Abstract
An amphiphilic poly((lactic acid)-b-hyaluronic acid) diblock copolymer, poly(LA-b-HA), was synthesized from short-chain hyaluronic acid and poly(lactic acid). The synthesis was conducted by coupling the N, N′- dicyclohexylcarbodiimide activated poly(lactic acid) to a short-chain hyaluronic acid which was pre-aminated with 1, 2-ethylenediamine at the reducing end followed by NaCNBH3 reduction. The poly (LA-b-HA) copolymers synthesized were verified by the spectral analyses of FTIR and 1H NMR. The poly(LA-b-HA) molecules can self-assemble into micelles in aqueous solution. The average diameters of polymeric micelles were estimated to be 116 ± 17 and 98 ± 11 nm for the polymeric micelles derived from the poly(lactic acid)s of MW 3,200 and MW 16,900, respectively. The poly(LA-b-HA) copolymeric material is non-cytotoxic and can be used as micellar drug carriers. The drug encapsulation capabilities of these poly(LA-b-HA) micelles were demonstrated by using ellagic acid and lidocaine chloride as model compounds. These new biodegradable micelles have a great potential to be used as drug delivery carrier for biomedical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One class of the drug and gene delivery systems that has received wide spread attention over the past few decades is the polymeric micelle delivery system. Polymeric micelles have recently emerged as a novel carrier for both hydrophobic and amphiphilic drugs [1]. AB and ABA type amphiphilic block-copolymers can spontaneously self-aggregate to form micelles in a selective solvent. Amphiphilic synthetic block copolymers have got tremendous impetus on the ongoing research in the area of drug delivery technology due to their capability to provide a delivery vehicle having a broad range of amphiphilic characteristics, as well as targeting the drugs to a specific site [2]. Amphiphilic block copolymer micelles are of special interest for a number of reasons. First of all, hydrophobic drugs can be physically entrapped in the core of micelle and transported at a concentration exceeding their intrinsic water solubility. Secondly, the hydrophilic blocks can form hydrogen bonds with the aqueous surrounding and form a tight shell around the micellar core. Lastly, the block copolymer micelle can also target their payload to specific tissues through either passive or active means [3]. The drug delivery from micelles can be divided into three different routes: (1) the micelles remain outside the cells where the drug is released [4, 5], (2) the micelles enter the cells and (3) the micelles enter the nucleus. The release rate of drug from micelles depends on the physical/chemical properties of both the drug and the block copolymer [6].
Poly(lactic acid) (PLA) has been widely studied for use in medical applications because of its bioabsorbable, biodegradable and biocompatible properties. Recently, biodegradable micelles prepared using the copolymers such as PLA or PLGA (poly(lactic-co-glycolic acid)) copolymerized with PEG (poly(ethylene glycol)) have been employed extensively to deliver bioactive ingredients to the cells. The polymeric micelles thus formed have a PEG outer corona, which results in a prolonged plasma circulation times. Drugs using this type of approach, commonly known as “stealth” therapeutic strategy, can be delivered to the non-RES (reticuloendothelial system) sites with improved efficiency of immunospecific targeting [7–10]. Conjugation of proteins with PEG has been proved to result in prolonged protein circulation life and reduced immunogenicity and antigenicity [11–14]. In addition, PEG conjugated with an anti-tumor drug loaded microspheres were also of great interest since it could improve the water-solubility and stability of such microspheres, thus eliminating undesired side effects.
Hyaluronic acid (HA), a linear polysaccharide composed of repeating units of N-acetyl-glucosamine and D-glucuronic acid, is a major component of ECM. It has been widely used in biomedical applications such as scaffolding for wound healing and tissue engineering, ophthalmic surgery, arthritis treatment and as a component in implant materials [15–17]. Copolymer of HA and PLA is expected to be of great use for medical applications since both HA and PLA can be degraded into the metabolites. Recently, a graft copolymer consisted of HA as a hydrophilic backbone and PLA as hydrophobic polyester branches has been synthesized by Fabio et al. [18]. However, the capability of micelle formation of this branched copolymer has never been demonstrated.
