Abstract
Two novel poly(butylene succinate-co-2-methyl-1,3-propylene succinate)s, PBMPSu 95/5 and PBMPSu 90/10, were characterized as having 6.5 and 10.8 mol% 2-methyl-1,3-propylene succinate (MS) units, respectively, by 1H NMR. A differential scanning calorimeter (DSC) and a polarized light microscope (PLM) employed to investigate the nonisothermal crystallization of these copolyesters and poly(butylene succinate) (PBSu). Morphology and the isothermal growth rates of spherulites under PLM experiments at three cooling rates of 1, 2.5 and 5 °C/min were monitored and obtained by curve-fitting. These continuous rate data were analyzed with the Lauritzen-Hoffman equation. A transition of regime II→III was found at 96.2, 83.5, and 77.9 °C for PBSu, PBMPSu 95/05, and PBMPSu 90/10, respectively. DSC exothermic curves at five cooling rates of 1, 2.5, 5, 10 and 20 °C/min show that almost all of the nonisothermal crystallization occurred in regime III. DSC data were analyzed using modified Avrami, Ozawa, Mo, Friedman and Vyazovkin equations. All the results of PLM and DSC measurements reveal that incorporation of minor MS units into PBSu markedly inhibits the crystallization of the resulting polymer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the use in a large amount of the chemosynthetic polymers has caused a global environmental problem because these polymers are difficult to be decomposed. Biodegradable polymers which can be degraded to CH4, CO2 and H2O by enzymes or microorganisms have received much attention as ecological materials [1]. Aliphatic polyesters have been a good choice among many biodegradable polymers. Poly(butylene succinate) (PBSu) which is a chemosynthetic aliphatic polyester has relatively high melting point and comparable mechanical properties with those of widely used polymers such as low-density polyethylene (LDPE) and poly(propylene) [2]. Because it can be processed using conventional equipments, it becomes the hopeful substitute for LDPE.
Fundamentally, the characterization for the enzymatic degradation of polyester films demonstrated that the degree of crystallinity, morphology and crystal structure play an important role in the mechanical properties and biodegradability of polymers [3]. In many literatures, the crystalline structure, crystallization and melting behavior of neat PBSu [4–15] have been investigated extensively. PBSu has a relatively low biodegradation rate because of its high crystallization rate and high crystallinity. To improve the biodegradability of PBSu, physical blending and copolymerization have often been adopted. About copolymers, the degradation rates of PBSu can be increased by incorporating minor amounts of diols or diacids [10, 16, 17] because of the reduced crystallinity.
Poly(propylene succinate) (PPSu) derived from 1,3-propanediol with an odd number of carbon atoms in the backbone has gained increasing attention, because it has a higher biodegradation rate than that with two or four carbon atoms in the diol monomer [18–20]. Poly(butylene succinate-co-propylene succinate) (PBPSu) copolymers have been synthesized and characterized by combining PBSu with a high melting point and PPSu with high biodegradability [21–25]. The crystallization rate of PBPSu decreases as the mol% of propylene succinate unit increases.
In contrast with the high cost and the limited availability of 1,3-propanediol, 2-methyl-1,3-propanediol (MPD) is commercially available in early 1990s. It is well known that the linearity and conformation of polymer backbone have a significant effect on the crystallization behavior of the polymer. Upon introducing the MPD units into the backbone, methyl group on the side chain of a polymer will further retard the crystallization [26–30]. No earlier study of PBSu-based copolymers involving MPD units could be found in the literatures. Furthermore, the crystallization kinetics under nonisothermal conditions has not been studied.
In a previous study [31], PBSu and PBSu-rich copolyesters [poly(butylene succinate-co-2-methyl-1,3-propylene succinate), PBMPSu] with high molecular weight were synthesized via a direct polycondensation reaction in the presence of a highly effective catalyst, titanium tetraisopropoxide. The distribution of 2-methyl-1,3-propylene succinate (MS) unit in these copolymers was characterized to be random from the 13C NMR spectrum. The heating thermograms of quenched specimens and the WAXD patterns of melt-crystallized specimens show that incorporating MS units into PBSu markedly inhibits its crystallization rate.
