Abstract
Nanocomposite superabsorbents were synthesized by graft copolymerization of mixture of acrylamide (AAm) and acrylic acid (AA) onto collagen using potassium persulfate (KPS) as a free radical initiator and methylenebisacrylamide (MBA) as a crosslinker. Nanoclay sodium montmorillonite (MMt) was introduced as filler into superabsorbent. The chemical structure of the Collagen-g-poly(Sodium Acrylate-co-Acrylamide)/MMt nanocomposite was characterized by means of FTIR spectroscopy, XRD patterns, and TGA thermal methods. Morphology of the sample was examined by scanning electron microscopy (SEM). The effects of reaction variables were systematically optimized to achieve a superabsorbent with swelling capacity as high as possible. Under the optimized conditions concluded, the maximum swelling capacity in distilled water was 950 g/g. Dewatering of nanocomposite and clay-free superabsorbent revealed that inclusion of nanoclay into superabsorbents can improve water retention of superabsorbent under heating. The swelling ratio in various salt solution and kinetic of dewatering was also determined and additionally, the swelling of nanocomposite superabsorbent was measured in solution with pH ranged 1–13. The synthesized nanocomposite exhibited a pH-responsive characteristic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Superabsorbents are loosely crosslinked hydrophilic polymers that in contact with aqueous solution can absorb water and swell to several times to their initial volume without dissolving in aqueous media [1]. Superabsorbents are type of hydrogels capable to swell and absorb a large amount of water [2]. While the shapes of hydrogels do not change extensively during swelling, because of highly swelling capacity of superabsorbent, their shape will extensively change. Because of their excellent response to changing environment conditions such as temperature [3], pH [4], and solvent composition [5], superabsorbents have been attracting in many industrial applications [6, 7]. For example, water retention property and subsequently, the slow release of water from swollen superabsorbent, superabsorbents with high swelling capacity are of special interest as potential water retainer systems for agriculture fields. Also, in the field of agriculture, the slow release of water from the polymeric matrix opens another potential area of application that is related to load of agrochemicals into superabsorbents. In the swollen superabsorbents containing agrochemicals, not only the water releasing takes place, but also the agrochemical will be released together with water [8–11].
The higher production cost and low gel strength of these superabsorbents, however, restrict their application widely. To improve these limitations, inorganic compounds with low cost can be used. The introduction of inorganic fillers to a polymer matrix increases its strength and stiffness properties [12]. Conventional superabsorbent composites have been reported by scientists [13–15]. Inclusion of conventional micro-scale clays into superabsorbents produces aggregated and agglomerated points. This is attributed to the heterogeneous dispersion of clay particles [16]. It has been reported that the type of dispersion of clay in composites determines the properties of polymer composites [17]. In addition to aggregated clay-polymer composites, intercalated and exfoliated clay-polymer composites can be produced using suitable methods [18]. It is required to use nano-scale clays to achieve intercalated or exfoliated composites [16, 17]. The resulting materials are known as nanocomposites. Among these materials, nanocomposite superabsorbents are a class of crosslinked polymers as they can be synthesized through insertion of water soluble polymers into layered nano-clays through polymerization of hydrophilic monomers in the presence of layered nano-clays. Because of its low cost and unique characteristic such as its good water adsorption, extensive swelling in water and cation exchange capacity, montmorilonite as inorganic layered clay is the interest of researchers [19, 20]. Biocompatibility, non-toxicity, and biodegradability of renewable materials, superabsorbents and nanocomposite superabsorbents based on biopolymers such as proteins, starch, chitosan, carboxymethylcellulose, carrageenan, and sodium alginate have been widely studied [21–26].
In the present study, we attempted to synthesize a novel collagen-based superabsorbent nanocomposite using the AAm and AA monomers a well as Na-MMt clay, and investigated the effect of reaction variables on the equilibrium swelling capacity. The swelling ratio in various salt solutions was also determined and additionally, the swelling of the superabsorbents was measured in solutions with pH ranged 1–13. Water retention capacity under heating was also measured.
Experimental
Materials
Hydrolyzed collagen (Parvar Novin-E Tehran Co., Mw = 2000 to 20000 Da) was industrial grade which is available in market and has insoluble phosphate salts. To determine the amount of these salts, three samples (each sample 2.0 g) were examined for determination of these insoluble materials. After dissolving industrial collagen in water, it was filtered and the remaining insoluble salts dried at 50 °C to constant weight. The content of insoluble salts was determined to be 25 ± 1% [26]. Natural Na-MMt (Si4[Al1.67 Mg0.33]O10(OH)2.nH2O.Na0.33) as a clay with cation exchange capacity of 92 meq/100 g of clay was provided by Southern Clay Products. 90 vol% of its dry powder has particle size less than 13 μm, according to the manufacturer’s information.