The goal of our research was to synthesize the novel poly(LA-b-HA) block copolymers with unique physicochemical property. PLA is a hydrophobic, biodegradable polymer which will become amphiphilic when covalently bonded with HA. We believed that these amphiphilic diblock copolymers could self assemble into micelles of hydrophilic shell and hydrophobic core and thus useful as potential drug carriers. Two poly(LA-b-HA) copolymers were synthesized and designated as poly(sLA-b-HA) and poly(lLA-b-HA) derived from two different molecular weights of PLA (sPLA, MW 3,200 and lPLA, MW 16,900). The synthesized poly(LA-b-HA) linear diblock copolymers were characterized and used to encapsulate ellagic acid and lidocaine chloride to demonstrate their potential biomedical applications.
Materials and methods
Materials
Stannous octoate, N, N′-dicyclohexylcarbodiimide ,1,2-ethylenediamine, sodium cyanoborohydride, and MTT(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide thiazolyl blue) were all purchased from Sigma-Aldrich (St. Louis, MO) and used without further purification. Hyaluronic acid (HA) of low molecular weight (∼MW 6,500) was obtained from Lifecore Biomedical Inc. Dialysis membranes (Spectra Por_6 regenerated cellulose, MWCO 1,000 and 6,000 ∼ 8,000) were purchased from Spectrum Laboratories, Inc. (Rancho Dominguez, CA). Poly(lactic acid) with different molecular weights were synthesized by the direct condensation of lactic acid using stannous octoate as a catalyst [19, 20] and the molecular weight distributions of the polymers were determined in tetrahydrofuran by size exclusion chromatography using polystyrene as the standard (sPLA; MW ∼ 3,200 and lPLA; MW ∼ 16,900).
Experimental procedures
Poly(LA-b-HA) linear diblock copolymers were synthesized by two steps (Scheme 1), a reductive amination of hyaluronic acid by 1,2-ethylenediamine followed by the coupling of aminated HA with PLA through the amide bond coupling reaction. For the coupling reaction of diamine to hyaluronic acid, there is only one coupling site at the reducing end of HA. The occurrence of the HA dimerization by 1,2-ethyldiamine could be eliminated by raising the molar ratio of diamine to HA in the reaction mixture [21]. In this study, an excess amount of 1,2-ethyldiamine was reacted with hyaluronic acid.
Poly(lactic acid) activated by DCC/NHS
Poly(lactic acid)(PLA) and DCC (N,N′-dicyclohexylcarbodiimide,1.1 equiv.) were loaded into a flask, dissolved by DMSO (dimethyl sulfoxide) and then mixed with a magnetic stirrer. After 4 h mixing, an aliquot of NHS (N-hydroxy-succinimide, 1.1 equiv.) was added to activate the PLA and then the reaction was conducted overnight at ambient temperature.
Reducing-end amination of hyaluronic acid
An aliquot of 1, 2-ethylenediamine (∼10 equiv of the HA) was first dissolved in PBS buffer (pH ∼ 9.5) and the HA (MW ∼ 6,500) solution (10 mg/ml) was then added dropwisely into the pre-mixed solution and stirred for more than 4 h [22–25]. Sodium cyanoborohydride was added into the mixture incubated in an ice-bath and then the reaction proceeded at room temperature for overnight. The excess diamine and water were evaporated by rotary evaporator and then dialyzed (Spectra Por_6 regenerated cellulose, MWCO 1000) in PBS buffer solution for two days. After dialysis, the solution was dried by lyophilization to obtain the modified HA oligomers (hyaluronic acid-amine).
Coupling reaction of PLA with hyaluronic acid-amine
The coupling reaction of the hyaluronic acid-amine reactant (∼1.1 equiv of the activated PLA ) with the NHS-activated PLA was carried out in a mixing solution of DMSO (dimethyl sulfoxide) and PBS (phosphate buffered saline) (1/10; v/v) at room temperature for 24 h to obtain poly(LA-b-HA) linear diblock copolymer. The product compound was precipitated by methanol, dialyzed (Spectra Por_6 regenerated cellulose, MWCO 6,000 ∼ 8,000) for another two days and then lyophilized. The finished products were characterized both by FTIR (Perkin Elmer T1 type FTIR spectrophotometer) and 1H NMR (Bruker Avance 300-MHz NMR spectrometer).