In this study, the growth rates and the morphology of spherulites were obtained by performing nonisothermal experiments under a polarized light microscope (PLM). Three cooling rates (ϕ) of 1, 2.5 or 5 °C/min were employed. Curve-fitting methods allow the isothermal growth rates of PBSu and PBMPSu spherulites to be determined. Continuous growth rates were analyzed using the Lauritzen–Hoffman (LH) equation [32]. Nonisothermal crystallization of PBSu and its PBMPSu copolymers was also examined using a differential scanning calorimeter (DSC) at five different cooling rates. A time-temperature-transformation (TTT) diagram was superposed on the continuous-cooling-transformation (CCT) curves of PBSu and its copolymers [33]. The crystallization kinetics of the exothermic enthalpy were analyzed and compared using modified Avrami [34–36], Ozawa [37], and Mo [38] models. Finally, the activation energy of crystallization was evaluated using the isoconversional methods of Friedman [39] and Vyazovkin [40, 41]. These results are compared with the results of kinetics studies.
Experimental
Materials and preparation of specimens
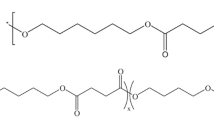
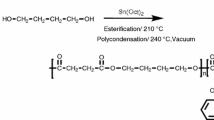
PBSu and PBSu-rich copolymers with minor amounts of MS units were synthesized by a two-stage esterification reaction of succinic acid, 1,4-butanediol (BD) and MPD [31]. The copolyesters used in this study are PBMPSu 95/05 and PBMPSu 90/10 where the numerical values represent the feed ratios of the diols (BD/MPD).
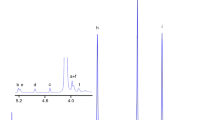
NMR spectra of d-chloroform solutions were obtained using tetramethylsilane as the reference standard on a Varian UNITY INOVA-500 NMR at 295.5 K. Figure 1 displays the 1H NMR spectrum of the PBMPSu 90/10 copolymer and the peak assignments. The two chemical shifts at 4.09–4.15 and 1.68–1.74 ppm are associated with the methylene protons that are α (H1) and β (H2) bonded to the ester oxygen in the butylene succinate (BS) units. The chemical shift of the protons of the succinic moiety (H3) appears at 2.61–2.66 ppm, whereas those of the H4, H5 and H6 protons derived from the MPD monomer are at δ = 4.01–4.06, 2.12–2.22 and 0.97–1.01 ppm, respectively. A magnified view of the protons is presented in the inset spectra in Fig. 1. H1, H4 and H5 appear as triplet, doublet and octet, respectively. The compositions of these copolymers were determined from the relative integration areas under the proton peaks of BS and MS units. The mol% ratio of BS/MS in PBMPSu 95/05 was 93.5/6.5. That in PBMPSu 90/10 was characterizing as having 89.2/10.8. The analysis of the 13C NMR spectra of the carbonyl carbon indicates a random distribution of the comonomers in these two copolymers [31].
The number average molecular weight of PBSu, PBMPSu 95/05, and PBMPSu 90/10 were 3.0 × 104, 2.7 × 104, and 2.4 × 104 g/mol, respectively, relative to poly(methyl methacrylate) which were obtained from gel permeation chromatography at 40 °C using hexafluoroisopropanol as the solvent [31]. Their molecular weight distribution values were 2.5, 2.7 and 2.2. The corresponding values of glass transition temperature (T g ) were –41.1, –39.9 and –38.3 °C, respectively, and those of melting temperatures (T m ) were 113.1, 107.4 and 102.5 °C, respectively, which were measured at 10 °C/min using a Perkin-Elmer Pyris 1 DSC [31].
Dried polyesters were sandwiched between two polyimide films and two copper plates which were then hot pressed at a preset temperature 20~30 °C above T m under 2.5 and 5 kg/cm2 pressure. Compressed sheets with a thickness of about 0.2 mm were made and then dried in vacuo overnight at room temperature before use.