N,N′-methylenebisacrylamide (MBA, from Merck), Potassium persulfate (KPS , from Merck), Acrylic acid (AA, from Merck), acrylamide (AAm, from Merck) were of analytical grade and used without further purification. All other chemicals were also analytical grade. Double distilled water was used in order to superabsorbent preparation and swelling measurements.
Preparation of superabsorbent
Table 1 represents reaction condition to synthesize the nanocomposites. Hydrolyzed collagen (1.33 g) was dissolved in 50 ml distilled water and filtered to remove the insoluble phosphate salts. It may be noted that taking into account of the 25 wt% of insoluble salts, the content of pure collagen in solution was 1.0 g. The solution was added to a two-neck reactor equipped with a magnetic stirrer and then clay was added to collagen solution and allowed to stir for 24 h. After this time, the reactor was immersed in a water bath preset at a desired temperature (80 °C). Then the crosslinker (dissolved in 5 ml H2O) and certain weight ratio of AAm/AA (dissolved in 10 ml H2O) were simultaneously added to the reactor. The mixture was stirred for 10 min and then the initiator solution (dissolved in 5 ml H2O) was added to the mixture. After the completion of the reaction, (1 h), a certain weight amount of sodium hydroxide (0.277–0.499 g) was added to the reactor as well as vigorously stirring to neutralize (50–90%) carboxyl groups.
The produced superabsorbent was poured to excess non solvent ethanol (200 ml) and remained for 3 h to dewater. Then ethanol was decanted and the product scissored to small pieces (~5 mm diameter). Again, 200 ml fresh ethanol was added and the superabsorbent was left for 24 h. Finally the filtered superabsorbent is dried in oven at 60 °C for 48. After grinding, the powdered nanocomposites were stored away from moisture, heat and light.
Measurement of gel content
To determine the gel content values, 0.05 g of dried sample was dispersed in double distilled water to swell for 72 h. After filtration, the extracted gel was dewatered by non solvent ethanol, dried for 5 h at 70 °C, and then reweighed. Gel content (Gel %) was calculated by Eq. 1:
where m and M stand for initial weight of sample and final weight of sample, respectively.
Swelling measurements
A tea bag (i.e. a 100 mesh nylon screen) containing an accurately weighted powder sample (0.05 ± 0.001 g) was immersed entirely in 250 ml distilled water and allowed to soak for 2 h at room temperature. The sample particle sizes were 40–60 mesh. The tea bag was hung up for 15 min in order to remove excess water. The equilibrium swelling (ES) was calculated by the following Eq.:
The accuracy of the measurements was ±3%. The water absorbency of the nanocomposite in salt solutions and various pHs was determined according to Eq. 2.
Swelling kinetics
To study the rate of absorbency of the synthesized nanocomposite superabsorbents, approximately (0.05 ± 0.001 g) of sample with various particle sizes (40–60 mesh) were poured into weighted tea bags and immersed in 250 ml distilled water. The water absorbency was measured as a function of time.
Swelling in various salt solutions
Absorbency of the optimized sample of collagen-g-poly (AAm-co-AA)/MMt superabsorbent (0.05 ± 0.001 g) was evaluated in 0.15 M solutions of NaCl, AlCl3, KCl, CaCl2, BaCl2.2H2O, LiCl, and MgCl2.6H2O according to the method described above for swelling measurement in distilled water.
Dewatering kinetics of swollen superabsorbent in various salt solutions
To study the rate of water lost of the swollen superabsorbent composite in salt solutions, the dry sample (0.05 ± 0.001 g) were dispersed in 250 ml distilled water for 2 h. Then, the swollen superabsorbent immersed in 0.15 M solution of NaCl, CaCl2, and CuCl2. Water lost of the superabsorbent was measured as a function of time.
Water lost and water uptake of swollen superabsorbent nanocomposite
0.05 ± 0.001 g of dried optimized nanocomposite were dispersed in 250 ml NaCl solution (0.15 M). Then, the swollen nanocomposite was transferred in CaCl2 solution (0. 15 M) and again in NaCl solution. Water uptake and water lost of the nanocomposite was measured as a function of time.