Dynamic light scattering measurements (DLS)
Aqueous dispersions of the polymeric micelles were prepared by a precipitation/solvent evaporation technique without any added surfactants for the investigation of their ability of forming polymeric micelles. A diblock copolymer solution in dichloromethane was added dropwisely to double de-ionized water under ultrasonication. Dichloromethane was removed at lower pressure. The solution was sufficiently diluted so that the multiple scattering due to high concentration of micelle may not occur. All dispersions were filtered using disposable 0.80 mm Millipore filters to avoid any effect on the particle yield or size distribution. The mean size and size distributions of empty and loaded micelles were determined by a DLS Instrument (Brookhaven 90Plus) using 35 mW diode laser with a scattering angle of 90°. The zeta potential of the micelles was measured by 90 Plus Zeta Size Analyzer (Brookhaven Instruments Corporation). All analyses were performed on samples diluted with PBS solution (pH 7.4) in order to maintain a constant ionic strength. For each sample, the mean value SD of three determinations was established.
Transmission electron microscopic (TEM) observation of dispersed micelles
The morphology of poly(LA-b-HA) micelles were examined by using a transmission electron microscopy (TEM) instrument (JEOL). Specimens were prepared by discharging a drop of the micelle solution onto carbon coated EM grids. The solution on the grid was frozen in liquid nitrogen and lyophilized. The micelles on the grid were stained by 2 wt% of phosphotungstic acid and the specimen was then vacuum dried before examination.
Determination of the drug loading content (DLC)
Ellagic acid (EA, an antioxidant) and lidocaine (LD, an anesthetic) were chosen as model drugs to study the drug encapsulation capability of the polymeric micelles because of both drugs had solubility problems. Apart from poor solubility, EA also had poor stability at a physiological pH of 7.4 [26, 27]. To overcome these limitations, encapsulations of these two compounds with poly(LA-b-HA) micelles were studied. The two series of poly(LA-b-HA) block copolymers of 0.2 g were dispersed in 20 ml of double de-ionized water together with 0.01 g of EA and LD to give a drug loading content of 5% (w/w) and a final drug solution with a concentration of 0.05% (w/v) . Excess EA and LD were removed by ultra-filtration (MW 1,000) in a buffered mixture (phosphate buffer/ethanol (7:3), pH 7.4) for 2 days. The UV absorbance of the solution was measured at 230 nm and 210 nm to determine EA and LD concentrations, respectively. The amount of drug loading content (DLC) was calculated using the equation below:
Determination of drug released from the polymeric micelles
Test of the EA released from polymeric micelles was carried out by incubating the solution of EA-loaded polymeric micelles at 37 °C in a shaking water bath reciprocating with a speed of 100 rpm. Approximately 1 mg ellagic acid was encapsulated in the 10 mg of poly (LA-b-HA) micelles and then dispersed into a 10 ml phosphate buffer (pH 7.4). At the designated time interval, the sample solution was centrifuged at 5,000 rpm for 5 min and a 200 μl aliquot was removed from the tube and assayed using Beckman Coulter DU800 UV–VIS spectrophotometer. The amount of EA released into the aqueous solution was determined using the EA calibration curve (230 nm). The percentage of released EA was calculated by dividing the amount of EA released in the solution by that of the initial loaded. These tests were carried out in triplicate. The data represents the means from three independent experiments.
In vitro cytotoxicity test
A MTT assay using a tetrazolium dye was conducted to evaluate the in vitro cytotoxicity of the polymeric micelles. For each well of a 96-well plate, 100 μl of L929 fibroblast cells (105 cells/ml) in Minimum Essential Medium (MEM), was added. After cultured for 24 h in an incubator (37 °C, 5% CO2), 100 μl culture medium (MEM) containing 1 mg/ml of poly (LA-b-HA) block copolymers was charged to each well and further incubated for 24 h and 48 h. After that, each well was rinsed and replaced by fresh MEM and 50 μl of MTT solution (5 mg/ml) was added to the L929 cells. After incubation for 4 h, 100 μl of DMSO was added and the mixture was agitated in a shaker at room temperature. The optical density (OD) was measured at 570 nm with a Microplate Reader Model Powerwave XS (BIOTEK). The cell viable rate was calculated by the following equation: \( {\text{viable}}\,{\text{rate = }}\left( {{\text{OD}}_{\text{treated}} /{\text{OD}}_{\text{control}} } \right) \times \,100\% \), where ODtreated was obtained in the presence of copolymers and ODcontrol was obtained in the absence of copolymer.