PLM measurements
The spherulites growth rates of polyesters were determined by a Nikon Optiphot-pol polarizing microscope which equipped with a Linkam THMS-600 heating stage and a TMS-91 temperature controller. The video photograph system was adopted with a Sony DXC-755 CCD color video camera, a Pioneer DVR-510H DVD recorder and a Nikon COOLKPIX P510 digital camera. Fresh specimen was sandwiched between two microscopic covers and heated at 20 °C/min under nitrogen to 70–80 °C above melting temperature, at which temperature it was maintained for 5 min to melt the crystalline residues. In the nonisothermal experiment, the premolten specimen of PBSu was cooled rapidly from 190 to 89 °C (to 87 °C for PBMPSu 95/05 and 85 °C for PBMPSu 90/10, respectively), held for 4 min for the development of nuclei, heated at 100 °C/min to 99 °C (to 97 °C for PBMPSu 95/05 and 92 °C for PBMPSu 90/10, respectively), and held for another 2 min to yield suitably sized spherulites. Then, a scan to room temperature at a cooling rate of 1, 2.5 or 5 °C/min was conducted for subsequent analysis. The final pictures of these specimens were taken using digital camera. In the isothermal experiments, the premolten specimen was cooled rapidly to the specific crystallization temperature (T c), which ranged from 97 °C to 75 °C. The sizes of the spherulites during crystallization were recorded versus time, and the growth rates of the spherulites were calculated from the slopes of the plots of radius versus time.
Measuring DSC
A TA instrument DSC 2910 with a refrigerating system was used to investigate the nonisothermal crystallization of these polyesters from the melt state under a dynamic atmosphere of nitrogen at 50 ml/min. Approximately 10 mg of fresh sample was used in each case. The samples started from 30 °C up to 190 °C at a heating rate of 10 °C/min and held for 5 min to eliminate thermal history. Then the samples were cooled down to 10 °C at a rate of 1, 2.5, 5, 10 or 20 °C/min, respectively. The exothermic crystallization curves were recorded as a function of temperature or time, and the nonisothermal crystallization kinetics was analyzed by different models [34–41].
Results and discussion
Determination of growth rates by the non-isothermal method
In general, the growth rate (G) of spherulites is determined by measuring the spherulitic radii (r) from PLM micrographs that are taken at time (t) intervals before spherulitic impingement during the isothermal crystallization experiments. Although this method is easy and accurate, it is time consuming. The nonisothermal method for estimating the G values of polymers is an alternative means of solving this problem. Combining self-nucleation and nonisothermal temperature programs [42–45] widens the range of temperatures (T) over which the growth rate can be measured. When the melt of the specimen is solidified at a constant cooling rate (dT/dt, ϕ), the G values can be estimated from the following equation:
Different cooling rates were used to determine the growth rates of PBSu and its copolymers. Slower cooling rate allows G to be measured over a narrow range near higher T c. In the contrast, faster cooling rate can be applied to a wider T c range. Figure 2 displays the crystal growth rates of PBMPSu 90/10 obtained by nonisothermal crystallization at cooling rates at 1, 2.5 or 5 °C/min. These three continuous curves are in good agreement. Properly fitting these three curves from different cooling rates gives a curve for the growth rate vs. temperature, which is shown as a short dash line in Fig. 3. The other two curves for PBSu and PBMPSu 95/05 are also plotted in Fig. 3 for comparison. The growth rates of spherulite for PBMPSu 90/10 increase from 0.02 μm/s at 92 °C to a value of 0.3 μm/s at 74 °C. The growth rate of spherulite for PBMPSu 90/10 is the lowest and that for PBSu is the highest. It reveals that the growth rate decreases gradually with the amount of minor MS units incorporated into PBSu. Symbol points in Fig. 3 were obtained under the isothermal crystallization experiments at various T c values. It can be found that the growth rates of spherulite measured using isothermal method are very close to those results obtained using nonisothermal method. The maximum growth rate of these polyesters can not be observed because the crystallization rate of them is too fast to be measured. The growth rate of PBMPSu 90/10 spherulites is about one ninth of that of PBSu at 82 °C; that of PBMPSu 95/05 is about one third of PBSu. These results reveal that an efficient and reliable non-isothermal method can be adopted to obtain the isothermal growth rate of spherulites.
