Abstract
A novel phosphorus-containing intumescent flame retardant, triazine oligomer poly (2-morpholinyl-4- pentaerythritol phosphate-1,3,5-triazine) (PMPT), was used to improve the flame retardancy of PP. Effects of PMPT content on the thermal stability, mechanical properties and flammability of PP were discussed, respectively. The morphology of the char residue was observed by SEM. The thermogravimetric (TGA) curves showed that adding PMPT reduced the initial thermolysis temperature of flame retarded PP (FR-PP) while enhanced the thermal stability at high temperature. The slight decline on the mechanical properties of FR-PP suggested that the compatibility of PMPT and PP was good. PP/30% PMPT composite can achieve UL-94 V-0 rating, which revealed that PMPT was an efficient monocomponent intumescent flame retardant. There were many cavities on the compact char layer of FR-PP and the gases generated from PMPT was a critical factor for the flammability properties of FR-PP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, intumescent flame retardant (IFR) system is mainly used in flame retarded PP (FR-PP) as a kind of halogen-free flame retardant. The basic components of a multicomponent IFR system compose three parts: acid source, carbon source and gas source, such as ammonium polyphosphate (APP), pentaerythritol (PER) and melamine. It is generally agreed that APP usually affects the thermal stability and mechanical property of polymers because it promotes acid hydrolytic reaction of the substrates [1, 2].
Monocomponent IFR is the new way to develop, which contain three parts of IFR in a compound. It can not only reduce the amount of flame retardants and improve the thermal stability of FR-PP [3, 4], but also improve the compatibility between IFR and polymers by copolymerization with the monomer of polymer. Nitrogen element is the main component of IFR and it has been found that triazines and their derivatives are good charring agents because of their abundant nitrogen and structure of tertiary nitrogen [5, 6]. Phosphor has good synergic effect with nitrogen as flame retardant, especially when phosphor atom is introduced into the main chain of the molecular [7, 8]. Liu synthesized the phosphorus-containing triazine oligomer poly (2-piperazinylene-4-morpholino-1, 3, 5 – triazine) (PPMT) and found that PPMT had good flaming effect for PP [9]. But there was no carbon atom in the main molecular chain of PPMT, and in order to gain good flame retardancy PPMT must be used together with PER.
In our former work, a novel phosphorus-containing monocomponent IFR, triazine oligomer Poly (2-morpholinyl-4- pentaerythritol phosphate-1, 3, 5-triazine) (PMPT), was synthesized by copolymerization with carbon-containing compound [10]. In this paper, PMPT was used to prepared FR-PP and thermal stability and flammability properties were investigated by thermo gravimetric analysis (TGA), the limited oxygen index (LOI), and the UL-94 test. Moreover the possible pyrolysis mechanism of PMPT was deduced.
Experimental
Materials
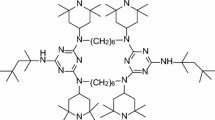
Polypylene (K8303) with a 0.8 g/10 min melt flow rate was obtained commercially from Beijing Yanshan Petrochemical Co. Ltd. Triazine oligomer PMPT was self-prepared in our laboratory. The concrete chemical reaction can be seen in the former document [10]. The chemical structure of PMPT is shown in Scheme 1.
Samples preparation
FR-PP was prepared in a twin screw extruder (SJSH-Z-30, Nanjing Rubber and Plastics Machine Factory) with the mass content of PMPT from 10% to 30%. The processing was carried out at a temperature of 230 °C and a rotor speed of 200 rpm. The obtained pellets were used to machine the samples in the injection molding machine (TTI2160F, China Huada Machine Corporation).
Measurements
Tensile strength test was measured on the material test machine (AG-1, SHIMADZU Co., Ltd, Japan) according to ASTM D638; Notched impact strength was obtained on the pendulum impact tester (B5113.300, Germany Zwick Co., Ltd) according to ASTM D256.
Thermogravimetric Analysis (TGA) was done in a NETZSCH Q209 thermal analyzer at a scanning rate of 20 °C/min in air condition, from 50 °C to 900 °C.
IR spectroscopy was applied with a Vector-33 FTIR spectrometer using KBr pellets. PMPT was heated up to the different temperature (200 °C, 400 °C, 500 °C and 600 °C) and then the residues of PMPT were measured by FTIR Spectrometer.