Cellular binding of poly(LA-b-HA)polymeric micelles
MDA-MB-435S cells, highly expressed in HA-receptor, were cultured on coverslips and then incubated with poly(LA-b-HA) polymeric micells for 3 h. For subsequent observation, the cells were fixed in 1% paraformaldehyde in PBS followed by washes in PBS containing 5 mM NH4Cl. The cells were examined by confocal microscopy [22]. Confocal microscopic images of the cells were obtained using a computer-interfaced, laser-scanning microscope (Leica TCS4D).
Skin penetration experiments
Transdermal drug delivery systems offer many advantages over conventional dosage forms in improving patient compliance and reducing side effects. In this study, we have evaluated the skin penetration rate of polymeric micelles by using lidocaine as a model drug to find out the potential of polymeric micelles as a skin permeation enhancer.
Animals
Adult female hairless mice (BALB/c, 18–22 g) were used in this study and they were obtained from Charles River Laboratories, Japan. They were housed and fed with a standard diet.
In vivo nude mice fluorescence image observation
Prior to experiments, animals were anaesthetized with ketamine (2 mg/kg) and their backs were cleaned by an electric shaver and ethanol solution (70%, v/v). A fixed concentration of NR (nile red) (0.1% w/w) was used in all experiments. Freshly prepared topical formulations were uniformly applied on the back of the mice, each covering an area of approximately 1 cm2. After an application time of 3 h, the excess of the formulation was washed off, the mice were sacrificed and treated skin samples excised.
In vitro Franz-type diffusion cells preparation
The hairs of the mice were shaved and their skins excised from the dorsal regions with the fatty layers removed before use. The skin specimens were placed on Franz-type diffusion cells, with a nominal diffusion area of 0.75 cm2 and a receptor chamber containing 10 mL of buffer solution (phosphate buffer/ethanol (7:3), pH 7.4). The receptor medium was maintained at 37 ± 1 °C and magnetically stirred. At each designated time interval, an aliquot of 200 μL were collected and the same volume of fresh phosphate buffer solution (pH 7.4) was refilled. Three replicates were determined for each formulation.
Results and discussion
In this study, we have synthesized a new amphiphilic poly(LA-b-HA) copolymer by selective reducing-end amination of HA and then coupled with NHS-activated PLA (Scheme 1). The final products were verified by the spectra of FTIR and 1H NMR. To determine the size effect of hydrophobic chain on the properties of copolymers, two poly(LA-b-HA)s, poly(sLA-b-HA) and poly(lLA-b-HA), were synthesized by coupling HA to PLA of average molecular weights of 3,200 and 16,900, respectively.
IR and NMR spectroscopic analyse of the poly(LA-b-HA) diblock copolymer
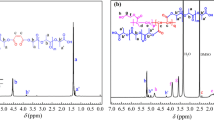
The FTIR and NMR spectra of poly(LA-b-HA) are shown in Fig. 1. For FTIR spectra (Fig. 1a) of poly(LA-b-HA) copolymer, there exist extra absorbance peak at 1,650 cm−1 assigned to C-N of HA, as well as a broad stretching absorbance at 3,200–3,400 cm−1(νas OH + νas NH of HA). These results of FTIR spectra demonstrate that HA has been coupled to PLA. To further characterize micelle formation, we also analyzed the 1H-NMR spectra of the poly(LA-b-HA) block copolymers, both in D2O (Fig. 1b) and DMSO-d6/D2O mixed solvents (30/70) (Fig. 1c). The characteristic features (Fig. 1b and c) are almost the same except a little chemical shift caused by solvent effect: δ = 3.21 ∼ 4.67 (4H, OH of HA), δ 4.20 (br, 2H, NCH 2 –CH2O), δ 3.39 (br, 2H, NCH2CH 2 O), δ 1.85 (s, 3H, NHCOCH 3 of HA), δ 1.32 and δ 1.26 (d, 3H, CHCH 3 of PLA) ppm (Fig. 1c) [28]. Note that the peaks of the poly(lactic acid) repeat units are not obviously visible in D2O as compared with that in the DMSO-d6/D2O mixing solvent. These results are in agreement with the view that the structure of the micelles, when dispersed in an aqueous solution, is indeed of the core-shell type with the HA chains extending out into the aqueous environment [29]. This is also an evidence that the block copolymer of poly(LA-b-HA) has been successfully synthesized.