Absorbency at various pHs
Individual solutions with acidic and basic pH were prepared by dilution of NaOH (pH = 13.0) and HCl (pH = 1.0) solutions to achieve pH 8.0 and pH < 8.0, respectively. The pH values were precisely checked by pH-meter (Metrohm/827, accuracy ± 0.1). Then 0.05 gr (±0.001 g) of the dried optimized superabsorbent was used for the swelling measurements according to Eq. 1.
pH-sensitivity
pH-sensitivity of the optimized sample was investigated in terms of swelling and deswelling of the final product at two basic (pH 8.0) and acidic (pH 2.0) solutions, respectively. Swelling capacity of the superabsorbents at each pH was measured according to Eq. 1 at consecutive time intervals and using a fresh solution for each cycle.
Thermal stability of swollen nanocomposite
To study the rate of water lost of the swollen nanocomposite approximately 0.05 ± 0.001 g of dried optimized superabsorbent were dispersed in 250 ml distilled water for 2 h. Then in an oven and at 60 and 80 °C water lost the swollen superabsorbent was measured as a function of time.
Swelling and reswelling capacity
0.05 ± 0.001 g of dried composite was immersed in known volume of de-ionized water to make sure that the swelling equilibrium was achieved. The swollen superabsorbent was placed in an oven at 100 °C until the superabsorbent was dried completely. Then, an equal volume of water was added to the dried superabsorbent and after reaching the equilibrium, it was placed in the oven again at same temperature. A similar procedure was repeated and the saturated absorbency of the sample after several times of reswelling was thus obtained.
Instrumental analysis
FTIR spectra of sample were taken in KBr pellets using a Perkin Elmer Precisely-100 spectrophotometer. The surface morphology of the gel was examined using scanning electron microscopy (SEM). Dried superabsorbent composite powder were coated with a thin layer of gold and imaged in a SEM instrument (S360 CAMBRIDGE). A simultaneous thermal analyzer (STA-625, Reometric Scientific) was used for TGA of samples under nitrogen atmosphere. The heating rate was 20 °C min−1. One-dimensional, wide angle X-ray diffraction patterns were obtained by using a Philips X’Pert MPD X-ray diffractometer with wavelength, λ = 1.54 Å (Cu-Kα), at a tube voltage of 40 KV, and tube current of 40 mA.
Results and discussions
Synthesis and characterization
The nanocomposite superabsorbents were prepared by simultaneously graft copolymerization of AA and AAm onto collagen backbones in the presence MBA and sodium MMt as crosslinker and clay, respectively. KPS was used as initiator. A simple mechanism was illustrated in Scheme 1. The persulfate can decompose on heating and produce sulfate anion-radicals that abstract hydrogen from one of the existing functional groups in the protein backbone (i.e. COOH, SH, OH, and NH2) to form corresponding macro-initiator. These macro-radicals initiate AA/AAm grafting onto the nanoclay and collagen backbone, leading to a graft copolymer. Since a crosslinking agent, e.g. MBA, is presented in the system, the copolymer comprises a crosslinked structure. It may be noted that not only crosslinked graft copolymer is produced, but also crosslinked poly(AAm-co-AA) is formed. In fact, the nanocomposite comprises crosslinked graft and un-graft poly(AAm-co-AA) that the latter section is captured inside nanocomposite and it is impossible to separate this component from graft copolymer. The AAm monomer and collagen biopolymer can intercalate into clays layers and under polymerization of the monomers, an intercalated superabsorbent nanocomposite will be achieved.
The XRD patterns of pristine clay and nanocomposites containing 2.9, 5.6, and 12.3 wt% of clay were collected from 2 to 10° (2θ) and are shown in Fig. 1. Because of ionic structure of AA monomer, monomer composition (AA/AAm weight ratio) can affect type of clay dispersion in nanocomposite. In this study, we only studied the XRD patterns of nanocomposites by varying clay content and using 0.25 of AAm/AA weight ratio. As can be seen from this figure, the XRD profile of pristine Na-MMt (Fig. 1-a) shows a diffractive peak at 2θ = 7.6 corresponding to the distance of clay sheets with d spacing 11.61 nm. Stirring of clay for 24 h subsequently in situ polymerization of AA and AAm in the presence of MBA crosslinker leads to a nanocomposite superabsorbents that the XRD profile of these nanocomposite shown in Fig. 1-(b-d). The results showed that the clay content affects its dispersion type in nanocomposite. It was observed that there is no diffraction peak in nanocomposite containing 2.9 wt% of clay and it can be concluded that the clay layers are completely exfoliated and uniformly dispersed in organic network (Fig. 1-b). However, nanocomposite containing 5.6 wt% of clay shows a relatively broad peak at around 2.5–5.5°. This broad peak indicates intercalated and exfoliated Na-MMt in organic network [27]. When the clay content reaches 12.3 wt%, MMt characteristic peak shifted to 4.5°. In comparison to nanocomposite containing 2.9 wt% of clay, the peak intensity has been increased suggesting intercalated Na-MMt structure.