The limited oxygen index (LOI) values were measured on a FTT oxygen index meter (England Fire Testing Technology Co., Ltd) with sheet dimensions of 130 × 6.5 × 3 mm according to ISO 4589-1984. The vertical flammability test is measured according to UL-94 test using 150 × 13 × 3 mm3 specimens.
The char residual morphology of FR-PP was viewed by scanning electron microscope (SEM) (JSM-638OLA, Japan Electron Optics Laboratory Co, Ltd). The char residual after LOI test were gold-coated before observing.
Results and discussion
Thermal degradation behaviors of PMPT and PP/PMPT
Thermal stability of flame retardant, especially for IFR, is important for the processing of the blends. IFR is required to not only endure the processing temperature, but also pyrolysis to a certain extent before thermal degradation of polymers. Figure 1 shows the TGA thermogram of PMPT. The results show that the thermal degradation of PMPT can fall into two main stages. The first stage starts at about 230 °C and the mass degradation rate reaches the maximum at about 300 °C. Triazine derivative and polyphosphoric acid may be generated in order to accelerate the reaction of esterification and carbonization in this stage. The second stage is the charring formation of skeleton molecular and the incombustible gases, such as N2, CO2 and NO2, are released, from about 550 °C to 800 °C. PMPT had the residual mass about 43% at 600 °C in air condition and the final char residue of 10% at 900 °C. The initial decomposing temperature and char residue are both close to those of other IFR, but the test condition is stricter (in air condition) [4, 9]. Therefore PMPT has a better thermal stability and can meet the processing temperature of engineering plastics.
The FTIR spectrums of the intumescent residual char for PMPT after heat-treatment at different temperature (room temperature, 200 °C, 400 °C, 500 °C and 600 °C) are given in Fig. 2. As increasing the heat-temperature the band at 857 cm-1 attributed to the stretching vibration of P - C and at 1267 cm−1 attributed to P = O gradually decreased and at last dispersed until 400 °C. It showed that PMPT decomposed and generated phosphate and triazine oligomer during this stage. It coincides with the first thermal degradation stage of PMPT. The band at 1560 cm−1 at 500 °C was attributed to the skeleton vibration of C=N and this vibration absorption peak completely dispersed at 600 °C [11]. It was probably happened that triazine structure had been destroyed and the incombustible gases were generated. Therefore it can provide the positive basis for the pyrolysis mechanism of PMPT.
Figure 3 shows TG curves for pure PP and FR-PP with various PMPT content. The initial degradation temperatures (T onset) of FR-PP are all lower than that of PP due to the earlier degradation of PMPT, however, the maximum weight-loss rate (T max) of FR-PP increases in the main thermal degradation (350~450 °C). It suggests that PMPT improves the thermal degradation of FR-PP. The proper gap of initial degradation temperature between polymers and IFR is necessary because phosphoric and polyphosphoric aids have to be produced in the beginning of combustion in order to accelerate the reaction of esterification and carbonization. It also can be seen from Table 1 that the char residue at 600 °C of PP/PMPT composites increases with increasing the content of PMPT. Moreover the char residue weight of PP/30% PMPT sample is about 2.8 times than that of pure PP. The reason might be that the incombustible gases decomposed from PMPT promote the charring formation at higher temperature. The formation of intumescent chars is responsible for the thermal stability and flame retarding property of FR-PP.
Mechanical property of FR-PP
The molecular weight of IFR is always small and the main molecular chains are heterochain structure. Therefore the compatibility of IFR and polymers is poor and IFR usually depresses the mechanical properties of polymers. The mechanical properties of FR-PP are listed in Table 2. It can be seen that adding PMPT makes the mechanical properties of FR-PP slightly decrease compared with those of pure PP. The better mechanical properties were attributed to the higher molecular weight of PMPT and better compatibility with PP. The number average molecular weight of PMPT is about 104 and the powder of PMPT is grinded before blending with PP. In addition the good thermal stability of PMPT is also one of the reasons.