Characteristics of the poly(LA-b-HA) polymeric micelles
Average size of micelles
Amphiphilic block copolymers have the capability to self-associate when placed in a specific solvent. The size distribution of polymeric micelles is an important parameter in controlling the rate of drug release from the micelles. The size distribution of poly(sLA-b-HA) polymeric micelles was determined and shown in Fig. 2. The mean size of the poly(sLA-b-HA) polymeric micelles was 116 ± 17 nm (Fig. 2(a)) and increased to 265 ± 34 nm (Fig. 2(b)) and 193 ± 26 nm after the entrapment of ellagic acid (EA) and lidocaine chloride (LD). The morphology of the poly(sLA-b-HA) polymeric micelle was examined using a transmittance scanning electron microscopy (TEM JEOL JSM-6335F). As shown, the polymeric micelles prepared from the diblock copolymer of poly(LA-b-HA) are almost spherical with a core—shell structure.
TEM images of the poly(LA-b-HA) polymeric micelles in the aqueous solution (left) and the size distribution determined with a dynamic light scattering (DLS) instrument (right). a poly(sLA-b-HA)/micelles, the size is 116 ± 17 nm; b poly(sLA-b-HA)/micelles loaded with EA (ellagic acid), the size is 265 ± 34 nm. (bar = 2 μm)
Zeta potential analyses
Zeta potentials of poly(LA-b-HA) polymeric micelles were listed in Table 1. All micelles had a negative zeta potential, which may be attributed to the presence of ionized carboxyl groups of HA on the micelle surface.
The drug loading content (DLC) of polymeric micelles
The total ellagic acid (EA) contents in the micelles obtained from a constant initial drug loading (5% w/w) were 2.8 ± 0.3% w/w (280 μg/ml) and 3.6 ± 0.4% w/w (360 μg/ml) for the poly(sLA-b-HA) and poly(lLA-b-HA) polymeric micelles, respectively. The drug loading content and entrapment efficiency depend on the composition of the copolymer, but the effects are rather complicated and could be affected by many factors, such as molecular weights, the ratio of hydrophobic segment to hydrophilic segment and crystallinity. As compared with poly(sLA-b-HA), poly(lLA-b-HA) yielded a higher drug loading level, driven by the more stronger hydrophobic interactions between the drug and hydrophobic block of polymers. Strong polymer—drug interactions might be part of the reasons that the EA-loaded micelles prepared by poly(lLA-b-HA) achieved a higher drug loading level.
In vitro release studies
The in vitro drug release profiles of the EA from the poly(LA-b-HA) copolymer micelles were shown in Fig. 3. As shown, a slightly burst release (∼13.8%) was observed at the beginning followed by a slower release from poly (sLA-b-HA) polymeric micelles. After the initial burst, the release of EA exhibited an initial rapid release followed by a slower and linear release behavior. The release profile was biphasic with a higher release rate in the first 24 h. In this fast releasing period, less than half (about 44.5 ± 1.1% of EA loaded) was released from the poly(sLA-b-HA) micelles. The remaining amount of drug was released during the second phase (24–168 h, about 85.8 ± 0.9% of EA loaded). On the other hand, the extent of the initial burst release of EA from poly(lLA-b-HA) micelles was slightly higher than that from poly(sLA-b-HA) micelles, poly(lLA-b-HA) micelles exhibited a slower release of 33.8% of drug within 24 h and 73.5% in 7 days. It appears that as the chain length of PLA block (the core-forming block) increases, the release became slower. Larger PLA segment could cause greater polymer aggregations and enhanced the interactions between block copolymers and drugs, resulting in a stronger PLA–EA interaction and thus decreased the drug release rate [30, 31]. The drug release profile is governed by many factors such as solubility of drug, degradation of polymer, and polymer–drug interaction, and thus difficult to predict a drug release profile under the current conditions [32]. These experimental results suggest that both poly(LA-b-HA)s are good candidates for drug delivery carriers.