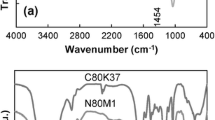
The FTIR spectroscopy was carried out to confirm the chemical structure of the nanocomposite superabsorbent. In Fig. 2(a), the band observed at 1686 cm−1 can be attributed to carbonyl stretching in carboxamide functional groups of collagen. The broad band at 3200–3600 cm−1 is due to stretching of hydroxyl groups of the hydrolyzed collagen. The FTIR of clay-free superabsorbent was shown in Fig. 2(b). In the spectrum of graft copolymer, 2 band peaks at 3197 and 1660 cm−1 correspond to the primary amides and amide –NH stretching vibrations, respectively. The other intense characteristic band at 1548 cm−1 is due to C=O asymmetric stretching in the carboxylate anion that is reconfirmed by another sharp peak at 1407 cm−1, which is related to a symmetric stretching mode of the carboxylate anion.
In the Fig. 2(c), the characteristic vibration bands of the nanoclay (-OH stretch from lattice hydroxyl, -OH stretch from free H2O, -OH bending and Si-O stretch) are shown at 3635, 3448, 1639 and 1040 cm−1, respectively. In the spectrum of the nanocomposite (Fig. 2(d)), the characteristic band at 1667 cm−1 can be attributed to C=O stretching in the carboxamide functional groups and also, two absorption peaks at 1560 and 1405 cm−1 were attributed to asymmetric and symmetric stretching of the carboxylate groups, respectively. In addition, by compared with Fig. 2-c, the absorption peaks at 3635 cm−1 and 3448 cm−1 attributed to the OH groups and intense band at 1040 cm−1 attributed to the Si-O stretching on the nanoclay disappeared after reaction. Therefore, it can suggest that the strong chemical interaction take place between the Si-O and -OH groups of the nanoclay particles with functional groups of AA and AAm monomers during the graft copolymerization reaction. As we can see from FTIR of clay-free and nanocomposite superabsorbents, the characteristic peaks at 1660 (C=O of carboxamide) and 1548 cm−1 (carboxylate anion) for clay free superabsorbents have been shifted to 1667 and 1560 cm−1 for nanocomposite superabsorbent. This may be attributed to interaction of these functional groups with clay.
One of the most important properties of nanocomposites that can be considered is superabsorbent microstructure morphology. Figure 3-a and b show surface morphology of optimized nanocomposite superabsorbents with 1 and 0.5 μm of scale bars, respectively. According to these images, nanocomposite superabsorbent contains a highly porous structure. As can be seen, the pores size is relatively in nano size.
TGA trace is presented in Fig. 4. The grafted collagen containing clay has shown enhancement in thermal stability as clear from TGA curve. As shown in the figure, the initial decomposition temperature increased from 231 °C in collagen to 276 °C in optimized superabsorbent nanocomposite. In comparison to original decomposition temperature of 320 °C of collagen, maximum decomposition rate shifted to 402 °C in synthetic sample. These observations have clearly indicated that grafting has improved the thermal stability of collagen and thermal stability was found to be dependent on degree of grafting onto collagen.
Effect of reaction variables on the swelling
Effect of MBA concentration
To study the effect of crosslinker concentration on the swelling of the resultant nanocomposite superabsorbents, MBA concentration was varied from 3.7 to 12.9 mmol/L. When the MBA concentration was lower than 3.7 mmol/L, nanocomposites did not possess good dimensional stability. As it clear from Table 1, increase in crosslinker content results in a decrease in water absorbency. 3.7 mmol/L of MBA concentration of provides the best swelling (857 g/g). In fact, a high crosslinker concentration decrease the free space between the copolymer chains and consequently the highly crosslinked rigid structure cannot be expanded to hold a large quantity of water. The following power law relationship between equilibrium swelling (ES) capacity and MBA concentration (Eq. 3) can deduce from plotting of swelling against MBA concentration.
K and n in Eq. 3 are constant for an individual superabsorbent [28]. The n value represents the sensitivity of the superabsorbent to the crosslinker content, while the K value gives a useful criterion for comparing the extent of swelling at a fixed crosslinker content (i.e. the higher the K value, the higher the equilibrium swelling capacity). The values K = 2653 and n = 0.75 were obtained from the curve fitted with Eq. 3.