Char residue morphology of FR-PP
The structure and formation of char layer are critical factor for the flame retardancy of polymers. The surface morphology of char residue observed by SEM is shown in Fig. 4. The surface of char residue is more cohesive while the char residue of pure PP is little. Moreover there are many cavities inside of char layer [4]. These cavities are formed when the incombustible gases generated from PMPT during burning process. The amount of the gases generated from PMPT has an important effect on the formation of char layer and flame retardancy of FR-PP. The more gas will destroy the compact char layer while the little gas can’t make the char layer effectively swell. In Fig. 4, the cavities on the surface of char layer are bigger than those of flame retarded ABS prepared by Ma HY and there are more gases generated from PMPT [4]. It may be the reason that the proportion of gas source in PMPT is bigger than that in the conventional IFR. The mass proportion of acid source, carbon source and gas source in the conventional IFR (APP/PER/melamine) is about 3/1/1. But for PMPT in Scheme 1, the proportion of every component is about 1/1.4/1.7. Therefore the char layer was easy to be destroyed by the more gases. This is the reason that the LOI values of PP/PMPT composites are not so high compared with that of other IFR composites.
Flame retardancy and possible mechanism of PMPT
The LOI of FR-polymers is an important parameter evaluating the flame retardancy of materials. A higher LOI value indicates that the polymer has a lower combustibility. Table 3 lists the LOI values of FR-PP with different amount of PMPT. The LOI values of FR-PP gradually improved with the increasing of the amount of PMPT. Compared with that of PP, the LOI value of PP/30 wt% PMPT composite increases about 59.7% and achieves UL-94V-0 rating. It suggested that PMPT had a good flame retarding effect on PP using lonely. The mass proportion of air source in PMPT is bigger than that in the conventional IFR (APP/PER/melamine). The synergistic using of PMPT as an air supply and other carbon source and acid source, such as APP and PER, will be hopeful to further improve the LOI of FR-PP.
Scheme 2 is the possible pyrolysis mechanism of PMPT. When PMPT were heated, it decomposed into two kinds of materials. One was phosphate compound which easily react with polyhydroxy compound and generate crosslinking polyester. Finally polyester was heated and carbonized at high temperature. The other material was triazinyl ring compound which can generate ammonia. The ammonia and water vapor as a blowing agent swelled the carbonaceous residue. The char residue layer could prevent the heat transfer between the flame zone and the substrate, and thus protected the underlying materials from further burning and pyrolysis.
Conclusions
PMPT could effectively reduce the rate of the thermal degradation of FR-PP and promote the formation of char residue, which suggested that PMPT had a good thermal stability. The slight effect of the PMPT contents on the mechanical properties of FR-PP suggested that the compatibility of PMPT and PP was good. The char layer of FR-PP was compact and there were many cavies inside of char layer. It revealed that de-phosphate reaction celebrated the forming of char layer and prevented the flammability of polymers. The LOI values of FR-PP were improved with the increase of the PMPT content. The possible pyrolysis mechanism was reduced and PMPT was confirmed to be an efficient monocomponent IFR.
References
Pal K, Rastogi JN (2004) J Appl Polym Sci 94:407–415
Camino G, Costa L, Trossarelli L (1984) Polym Degrad Stab 7:25–31
Wang DY, Liu Y, Wang YZ, Perdomo AC, Richard HT, Dennis P (2007) Polym Degrad Stab 92:1592–1598
Ma HY, Tong LF, Xu ZB, Fang ZP, Jin YM, Lu FZ (2007) Polym Degrad Stab 92:720–726
Chiu SH, Wang WK (1998) J Appl Polym Sci 67:989–995
Horacek H, Pieh S (2000) Polym Int 49:1106–1114
Wang HF, Zhu P, Zhang JB, Wang B (2005) Text Aux 22:15–17
Hu XP, Li YL, Wang YZ (2004) Macromol Mater Eng 289:208–212
Liu G, Zhao JQ, Zhang YH, Liu SM, Ye H (2007) Polym Polym Compos 15:191–198
Sheng Q, Liu G, Cheng XL, Liu SM, Ye H, Zhao JQ (2007) Petrochemical Technol 38:1244–1249
Ke G (2009) Chin Chem Lett 20:1376–1380
Acknowledgements
This project was supported by the Shenzhen Science and Technology Research Grant (CXB200903090012A) and Nature Science Foundation of Guangdong Province (No. 8451806001001924).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zuo, J., Su, Y., Liu, S. et al. Preparation and properties of FR-PP with phosphorus-containing intumescent flame retardant. J Polym Res 18, 1125–1129 (2011). https://doi.org/10.1007/s10965-010-9515-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-010-9515-0