Toxicity of poly (LA-b-HA) polymeric micelles
To examine the influence of poly(LA-b-HA) polymeric micelles on cell viability, several methods are available. These include exclusion of colloidal dyes [33], mitochondrial functionality [34] and cytoplasmic leakage assays of cell-introduced probes [35]. In this study, MTT assay was used for evaluation of poly (LA-b-HA) polymeric micelles toxicity. The cellular viability, as determined with an MTT assay, was normalized to the viability of cells cultured without polymeric micelles. Figure 4 displays the cell viability of L929 seeded with poly(LA-b-HA)/micelles as well as on TCPS. No significant cytotoxicity to L929 cells was observed for a particle concentration at 1 mg/ml for both polymeric micelles with seeding time intervals of 24 and 48 h.
Cell viability measured using MTT assay. The L929 cells were cultured with polymeric micelles of two different PLA chain lengths for 24 h and 48 h. Each cultured medium contain 1% poly(sLA-b-HA) polymeric micelles or poly(lLA-b-HA) polymeric micelles. Culture medium without any added polymeric micelle is designated as TCPS and used as a control
Binding and localization of poly(LA-b-HA) polymeric micelles to the cell membrane is hyaluronan-dependent
Experiments of amphiphilic diblock copolymers with a specific ligand at their hydrophilic chain ends have shown capable of recognizing and interacting strongly with the target cells. Saccharides are of great interest for drug targeting by playing an important role in cell–protein and cell–cell interactions. For example, Yonese prepared a sugar-substituted poly(g-methylglutamate)-b-poly(ethylene oxide) (PMG–PEO) block copolymer with a lactose residue for the targeted drug delivery [36]. Here, we demonstrated that our newly synthesized hyaluronic acid based polymeric micelles are capable of binding to MDA-MB-435S (ATCC No HTB-129; breast cancer cells) cells over-expressed with HA receptors. MDA-MB-435S cells were incubated with fluorescence encapsulated poly(sLA-b-HA) polymeric micelles (MW 9700) at 37 °C. The binding of poly(sLA-b-HA) micelles to the incubated cells could be detected under a confocal laser scanning microscope (Fig. 5a). If the cells pre-treated with 3 mg/ml hyaluronic acid for 1 h, the total number of polymeric micelles bonded decreased dramatically (Fig. 5b). These results demonstrate that binding of poly(sLA-b-HA) polymeric micelles to MDA-MB-435S cells was attributed to the hyaluronic acid residues and that the recognition was carbohydrate specific. This binding may take place via a hyaluronic acid receptor CD44 on the cell membrane of MDA-MB-435S cells.
Confocal images of 435S cells incubated with 1 mg/ml of fluorescent poly(sLA-b-HA)-polymeric micelles (MW ∼ 9,700) for 3 h: a cells cultured with poly(sLA-b-HA) micelles loaded with NR(Nile red), b cells pretreated with 3 mg/ml hyaluronic acid for 1 h before adding the NR-loaded poly(sLA-b-HA) micelles. Slides were sectioned optically at 3 μm intervals through the cell monolayer to obtain the appropriate focal depth. An argon/krypton mixed-gas laser with excitation lines at 560 nm was used to induce fluorescence. Excitation of the red fluorescence (Nile red signal) was achieved by the 560-nm excitation line. Images were digitized with Leica scanware
Skin penetration experiments: qualitatively and quantitatively
Qualitatively
Fluorescence photography in sections was obtained from skin treated with NR (nile-red) containing formulations (Fig. 6). After application of NR dispersion solution (Fig. 6a), there was generally confined to the stratum corneum (SC) only. On the contrary, poly(sLA-b-HA)/NR loaded micelles penetrated into the viable epidermis (Fig. 6b). In these two cases, accumulation of NR was also seen in hair follicles and in sebaceous glands.