Effect of AAm/AA weight ratio
In this series of experiments, wt% of AAm in monomer feed was changed from 12.5 to 87.5. It expects that because of non-ionic property of AAm monomer, increase in AAm content causes a decrease in water absorbency. But, according to Fig. 5, when AAm wt% was increased from 12.5 to 25, swelling capacity was increased. It may be attributed to presence of nano-clay in superabsorbent composition. In fact, increase in AAm content can cause insertion of polymer into MMt layers. On the other hand, by increasing AAm monomer ratio, distance of clay layers is increased subsequently counterions clay particles or between the plates contribute to the total osmotic pressure inside the superabsorbent. The higher the osmotic pressure, results in the higher the water absorbency [29]. Decrease in water absorbency beyond 25 wt% of AAm can be attributed to decrease in ionic property of nanocomposite.
Effect of KPS concentration
The relationship between the initiator concentration and water absorbency values was studied by varying KPS concentration from 1.05 to 7.39 mmol/L. According to Table 1, the swelling capacity of superabsorbent at 4.22 mmol/L provides the best swelling (952 g/g). According to table, the absorbency is decreased with increasing the KPS concentrations form 4.22 to 7.39 mmol/L. Initial increment in water absorbency may be attributed to increased number of active free radicals on the protein backbone. Subsequent swelling loss is originated from an increase in terminating step reaction via bimolecular collision which, in turn, causes to enhance crosslinking density. In addition, the free radical degradation is an additional reason for swelling-loss at higher KPS concentration.
Effect of nano-clay content on swelling
The swelling capacity of nanocomposite in de-ionized water as a function of nanoclay content was studied by varying the nanoclay amount from 2.9 to 12.3 wt% (Fig. 6). Maximum swelling (952 g/g) was obtained at 5.6 wt% of nanoclay. It is observed that the absorbency is substantially increased from 789 g/g for clay-free superabsorbent to 952 g/g for optimum clay content and then decreased. When the sodium MMt content in superabsorbent composition is low, the ionization of sodium MMt take place easily, and subsequent the osmotic pressure of inside of nanocomposite is increased. Enhancement of osmotic pressure in superabsorbent causes an increase in water absorbency of superabsorbent [30, 31]. However, the swelling-loss after the maximum may be originated from decrease in ionization of sodium MMt and subsequent decrease in osmotic pressure. On the other hand, when the content of clay is higher than 5.6 wt%, clay counterions remain in the local volume around the clay particles or between the plates and do not contribute to the total osmotic pressure inside the superabsorbent. Also, it has been reported that MMt can act as crosslinker in superabsorbent systems. It is clear that by increasing MMt content, crosslinking points increase and results in low swelling capacity [32, 33]. The effect of clay amount on the gel content was shown in this figure. According to results, it was observed that with increasing clay amount in superabsorbent composition, the gel content is increased. This observation can be attributed to the interaction between clay and soluble part of synthesized superabsorbent. In fact, the increase in gel content with increasing clay content may be due to acting of clay as crosslinker. A similar observation has been reported by Kokabi et al [34] in poly (vinyl alcohol) nanocomposite superabsorbents using Na-MMt clay.
Effect of temperature on the swelling
To investigate the effect of reaction temperature on the swelling of nanocomposite superabsorbents, four reactions with same feeding composition were carried out at 60, 70, 80, and 90 °C. The swelling data are illustrated in Table 1. The water absorbency is increased by increasing the reaction temperature from 60 up to 80 °C and then, it is decreased considerably with a further increase in the temperature. The maximum absorbency was obtained at temperature 80 °C. Since KPS is a thermally initiator, it efficiently dissociated at the temperatures higher than its dissociation temperature, i.e. 80 °C. So, the higher initiating radicals increased the extent of polymerization reaction. The absorbency loss after 80 °C can be ascribed to (a) oxidative degradation of collagen chains by sulfate radical-anions generated from thermally dissociation of KPS, (b) increasing the rate of termination and chain transfer reaction and (c) decomposition of KPS to give O2 (a radical scavenger), which reacts with primary free radicals [35], resulting in decreased molecular weight and decreased swelling ratio.
Effect of neutralization percent on swelling
In this series of experiments, after completion of reaction, the carboxylic groups were neutralized to carboxylate anions by NaOH solution. Without the neutralization stage, the carboxylate anions are protonated, so the main anion-anion repulsive forces are eliminated and consequently the water absorbency is decreased. According to Fig. 7, the best neutralization percent was found to be 70%. In high neutralization percent of the carboxylic groups, due to different phenomena that is related to “charge screening effect” of excess Na+ ions in the swelling media, reduction in swelling is observed. The excess cations shield the carboxylate anions and prevent effective anion-anion repulsion (screening /shielding effect) [35]. Also with increasing the NaOH concentration the ionic strength of the swollen solution is increased. As a result, the osmotic pressure between the aqueous and the gel phases is reduced and the swelling is consequently decreased.
