synth Met 159(7–8):632–636. doi:10.1016/j.synthmet.2008.12.007
Huang JX, Virji S, Weiller BH, Kaner RB (2003) Polyaniline nanofibers: facile synthesis and chemical sensors. J Am Chem Soc 125(2):314–315. doi:10.1021/Ja028371y
Li W, Zhang QH, Chen DJ, Li L (2006) Study on nanofibers of polyaniline via interfacial polymerization. J Macromol Sci Part A Pure Appl Chem 43(11):1815–1824. doi:10.1080/10601320600941029
Huang JX, Kaner RB (2004) A general chemical route to polyaniline nanofibers. J Am Chem Soc 126(3):851–855. doi:10.1021/Ja0371754
Li Y, Wang BC, Feng W (2009) Chiral polyaniline with flaky, spherical and urchin-like morphologies synthesized in the L-phenylalanine saturated solutions. Synth Met 159(15–16):1597–1602. doi:10.1016/j.synthmet.2009.04.023
Jin E, Wang X, Liu N, Zhang WJ (2007) Self-assembled microspheres of glucose-containing polyaniline by alkali-guided method. Mater Lett 61(27):4959–4962. doi:10.1016/j.matlet.2007.03.080
Laslau C, Zujovic ZD, Travas-Sejdic J (2009) Polyaniline “Nanotube” self-assembly: the stage of granular agglomeration on nanorod templates. Macromol Rapid Commun 30(19):1663–1668. doi:10.1002/marc.200900244
Konyushenko EN, Stejskal J, Sedenkova I, Trchova M, Sapurina I, Cieslar M, Prokes J (2006) Polyaniline nanotubes: conditions of formation. Polym Int 55(1):31–39. doi:10.1002/Pi.1899
Fu YP, Elsenbaumer RL (1994) Thermochemistry and kinetics of chemical polymerization of aniline determined by solution calorimetry. Chem Mater 6(5):671–677
Zujovic ZD, Laslau C, Bowmaker GA, Kilmartin PA, Webber AL, Brown SP, Travas-Sejdic J (2010) Role of aniline oligomeric nanosheets in the formation of polyaniline nanotubes. Macromolecules 43(2):662–670. doi:10.1021/Ma902109r
Zimmermann A, Kunzelmann U, Dunsch L (1998) Initial states in the electropolymerization of aniline and p-aminodiphenylamine as studied by in situ FT-TR and UV-Vis spectroelectrochemistry. Synth Met 93(1):17–25
Venancio EC, Wang PC, MacDiarmid AG (2006) The azanes: a class of material incorporating nano/micro self-assembled hollow spheres obtained by aqueous oxidative polymerization of aniline. Synth Met 156(5–6):357–369. doi:10.1016/j.synthmet.2005.08.035
Acknowledgement
The authors greatly thank undergraduate student Mr. Kai Yao (Chemical Engineering, Xi’an Jiaotong University) for the helpful assistance with experiments and Mr. Zhuang Miao (Northwest Institute for Nonferrous Metal Research) for help with the SEM images.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(DOC 38 kb)
S-Figure 1
Entangled PANI nanofibers prepared in the interfacial polymerization system with aqueous phase possessing an initial pH of ca. 1.7. (JPEG 28 kb)
S-Figure 2
PANI nanofibers mixed with a very small portion of nanorods with smooth surfaces prepared in the interfacial polymerization system with aqueous phase possessing an initial pH of ca. 2.6. (JPEG 24 kb)
S-Figure 3
The FTIR spectra of PANI obtained in the static interfacial polymerization system and in the mildly stirred interfacial polymerization systems (corresponding to the samples shown in Figure 6). (JPEG 21 kb)
S-Figure 4
The in-situ recorded pH profile in the aqueous phase of the interfacial polymerization system with a much lower APS concentration (8.3×10−3 M). The molar ratio of aniline and APS was 3:1. (JPEG 11 kb)
S-Figure 5
The in-situ recorded pH profiles in aqueous phase of aniline polymerization systems with initial pH of ca 0.4 (a) and ca.4.0 (b). (JPEG 20 kb)
S-Figure 6
The original SEM image with a larger magnification of microscale spherical PANI shown in Figure 1 (d). (JPEG 16 kb)
S-Figure 7
The original SEM image with a larger magnification of microscale spherical PANI shown in Figure 1 (e). (JPEG 17 kb)
S-Figure 8
The original SEM image with a larger magnification of microscale spherical PANI shown in Figure 1 (f). (JPEG 12 kb)
S-Figure 9
The original SEM image with a larger magnification of microscale spherical PANI shown in Figure 3 (b). (JPEG 17 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Wang, Y., Jing, X. et al. Early stage pH profile: the key factor controlling the construction of polyaniline micro/nanostructures. J Polym Res 18, 2119–2131 (2011). https://doi.org/10.1007/s10965-011-9622-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-011-9622-6