Skin penetration testing design (left) and penetration of NR (Nile red)-loaded micelles into nude mice skin after 6 h. a NR only. b Poly(sLA-b-HA)/NR. Fluorescence pictures were taken (10_ magnification) using an inverted fluorescence microscope (Axiovert200, ZEISS) equipped with a monochrome camera (AxioCam HR, ZEISS)
Quantitatively
The quantitative analysis of the free and micelle-entrapped lidocaine penetrated through the skin was conducted by employing a Franz type diffusion cell. The amount of the lidocaine in the receptor chamber was measured by the light absorbance at 210 nm. A calibration curve (peak area versus drug concentration) was constructed by running standard lidocaine solutions in a mixed solution (phosphate buffer/ethanol (7:3), pH 7.4) for each series of chromatographed samples. As shown in Fig. 7, the loaded micelle is a better promoter of percutaneous lidocaine chloride penetration than the unloaded one. By using poly(sLA-b-HA), the total amount of lidocaine penetrated through the skin increased 1.2 times after administration of 6 h.
Conclusion
We have synthesized poly(LA-b-HA) linear diblock copolymers by coupling short chain HA to PLA. These hyaluronic acid-conjugated polymeric micelles have the ability to encapsulate drug with higher capacity when PLA of higher molecular weight was used. The polymeric micelles can bind to the membrane and transported inside the cells expressed with HA-receptors, furthermore the block copolymer with a HA moiety may also exhibit an enhancing effect on the permeation the drug through the skin. Consequently, this HA-conjugated polymeric micelle material has a great potential for biomedical application.
References
Jones M-C, Leroux J-C (1999) Polymeric micelles—a new generation of colloidal drug carriers. Eur J Pharm Biopharm 48:101–111
Kumar N, Ravikumar MNV, Domb AJ (2001) Biodegradable block copolymers. Adv Drug Deliv Rev 53:23–44
Nishiyama N, Okazaki S, Cabral H, Miyamoto M, Kato Y, Sugiyama Y, Nishio K, Matsumura Y, Kataoka K (2003) Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res 63:8977–8983
Allen C, Han J, Yu Y, Maysinger D, Eisenberg A (2000) Polycaprolactone-b-poly(ethylene oxide) copolymer micelles as a delivery vehicle for dihydrotestosteron. J Contr Release 63:275–286
Lin W-J, Juang L-W, Lin C-C (2003) Stability and release performance of a series of pegylated copolymeric micelles. Pharmaceut Res 20(4):668–673
Kataoka K, Harada A, Nagasaki Y (2001) Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 47:113–131
Shin LL, Kim SY, Lee YM, Cho CS, Sung YK (1998) Methoxy poly(ethylene glycol) /e-caprolactone amphiphilic block copolymeric micelle containing indomethacin. I. Preparation and characterization. J Contr Release 51:1–11
Yokoyama M, Okano T, Sakurai Y, Suwa S (1996) Introduction of cisplatin into polymeric micelles. J Contr Release 39:351–356
Yokoyama M, Okano T, Sakurai Y, Fukushima S, Okamoto K, Kataoka K (1999) Selectivity delivery of adriamycin to loading level. A solid tumor using a polymeric micelle carrier system. J Drug Target 7:171–186
Yeh MK, Jenkins PG, Davis SS, Coombes AGA (1995) Improving the delivery capacity of microparticle systems using blends of poly(DL-lactide-co-glycolide) and poly-(ethylene glycol). J Contr Release 37:1–9
Abuchowski A, Van Es T, Palczuk NC, Davis FF (1977) Alteration of immunological properties of bovine serum albuminby covalent attachment of polyethylene glycol. J Biol Chem 252:3578–3581
Abuchowski A, McCoy JR, Palczuk NC, Van Es T, Davis FF (1977) Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver lacticcatalase. J Biol Chem 252:3582–3586
Davis S, Abuchowski A, Park YK, Davis FF (1981) Alteration of the circulating life and antigenic properties of bovine adenosine deaminase in mice by attachment of polyethylene glycol. Clin Exp Immunol 46:649–652
Harada A, Kataoka K (1997) JMS Pure Appl Chem A34:2119–2133
Miyauchi S, Horie K, Morita M, Nagahara M, Shimizu K (1996) Protective efficacy of sodium hyaluronate on the corneal endothelium against the damage induced by sonication. J Ocul Pharmacol Ther 12:27–34