Kinetic analysis of growth rates of spherulites
Based on the secondary nucleation theory of LH [32], the growth rates of spherulites can be expressed as
where G 0 denotes a preexponential factor, R represents the gas constant; U* is the activation energy of reptational diffusion; K g denotes the nucleation parameter, T ∞ is a hypothetical temperature below which motion ceases; \( \Delta T = (T_m^o - {T_c}) \) indicates the degree of undercooling, \( T_m^o \) is the equilibrium melting temperature, and f is a correction term of the order of unity, and is usually given by \( f = {2}{T_c}/(T_m^o + {T_c}) \). The continuous growth rate data in Fig. 3 was analyzed using Eq. 2. The regime transition analyses of the LH model were performed by both empirical (U * = 1,500 cal/mol, T ∞ = T g −30) and Williams–Landel–Ferry (WLF) (U * = 4,200 cal/mol, T ∞ = T g −51.6) values, respectively, as shown in Table 1. Figure 4 presents a typical regime analysis using the following values; U* = 35,000 cal/mol and T ∞ = T g −30 K with T g at 234.8 K. \( T_m^o \) = 391.5 K [Supporting Information Fig. S1] was used. The two optimally fit lines of logG + U*/[2.303R(T c −T ∞)] as a function of 1/(T c ∆Tf ) yield the values of K g (slope × 2.303) and log G 0 (intercept) in each regime for PBMPSu 90/10. A break in the curve occurs at T c = 77.9 °C and the ratio of the two slopes is 2.0, as shown in the 17th row of Table 1. Columns 4–9 in Table 1 present the calculated values of K g , the correlation coefficient (r 2) and regime II→III transition temperature (T II→III) as for various T ∞ and U* values (see columns 2 and 3). It is obvious that the regime transition temperature T II→III is almost independent of the values of U* for each sample. The results show that T II→III is 96.2, 83.5, and 77.9 °C for PBSu, PBMPSu 95/05, and PBMPSu 90/10, respectively. The T II→III for PBSu crystallization is very close to the value 96 °C in the literature [9, 23].
Morphology of spherulites
Figure 5 shows micrographs of PBSu, PBMPSu 95/05, and PBMPSu 90/10 that had been crystallized from the melt at cooling rates of 1 and 5 °C/min. The spherulite size increased as the cooling rate declined because the nucleation rate decreased, reducing the nucleation density. These spherulites displayed Maltese cross. Bands were observed at regular intervals in the radial direction in the extinction patterns of the PBSu spherulites and those copolyesters spherulites. The textures of the ringed band spherulites become more obvious as the cooling rate or the amount of MS units increases. The spacing between the ringed bands decreased as the cooling rate increased, while those near the center of the spherulites increased because of the slower growth rate at a higher temperature. It is well known that ringed bands are often found in many semicrystalline polymers and are produced by the periodic twisting of radiating lamellar crystals about their axes of fastest growth [46]. The spherulites of PBSu homopolymers are larger than those of these copolyesters, as revealed by the textures of Fig. 5. It indicated that incorporating minor MS units into PBSu substantially inhibits the crystallization behavior of PBSu, leading to a clear secondary crystallization phenomenon.
Nonisothermal crystallization
Figure 6 presents the nonisothermal melt-exothermic curves of PBMPSu90/10 recorded at five different cooling rates from 1 to 20 °C/min. It is apparent that these exothermic curves become broader and shift to the lower temperature region as the cooling rate increases. The times and temperatures of the starting, peak and ending of these nonisothermal exothermic curves were recorded. Table 2 lists the basic parameters of these polyesters, such as the peak temperature (T p ) and the enthalpy of nonisothermal crystallization (ΔH c ). The values of T p or ΔH c decreased as the cooling rate increased in each case and they followed the order of PBSu > PBMPSu 95/05 > PBMPSu 90/10 at a given cooling rate. Figure 7 displays a time-temperature-transformation (TTT) diagram superposed on the continuous-cooling-transformation (CCT) curves for PBMPSu 90/10 samples following the consolidation at 190 °C for 5 min. The CCT curves plot the value of temperature versus crystallization time (in unit min) at the five cooling rates. The TTT diagram is established by fitting the connecting points of the starting, peak and ending times for the nonisothermal crystallization processes. Based on the kinetic analysis of the growth rates of PBMPSu 90/10 spherulites in the previous section, it shows that T II→III is 77.9 °C (a horizontal dash line), which exceeded the starting temperatures of nonisothermal crystallization, except T II→III was only a few degrees below the starting temperatures at the cooling rates of 1 and 2.5 °C/min, as shown in Fig. 7. It indicates that most of the nonisothermal crystallization of PBMPSu 90/10 in this study occurred in regime III. PBSu and PBMPSu 95/05 had all of the nonisothermal crystallization occurred in regime III. In regime III, the crystallization rate is relatively high, and the polymer chains do not undergo repeated adjacent reentry into the lamellae [47]. The secondary crystallization may be more likely to occur in regime III than in regime II.