Swelling kinetic
Figure 8 represents the dynamic swelling behavior of the optimized nanocomposite with various particle sizes in water. Initially the rate of water uptake increases sharply and then begins to level off.
The data may be well fitted with a voigt-based equation [28]:
Where S t is swelling at time t, S e is equilibrium swelling (power parameter), t is time (min) for swelling S t , and τ (min) stand for the rate parameter. τ (min) for (100–250 μm), (250–400 μm) and (400–550 μm) of particle size was 1.19 min, 1.38 min and 2.12 min respectively. It is well-known that the swelling kinetics for the superabsorbent composite polymers is significantly influenced by particle size of the samples. With a lower the particle size, a higher rate of water uptake is observed. An increase in the rate of absorption would be expected from the increase in surface area with decreasing particle size of nanocomposite superabsorbent.
Swelling in various salt solutions
It is important to know the swelling behavior of superabsorbents in salt solution for many applications, especially agricultural and horticultural ones. It is well known that water absorbency decreases with increasing ionic strength of salt solutions. This result may be attributed to the reduction in the osmotic pressure difference between the superabsorbent and the external salt solution with increasing ionic strength. Also, the screening effect of the additional cations on the anionic groups causes a non-perfect anion-anion electrostatic repulsion and reduces water absorbency [36]. In addition, ionic crosslinking of the superabsorbent in the multivalent cation solution is other cause for reducing of water absorbency. It was our interest to investigate the effect of clay content on the swelling of nanocomposite superabsorbents in salt solutions. The effect of clay content on the swelling of superabsorbents in 0.15 M of NaCl solution was illustrated in Fig. 9. As shown in this figure, the swelling of nanocomposite as a function of clay content is in agreement with swelling in distilled water. But, as it clear from figure, the swelling of all nanocomposites is higher than that of clay-free superabsorbent.
Figure 10 shows the effect of the various chloride salt solutions on the water absorbency of the nanocomposites. The water absorbency was decreased in salt solutions. It is apparent that the swelling decrease is strongly dependent on the charge and radius of the cation added to the swelling medium. With increasing the charge of cation, degree of crosslinking increased and swelling is consequently decreased. Therefore, the absorbency for the optimized superabsorbent in the studied salt solution is in the order of monovalent>divalent>trivalent cations. As a result, the absorbency in salt solutions is in the order of K+>Na+>Li+ and Mg2+ >Ca2+> Ba2+ and >Al3+, respectively. In the our previous work, the water absorbency of collagen-g-poly(acrylic acid) superabsorbent in Ca2+ solution was achieved ~8 g/g [26], but it was obtained ~34 g/g for nanocomposite superabsorbents.
Since the nanocomposites are comprised carboxylate groups, they exhibit various swelling capacity in different salt solutions with same concentrations. These swelling changes are due to valency difference of salts. The networks contain PAA chains with carboxylate groups that can interact with cations. As shown in Fig. 10, the swelling capacity of the superabsorbents in Ca2+ solution is lower than that of in Na+ solutions. In the presence of the bivalent calcium ions, the crosslinking density increases because of a double interaction of Ca2+ with carboxylate groups leading to “ionic crosslinking”. The swelling–deswelling cycle of the non-hydrolyzed superabsorbent in sodium and calcium salts are shown in Fig. 11. In sodium solution, swelling of the superabsorbent is increased with time. When this nanocomposite is immersed in calcium chloride solution, it de-swells to a collapsed form. When the shrinked superabsorbent is immersed in sodium chloride solution again, the calcium ions are replaced by sodium ions. This ion exchange disrupts the ionic crosslinks leading to swelling enhancement. As a result, when nanocomposite is treated alternatively with NaCl and CaCl2 solutions with equal molarity, the swelling reversibility of nanocomposite is observed. This chemical behavior of nanocomposite is resulted from the ion exchange ability of the carboxylate groups.
Figure 12 demonstrates the effect of salt solutions on dewatering of swollen superabsorbent. It is observed that the water loosing takes place with high rate in Ca2+ and Cu2+ solution. We know degree of crosslinking is increased with increasing the charge of cation and swelling is consequently decreased.