Miyauchi S, Iwata S (1986) Evaluations on the usefulness of viscous agents in anterior segment surgery. I. The ability to maintain the deepness of the anterior chamber. J Ocul Pharmacol 2:267–274
Liesegang TJ (1990) Viscoelastic substances in ophthalmology. Surv Ophthalmol 34:268–293
Palumbo FS, Pitarresi G (2006) New graft copolymers of hyaluronic acid and polylactic acid: synthesis and characterization. Carbohydr Polymer 66:379–385
Miyoshi R, Hashimoto N, Koyanagi K, Sumihiro Y, Sakai T (1996) Int Polym Process XI:320
Chen G-X, Kim H-S, Kim E-S, Yoon J-S (2006) Synthesis of high-molecular-weight poly(L-lactic acid) through the direct condensation polymerization of L-lactic acid in bulk state. Eur Polymer J 42:468–472
Raja RH, Leboeuf RD, Stone GW, Weigel PH (1984) Preparation of alkylamine and 125I-Radiolabeled derivatives of hyaluronic acid uniquely modified at the reducing end. Anal Biochem 139:168–l77
Roy R, Katzenellenbogen E, Jennings HJ (1984) Improved procedures for the conjugation of oligosaccharides to protein by reductive amination. Can J Biochem Cell Biol 62(5):270–251
Yoshida T, Lee YC (1994) Glycamine formation via reductive amination of oligosaccharides with benzylamine: efficient coupling of oligosaccharides to protein; corresponding author. Carbohydr Res 251(3):175–186
Stoll MS, Hounsell EF (1988) Selective purification of reduced oligosaccharides using a phenylboronic acid bond elut column: potential application in HPLC, mass spectrometry, reductive amination procedures and antigenic/serum analysis. Biomed Chrom 2(6):249–253
Evangelista RA, Fu-Tai AC, Guttman A (1996) Reductive amination of N-linked oligosaccharides using organic acid catalysts. J Chromatogr A 745(1):273–280
Bala V, Bhardwaj S, Hariharan SV, Kharade N, Kumar MNVR (2006) Sustained release nanoparticulate formulation containing antioxidant ellagic acid as potential prophylaxis system for oral administration. J Drug Target 14:27–34
Bala V, Bhardwaj S, Hariharan SV, Kumar MNVR (2006) Analytical methods for assay of ellagic acid and its solubility studies. J Pharm Biomed Anal 40:206–210
Klink D, Qian-Chun Yu, Glick MC, Scanlin T (2003) Lactosylated Poly-L-Lysine targets a potential lactose receptor in cystic fibrosis and non-cystic fibrosis airway epithelial cells. Mol Ther 7(1):73–80
Harkach JS, Peracchia MT, Domb A, Lotan N, Langer R (1997) Nanotechnology for biomaterials engineering: structural characterization of amphiphilic polymeric nanoparticles by 1H NMR spectroscopy. Biomaterials 18:27–30
Kim SY, Shin IG, Lee YM, Cho CS, Sung YK (1998) Methoxy poly(ethylene glycol) and ɛ -caprolactone amphiphilic block copolymeric micelle containing indomethacin II. Micelle formation and drug release behaviours. J Contr Release 51:13–22
Lin W-J, Juang L-W, Lin C-C (2003) Stability and release performance of a series of pegylated copolymeric micelles. Pharm Res 20:668–673
Lee J, Cho EC, Cho K (2004) Incorporation and release behavior of hydrophobic drug in functionalized poly(D, L-lactide)-block–poly(ethylene oxide) micelles. J Contr Release 94:323–335
Kamentsky LA, Melamed MR (1965) Spectrophotometer: new instrument ultrarapid cell analysis. Science 150:630–631
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Walum E, Peterson A (1982) Tritiated 2-deoxy-D-glucose as a probe for cell membrane permeability studies. Anal Biochem 120:8–11
Toyotama A, Kugimiya S, Yamanaka J, Yonese M (2001) Preparation of a novel aggregate like sugar-ball micelle composed of poly(methylglutamate) and poly(ethyleneglycol) modified by lactose and its molecular recognition by lectin. Chem Pharm Bull 49:169–172
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, C.C., Chang, K.Y. & Wang, Y.J. A novel biodegradable amphiphilic diblock copolymers based on poly(lactic acid) and hyaluronic acid as biomaterials for drug delivery. J Polym Res 17, 459–469 (2010). https://doi.org/10.1007/s10965-009-9332-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-009-9332-5