From the DSC thermograms in Fig. 6, the relative degree of crystallinity, X T , at various cooling rates can be expressed as a function of temperature (T) based on the following equation:
where dH denotes the enthalpy of crystallization released during an infinitesimal temperature interval dT, T 0 and T ∞ are the starting and ending temperatures of nonisothermal crystallization. The temperature abscissa can be transformed into a time scale, as shown in Fig. 8, based on the equation of t = (T 0− T)/ϕ, where T is the temperature at crystallization time t, and ϕ is the cooling rate. X T is replaced by X t after the transformation of abscissa from T to t. The half-time of crystallization (t 1/2—defined as the time required from the starting of crystallization to the time at which X t is 50%) was evaluated. Figure 8 and t 1/2 values in Table 2 indicate that increasing the cooling rate reduces the crystallization time. Column 7 in Table 2 shows the enthalpy of nonisothermal crystallization (ΔH c), which average value declines from 71.0 ± 3.5 J/g for PBSu, through 63.6 ± 1.4 J/g for PBMPSu 95/05, to 60.5 ± 1.1 J/g for PBMPSu 90/10. The degree of crystallinity was calculated by dividing ΔH c by 210 J/g [14], and is listed in the last column in Table 2. Incorporating minor 6.5 or 10.8 mol% MS units into PBSu markedly inhibits the crystallization behavior of PBSu, therefore, the secondary crystallization behavior in the copolymers cannot be ignored.
Avrami model
The most common model to describe the overall isothermal crystallization kinetics is the Avrami equation [34, 35]. It assumes that the development of the relative degree of crystallinity, X t , is related to the crystallization time t as,
Taking the double-logarithms form as follows:
where K and n are the crystallization rate constant and Avrami exponent, respectively. Avrami equation has been modified by Jeziorny. He proposed a method to determine the parameters characterizing the kinetics of nonisothermal crystallization [36]. Assuming constant cooling rate (ϕ), the final form is given as:
Given Eq. 5, Fig. 9 shows the plots of Avrami model with time in unit min. In the fitting, the relative crystallinity data between 0.5% and 99.5% were used. The slope and the intercept yield the values of the Avrami exponent n and the rate parameter K (K c from Eq. 6), which are given in Table 2. The n vales were found to be in the range of 3.7~4.4, 3.4~4.8 and 3.4~4.4 for PBSu, PBMPSu 95/05, and PBMPSu 90/10, respectively, suggesting that the crystallization proceeds by three-dimension spherical growth. The values of K c increased as the cooling rate increased. However, in nonisothermal crystallization, K c and n do not have the same physical significance as in the isothermal process because the temperature decreases continuously in a nonisothermal process. This change in temperature affects the rate of both nuclei formation and spherulite growth. Based on the values of K c and t 1/2, as shown in Table 2, the crystallization rate followed the order: PBSu > PBMPSu 95/05 > PBMPSu 90/10. In this study, the possible effect of molecular weight on the growth rate and the overall crystallization rate of these polyesters are not discussed because the intrinsic viscosity, \( {\overline M_n} \) and molecular weight distribution of these polyesters were close to each other [31].
Ozawa model
When the time variable in the Avrami equation was replaced by a cooling rate (by replacing t with T/ϕ), Ozawa [37] model can be expressed as follows:
Taking the double logarithm of Eq. 7 and rearranging yields the following form
where K(T) is a function of the cooling rate, and m is an Ozawa exponent. A plot of log [–ln (1–X t )] versus log ϕ at a given temperature should be a straight line if the Ozawa equation captures the nonisothermal crystallization process very well. K(T) and m are obtained from the intercept and the slope, respectively. According to the Ozawa model, the analyses of the data within the temperatures ranging from 63 to 73 °C are shown in Fig. 10 for PBMPSu 90/10. The plots of log [–ln (1–X t )] against log ϕ are not straight lines. Evidently, the Ozawa analysis does not accurately describe the nonisothermal crystallization kinetics of this copolyester because part of the crystallization is secondary crystallization, as discussed in the previous sections. Similar results are obtained for PBSu and PBMPSu 95/05, and are consistent with the result for PBSu in the literature [48].