Effect of pH on equilibrium swelling
Since the swelling capacity of all “anionic” superabsorbents is appreciably decreased by addition of counter ions (cations), stock NaOH (pH = 13.0) and HCl (pH = 2.0) solutions were diluted with distilled water to reach desired basic and acidic pH values, respectively. As shown in Fig. 13, the absorbency of clay-free and nanocomposite superabsorbents is increased as the pH increased from 3 to 4 and decreased in the pH range of 10–13. But, the increase and decrease in nanocomposite is sharper than that of clay-free superabsorbent.
This behavior may be clarified by a buffer action of the carboxylate group with an acid or base, as previously reported by Lee and Wu [36]. In an aqueous solution of a low pH, the carboxylate groups on the polymeric chain can turn into carboxylic groups. The carboxylic groups show neutral electric charge and lead to a reduction of the repulsion between groups on the polymeric chain. Therefore, the electrostatic repulsion of the chains of the polymer decreases, and this leads to a reduction of the water absorbency. At a high pH value, the carboxylic groups on the polymeric chain can turn into carboxylate groups. Consequently, the screening effect of the counter ion on the polyanion chain leads to a reduction in the expansion of the network. Therefore, the water absorbency is reduced. The swelling degree of superabsorbents in various pHs is in agreement to distilled water. In fact, the anionic carboxylate groups are the main factor in response to change of pH.
pH-responsive behavior of nanocomposite
The pH-reversibility of these superabsorbents was investigated in solutions with pH = 2 and pH = 8 (Fig. 14). At pH = 8.0, the superabsorbent swells due to anion-anion repulsive electrostatic forces, while at pH = 2.0, it shrinks within a few minutes due to protonation of carboxylate groups. Since the existence of counter ions in the buffer solutions, the swelling capacity of superabsorbent is appreciably decreased. This sharp swelling-deswelling behavior of the superabsorbents makes them as suitable candidate for controlled drug delivery systems. Such on-off switching behavior as reversible swelling and deswelling has been reported for other ionic superabsorbents [37]. While the pH-reversibility for nanocomposite and clay-free superabsorbent is similar, the rate of shrinking of nanocomposite in pH 2 is higher than that of clay-free superabsorbent.
Water retention under heating
Figure 15 illustrates the water retention capacity of swollen nanocomposite and clay-free superabsorbent at 80 °C. As can be seen from this figure, up to 3 h, the difference in water retention for nanocomposite and clay-free superabsorbent is low. After this time, the rate of water loosing for clay-free superabsorbent is increased. While water retention for sample without clay was reached zero after 7 h, nanocomposite was kept 25% of its absorbed water. This observation depicts that not only inclusion of nano-clay into superabsorbent composition can improve its water retention property under heating, but also thermal stability of nanocomposite will be increased.
Reswelling capacity
In some cases of applications such as agricultural uses, it is important to keep reswelling property after each deswelling. This property was examined six times for both optimized nanocomposite and clay-free superabsorbent. In fact, swollen samples were de-swelled at 100 °C and re-swelled in water. It can be seen from Fig. 16, after repeating the swelling-deswelling- re-swelling the nanocomposite can absorb a lot of water. The results showed that nanocomposite retains a good water absorbing ability. The nanocomposite retained approximately 83% of its initial water absorbency even after repeating the swelling-deswelling-swelling test six times at 100 °C. But, according to data, clay-free superabsorbent can retain only 18% of its initial water absorbency and this shows improvement of the swelling-deswelling-swelling property of superabsorbent by inclusion of nano-clay into superabsorbent composition.
Conclusion
Synthesis of superabsorbent nanocomposite was done by graft copolymerization of mixture of AAm and AA monomers and using nanoclay particles in aqueous media. Main conclusions may be summarized as follows:
-
The optimum reaction conditions to obtain improved water absorbency (950 g/g) were found to be: Purified hydrolyzed collagen 1.0 g, MBA 5.5 mmol/l, weight ratio of AAm/AA 0.25, KPS 4.2 mmol/l, nanoclay 0.3 g, neutralization percent of 70%, and temperature 80 °C.
-
XRD patterns showed that content of clay in nanocomposite composition can affect its type of dispersion in nanocomposite structure. While for 2.9 wt% of clay an exfoliated nanocomposite was achieved, higher than this content result in intercalated dispersion of clay in nanocomposite.
-
SEM micrograph of optimized nanocomposite showed a highly porous structure. This porosity caused high rate of swelling for nanocomposite with τ values of 1.19, 1.38, 2.12 min for the nanocomposites with particle sizes of 100–250, 250–400 and 400–550 μm, respectively.
-
High swelling capacity was achieved for nanocomposite in CaCl2 solution (34 g/g). This high swelling capacity can be attributed to presence of clay in superabsorbent composition.
-
pH-Dependent swelling of nanocomposite was obtained by varying pH of swelling media.