Mo model
Mo and coworkers [38] proposed a different approach for the nonisothermal crystallization by combining the Avrami equation with the Ozawa equation as below:
or equivalently
where the parameter F(T) = [K(T)/K]1/m, a is the ratio of the Avrami exponent n to the Ozawa exponent m, i.e., a = n / m. According to the Mo method, at a given degree of crystallinity, the plot of log ϕ versus log t should yield a straight line, as presented in Fig. 11 for PBMPSu 90/10. It is clear that Mo model describes the nonisothermal crystallization of PBMPSu 90/10 well with the correlation coefficient values larger than 0.995. The kinetic parameter F(T) and a value can be determined from the intercept and the slope, respectively. Table 3 lists the values of F(T) and a for PBSu, PBMPSu 95/05 and PBMPSu 90/10, respectively. The value of F(T) increases with increasing the degree of crystallinity, which indicated that at unit crystallization time, a higher cooling rate is required to reach a higher X t . To have the same X t , the required cooling rate followed the order: PBMPSu 90/10 > PBMPSu 95/05 > PBSu. This result reveals that the crystallization rate followed the order: PBSu > PBMPSu 95/05 > PBMPSu 90/10, again. The value of a exceeds 1, indicating that n is always larger than m, and suggesting that markedly secondary crystallization growth accompanies primary crystallization during the nonisothermal crystallization.
Effective activation energy
Kissinger’s method [49] has been adopted frequently for evaluating the effective activation energy during nonisothermal crystallization. This method has been formulated for heating experiments, but it has been demonstrated by Vyazovkin et al. that dropping the negative sign for the rate parameter (i.e., cooling rate) is a mathematically invalid procedure [40, 41]. The differential isoconversional method of Friedman [39] is as follows:
where dX t /dt represents the instantaneous crystallization rate as a function of time for a given value of X t , A is a preexponential constant, and \( \Delta {E_{{{X_t}}}} \)denotes the effective energy barrier in the process for a given X t . Plotting ln(dX t /dt) versus 1/\( {T_{{{X_t}}}} \)at a given degree of relative crystallinity (X t ) yields a straight line with a slope of –\( \Delta {E_{{{X_t}}}} \)/R. Figure 12 plots the dependence of the effective activation energy on the relative degree of crystallinity. \( \Delta {E_{{{X_t}}}} \) was found to increase with the relative crystallinity in these three polyesters, suggesting that as the crystallization proceeded polymer became more difficult to be crystallized. The values of \( \Delta {E_{{{X_t}}}} \) for PBSu, PBMPSu 95/05 and PBMPSu 90/10 were –180.3, –161.9 and –149.3 kJ/mol, respectively, at a given value of X t =10%. At all X t values, \( \Delta {E_{{{X_t}}}} \) followed the order: PBSu < PBPSu 95/5 < PBPSu 90/10.
The effective activation energy can be determined by the advanced integral isoconversional method of Vyazovkin [40, 41]. According to Vyazovkin’s method, the temperature dependence of the effective activation energy (ΔE T ) of the growth rate (G) is calculated as follows:
Activation energy (ΔE T ) can be obtained using the values of G ≈ 1/t 1/2 from nonisothermal crystallization experiment [50]. There is a temperature corresponding to t 1/2 from the DSC experiment for each cooling rate (see Table 2). According to Eq. 2, the value of K g for Vyazovkin equation can be obtained by five data points which were analyzed using the following values: U* = 6,500 cal/mol and T ∞ = T g −30 K for PBSu, PBMPSu 95/05 and PBMPSu 90/10, respectively. The values of ΔE T can be then calculated from Eq. 12 using these values of K g and U*. Figure 12 plots the conversion dependence of the effective activation energy (ΔE T ) from the Fridman and Vyazovkin methods for comparison. Figure 13 presents the temperature dependence of these ΔE T values from both methods. The values of ΔE T for PBMPSu 90/10 using Fridman method are very close to those values using Vyazovkin method. The values of ΔE T for PBSu were always smaller than those for the other two at the same conversion and followed the order: PBSu < PBPSu 95/5 < PBPSu 90/10. This result is in consistent with the analyses of Friedman model and the measured growth rates of these polyesters, as described above. It reveals that incorporating minor MS units into PBSu will result in a lower crystallinity and increase its biodegradability.
Conclusions
PBSu and two PBMPSu copolymers with high molecular weights were studied. The compositions of the PBMPSu 95/05 and PBMPSu 90/10 were characterized as having 6.5 and 10.8 mol% MS units, respectively. The isothermal growth rates of spherulites were determined using the nonisothermal method at cooling rates of 1, 2.5 and 5 °C/min, respectively. This method is efficient and reliable comparing with the isothermal method because the values of growth rates from the nonisothermal method fit very well with the values from the isothermal experiments. The growth rate of spherulites for PBMPSu 90/10 copolyester was about one ninth of that for PBSu, and that for PBMPSu 95/05 was about one third of that for PBSu at 82 °C. This result reveals that incorporating minor amount of MS units into the backbone of PBSu homopolymer substantially decreased the crystallization rate of the resulting copolymer. Based on the Hoffman–Lauritzen theory, the regime II→III transition temperatures of PBSu, PBMPSu 95/05 and PBMPSu 90/10 occurred at about 96.2, 83.5 and 77.9 °C, respectively.
Nonisothermal crystallization kinetics of neat PBSu and PBMPSu copolymers were investigated by DSC. The values of crystallization rates were found to be of the following order at a given cooling rate: PBSu > PBMPSu 95/05 > PBMPSu 90/10. The results indicated that the crystallization of PBSu copolyesters with minor amount MS units were retarded comparing with that of neat PBSu. The modified Avrami and Mo models were found to provide a good fit to the experimental data in this study. The results of kinetic analysis of these polyesters from the rate parameter K c (from the modified Avrami model), the kinetic parameter F(T) (from the Mo model), and the crystallization activation energies (from the Friedman and Vyazovkin models) give the same demonstration that neat PBSu was more crystallizable than its copolymers. The results of both PLM and DSC experiments reveal that the crystallization rate and the degree of cystallinity of PBMPSu decrease as the mole% of MS unit increases.
References
Mochizuki M, Hirami M (1997) Polym Adv Technol 8:203–209
Ishioka R, Kitakuni E, Ichikawa Y (2002) Biopolymers, vol 4. Wiley-VCH, pp 275–297
Kumagai Y, Kanesawa Y, Doi Y (1992) Macromol Chem Phys 193:53–57
Chatani Y, Hasegawa R, Tadokoro H (1971) Polym Prepr Jpn 20:420
Ichikawa Y, Suzuki J, Washiyama J, Moteki Y, Noguchi K, Okuyama K (1995) Polymer J 27:1230–1238
Ihn KJ, Yoo ES, Im SS (1995) Macromolecules 28:2460–2464
Miyata T, Masuko T (1998) Polymer 39:1399–1401
Yoo ES, Im SS (1999) J Polym Sci Part B: Polym Phys 37:1357–1366
Gan ZH, Abe H, Kurokawa H, Doi Y (2001) Biomacromolecules 2:605–613
Cao A, Okamura T, Nakayama K (2002) Polym Degrad Stab 78:107–117
Yasuniwa M, Satou T (2002) J Polym Sci Part B: Polym Phys 40:2411–2420
Qiu ZB, Komura M, Ikehara T, Nishi T (2003) Polymer 44:7781–7785
Yasuniwa M, Tsubakihara S, Satou T, Iura K (2005) J Polym Sci Part B: Polym Phys 43:2039–2047
Papageorgiou GZ, Bikiaris DN (2005) Polymer 46:12081–12092
Wang XH, Zhou JJ, Li L (2007) Eur Polym J 43:3163–3170