-
Heating of swollen nanocomposite and clay-free superabsorbent, it was observed that while water retention for sample without clay was reached zero after 7 h, nanocomposite was kept 25% of its absorbed water.
-
Reswelling property of nanocomposite and clay-free superabsorbent was examined and the results showed that by introducing of nano-clay, the reswelling capacity was increased from 18% for clay-free superabsorbent to 83% for nanocomposite.
References
Dimitrov M, Lambov N, Shenkov S, Dosseva V, Baranovski V (2003) Acta Pharm 53:25
Eshel H, Dahan L, Dotan A, Dodiuk H, Kenig S (2008) Polym Bull 61:257
Aoki T, Kawashima M, Katono H, Sanui K, Ogata N, Okano T, Sakurai Y (1994) Macromolecules 27:947
Hu Y, Horie K, Tori T, Ushiki H, Tang XC (1993) Polym J 25:123
Hirokawa Y, Tanaka T (1984) J Chem Phys 81:6379
Murali MY, Keshava MPS, Mohana RK (2005) React Funct Polym 63:11
Mohana RK, Padmanabha RM, Murali MY (2003) Polym Int 52:768
Rudzinski WE, Dave AM, Vaishnav UH, Kumbar SG, Kulkarni AR, Aminabhavi TM (2002) Des Monomers Polym 5:39
Bajpai AK, Giri A (2002) React Funct Polym 53:125
Mahdavinia GR, Mousavi SB, Karimi F, Marandi GB, Garabaghi H, Shahabvand S (2009) Express Polym Lett 3:279
Darvari R, Hasirci V (1996) J Microencapsul 13:9
Wang W, Wang A (2009) Carbohydr Polym 77:891
Qi X, Liu M, Chen Z, Liang R (2007) Polym Adv Technol 18:184
Bagheri Marandi G, Hosseinzadeh H (2007) Polym Polym Compos 15:395
Wan T, Wang W, Yuan Y, He W (2006) J Appl Polym Sci 102:2875
Jordan J, Jacob KI, Tannenbaum R, Sharaf MA, Jasiuk I (2005) Mater Sci Eng 393:1
Goettler LA, Lee KY, Thakkar H (2007) Polym Rev 47:291
Giannelis EP, Krishnamoorti R, Manias E (1999) Adv Polym Sci 138:108
Sun L, Boo WJ, Liu J, Tien CW, Sue HJ, Marks MJ, Pham H (2007) Polym Eng Sci 47:1708
Paranhos CM, Soares BG, Machado JC, Windmoller D, Pessan LA (2007) Eur Polym J 43:4882
Zeppa C, Gouanve F, Espuche E (2009) J Appl Polym Sci 112:2044
Lu J, Wang A (2008) J Appl Polym Sci 110:678
Wang W, Zheng Y, Wang A (2008) Polym Adv Technol 19:1852
Lai J, Fang R, Wang L, Tu K, Zhao C, Qian X, Zhan S (2009) J Appl Polym Sci 113:3944
Yu L, Gu L (2009) Polym Int 58:66
Bagheri Marandi G, Hariri S, Mahdavinia GR (2009) Polym Int 58:227
Liu KH, Liu TY, Chen SY, Liu DM (2008) Acta Biomater 4:1038
Omidian H, Hashemi SA, Sammes PG, Meldrum I (1998) Polymer 39:6697
Alaie J, Vashegani-Farahani E, Rahmatpour A, Semsarzadeh MA (2008) Eur Polym J 44:2024
Li A, Zhang J, Wang A (2007) J Appl Polym Sci 103:37
Xia X, Yih J, De Souza NA, Hu Z (2003) Polymer 44:3389
Xu K, Wang J, Xiang S, Chen Q, Zhang W, Wang P (2007) Appl Clay Sci 38:139
Haragachi K, Takehisa T (2002) Adv Mater 14:1120
Kokabi M, Sirousazar M, Mohammad-Hasan Z (2007) Eur Polym J 43:773
Branrup J, Immergut EH (1989) Polymer hand book, 3rd edn. Wiley, New York
Lee WF, Wu RJ (1996) J Appl Polym Sci 62:1099
Athawale VD, Lele V (1998) Starch/Starke 50:426
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bagheri Marandi, G., Mahdavinia, G.R. & Ghafary, S. Collagen-g-poly(Sodium Acrylate-co-Acrylamide)/sodium montmorillonite superabsorbent nanocomposites: synthesis and swelling behavior. J Polym Res 18, 1487–1499 (2011). https://doi.org/10.1007/s10965-010-9554-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-010-9554-6