Zhu CY, Zhang ZG, Liu QP, Wang ZP, Jin J (2003) J Appl Polym Sci 90:982–990
Tserki V, Matzinos P, Pavlidou E, Vachliotis D, Panayiotou C (2006) Polym Degrad Stab 91:367–376
Ranucci E, Liu Y, Lindblad MS, Albertsson AC (2000) Macromol Rapid Commun 21:680–684
Liu Y, Ranucci E, Lindblad MS, Albertsson AC (2001) J Polym Sci Part A: Polym Chem 39:2508–2519
Chrissafis K, Paraskevopoulos KM, Bikiaris DN (2006) Polym Degrad Stab 91:60–68
Xu YX, Xu J, Guo BH, Xie XM (2007) J Polym Sci Part B: Polym Phys 45:420–428
Xu YX, Wu J, Liu DH, Guo BH, Xie XM (2008) J Appl Polym Sci 109:1881–1889
Papageorgiou GZ, Bikiaris DN (2007) Biomacromolecules 8:2437–2449
Chen CH, Peng JS, Chen M, Lu HY, Tsai CJ, Yang CS (2010) Colloid Polym Sci 288:731–738
Lu SF, Chen M, Shih YC, Chen CH (2010) J Polym Sci Part B: Polym Phys 48:1299–1308
Sullivan CJ, Dehm DC, Reich EE, Dillon ME (1990) J Coat Technol 62:37–45
Bello P, Bello A, Riande E (1999) Macromolecules 32:8197–8203
Nalampang K, Johnson AF (2003) Polymer 44:6103–6109
Suh J, Spruiell JE, Schwartz SA (2003) J Appl Polym Sci 88:2598–2606
Lewis CL, Spruiell JE (2006) J Appl Polym Sci 100:2592–2603
Chen CH, Yang CS, Chen M, Shih YC, Hsu HS, Lu SF (2010) eXPRESS Polym Lett. doi:10.3144/expresspolymlett.2011.29
Hoffman JD, Davis GT, Lauritzen JI Jr (1976) Treatise on solid state chemistry, Vol 3, Chapter 7. Plenum, New York
Chen M, Chung CT (1998) Polym Compos 19:689–697
Avrami M (1940) J Chem Phys 8:212–224
Avrami M (1941) J Chem Phys 9:177–184
Jeziorny A (1978) Polymer 19:1142–1144
Ozawa T (1971) Polymer 12:150–158
Liu TX, Mo ZS, Wang SG, Zhang HF (1997) Polym Eng Sci 37:568–575
Friedman HL (1964) J Polym Sci Part C 6:183–195
Vyazovkin S (2001) J Comput Chem 22:178–183
Vyazovkin S, Sbirrazzuoli N (2004) Macromol Rapid Commun 25:733–738
Chung CT, Chen M (1992) Polym Prepr 33:420–421
Chen M, Chung CT (1998) J Polym Sci Part B: Polym Phys 36:2393–2399
Di Lorenzo ML, Cimmino S, Silvestre C (2000) Macromolecules 33:3828–3832
Tsai CJ, Chen M, Lu HY, Chang WC, Chen CH (2010) J Polym Sci Part B: Polym Phys 48:932–939
Keller A (1955) J Polym Sci 17:351–364
Sperling LH (2006) Introduction to physical polymer science, 4th ed, chapter 6. Wiley-Interscience, New York
Qiu ZB, Fujinami S, Komura M, Nakajima K, Ikehara T, Nishi T (2004) Polym J 36:642–646
Kissinger HE (1957) Anal Chem 29:1702–1706
Papageorgiou GZ, Achilias DS, Bikiaris DN (2007) Macromol Chem Phys 208:1250–1264
Acknowledgements
The authors thank the National Science Council of the Republic of China, Taiwan, for financially supporting this research under Contract No. NSC 98-2221-E-110-007.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Fig. S1
Hoffman−Weeks plot for determining the equilibrium melting temperature of PBMPSu 90/10 from DSC data at a heating rate of 10 °C/min. (GIF 40 kb)
Rights and permissions
About this article
Cite this article
Lu, JS., Chen, M., Lu, SF. et al. Nonisothermal crystallization kinetics of novel biodegradable poly(butylene succinate-co-2-methyl-1,3-propylene succinate)s. J Polym Res 18, 1527–1537 (2011). https://doi.org/10.1007/s10965-010-9558-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-010-9558-2