Abstract
The polycondensation of 2-hydroxy-4-ethoxybenzophenone with 1, 3 butane diol was carried out in presence of polyphosphoric acid as a catalyst at 155 °C for 10 h to synthesize desired resin. The resin was used to synthesize polymer-metal complexes with 4f-block elements. The resin and its polymer-metal complexes were characterized on the basis of elemental analyses, electronic spectra, magnetic susceptibilities, FTIR, NMR and Thermogravimetric analyses. Morphology of resin and its polymer-metal complexes was studied by SEM. Catalytic activity of selected polymer-metal complexes in organic synthesis was examined. Antimicrobial activity of polymer-metal complexes against Escherichia coli, Bacillus subtilis, Staphylococcus aureus (bacteria) and Saccharomyces cerevisiae (yeast) were measured. It is observed that polymer-metal complexes are efficient and effective catalysts and antimicrobial agents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Novel and fascinating types of entanglements of individual moieties are exhibited in coordination network polymers [1]. The incorporation of metals into the polymer chain offers potential for the preparation of processable materials with variety of applications, which differ significantly from those of conventional organic polymers. The diverse range of coordination and geometries of elements offers the possibility of accessing polymers with unusual conformational, mechanical and morphological character [2, 3]. Such polymers are of considerable interest in materials science because the metal atoms can also impart unique redox, electronic, optical and magnetic properties [4].
In the coordination polymers, metal atoms attached to polymer backbone are bound to exhibit characteristic antimicrobial [5] catalytic behavior [6], which are distinctly different from their low molecular weight analogue. Indeed, many synthetic polymer-metal complexes show chemical adsorption, UV- absorption [7, 8], high catalytic efficiency, in addition to semi conductivity, heat resistance and biomedical potentials [9, 10].
Selection or design of a ligand containing features such as flexibility, versatile binding modes and the ability to form hydrogen bonds is crucial to the construction of polymeric complexes [11]. Phenolic resin with pendant hydroxyl groups and carbonyl oxygen as donor sites yield metal coordinated polymers with interesting properties. The lanthanide ions are well known for variable coordination number, in the range of 3 to 12 [12] which will make lanthanide ions become excellent spacers in assembling fascinating framework.
An antimicrobial activity of Lanthanide chelates has great significance, due to greater biological and clinical aspects [13]. The insertion of metal ion in the polymeric system, changes the physico chemical as well as biological properties of the polymers. Coordination polymers are also used as biocidal coatings and a widely applied to prevent the growth of microorganisms on surfaces. e. g. Antifouling paints [14].
An attempt has been made to synthesize polymer-metal complexes of phenolic resin and their thermal, catalytic and antimicrobial behaviors have been studied. Resin possesses good thermal stability, while polymer-metal complexes are thermally less stable. Polymer-metal complexes shows excellent catalytic activity and very good bactericidal activity compared to the parent polymeric ligand.
Experimental
Chemicals required
2, 4 – dihydroxy benzophenone (DHBP, Aldrich), K2CO3 (Anhydrous) , n-bromo ethane (Aldrich), 1,3 Butane diol (1,3 BD, Aldrich), polyphosphoric acid (PPA, Lancaster), Methanol, Ethanol, Acetone, Dimethyl Sulfoxide (DMSO), Benzaldehyde, 4-Hydroxybenzaldehyde,Chlorobenzaldehyde, Vanillin, Urea, Acetoacetic ester, Acetyl acetone (AR – Grade), Hydrated metal acetates of Lanthanum, Praseodymium, Neodymium, Samarium, Gadolinium, Terbium and Dysprosium (Merck), Nutrient-Broth (Hi – media, M 002) and MGYP media (Hi – media).
Synthesis of monomer and resin
2, 4 – dihydroxy benzophenone (14.98 gm, 0.07 mole) was dissolved in 60 ml of acetone. Then anhydrous K2CO3 (19.32 gm, 0.14 mole) was added to it and stirred well. To this mixture, n-bromo ethane (5.18 ml, 0.07 mole) was added slowly with stirring. The reaction mixture was then refluxed at boiling temperature for 24 h. After 24 h, anhydrous K2CO3 (9.66 gm, 0.07 mole) was added and further refluxed for 24 h. The reaction mixture was then allowed to cool, poured on crushed ice. The pale yellow solid was separated out. It was collected by filtration and washed with cold water and recrystallized from acetone. The product is pale yellow in color, m.p. 58 °C, yield 13.23 gm (88%).
To a well stirred and ice-cooled mixture of 2-hydroxy-4-ethoxy benzophenone (14.52 gm, 0.06 moles) and 1,3 butane diol (5.38 ml, 0.06 moles), polyphosphoric acid (PPA) (20 gm) was added slowly with stirring as a catalyst. The reaction mixture was left at room temperature for half an hour and condensed on an oil bath at 155 °C for 10 hours. The reaction mixture was then cooled, poured on crushed ice and left overnight. A blackish-brown solid was separated out. It was collected by filtration and washed with cold water and methanol, to remove unreacted acid and monomer. The synthesized resin was further purified by reprecipitation from dimethyl formamide with water for three times and dried at 60 °C temperature. The synthesized resin is soluble in dimethyl formamide (DMF), dimethyl sulfoxide (DMSO), tetrahydrofuran (THF). The polymer was black in color, D.P. > 280 °C, yield 8.2gm (56.47 %). The reaction is shown in Scheme 1.
Synthesis of polymer-metal complexes
All polymer-metal complexes were synthesized by earlier reported general method [15]. Hydrated acetates of Lanthanum, praseodymium, neodymium, samarium, gadolinium, terbium and dysprosium were used for the preparation of the polymer-metal complexes. All the polymer-metal complexes are soluble in dimethyl formamide (DMF) and dimethyl sulfoxide (DMSO). The yield was in the range of 60–80 %.
Synthesis of substituted 3,4-dihydropyrimidin-2(1H)-ones
All substituted 3,4-dihydropyrimidin-2(1H)-ones were synthesized by following general method. Benzaldehyde, 4-Hydroxybenzaldehyde, 4-Chlorobenzaldehyde, Vanillin, Urea, Acetoacetic ester and Acetyl acetone were used to synthesize 3, 4-dihydropyrimidin-2(1H)-ones.
In a typical experimental reaction a solution of β-ketoester (0.1 moles), aldehyde (0.1 moles) and urea (0.1 moles) in ethanol (40 ml) was refluxed in presence of polymer-metal Complex as a catalyst, to give 3,4-dihydropyrimidin-2(1H)-ones as shown in Scheme 2. The reaction mixture was then allowed to cool; crude product obtained was separated by filtration. The crude product was dissolved in ethanol and filtered to remove insoluble catalyst from it and recrystallized. The separated catalyst can be used again by giving simple water treatment and after drying.
Antimicrobial testing
The antimicrobial activity of resin and its polymer-metal complexes was checked against Escherichia coli, Bacillus subtilis, Staphylococcus aureus and Yeast strains Saccharomyces cerevisiae. The antimicrobial effect of compound was investigated by standard microbiological parameters using Agar Diffusion Method [16]. The concentration of the compound tested for the antimicrobial activity was 500 ppm during the experiment. The bacterial culture was maintained on N-agar (N-broth, 2.5 % w/v agar). The yeast culture was maintained on MGYP in 3 % (w/v) agar.
For inoculum developments of bacterial and yeast culture, a loop of cell mass from pregrown slants was inoculated into sterile N-broth tubes containing 15 mL medium and incubated on a shaker at 150 rpm and 37 °C for 24 h, to obtain sufficient cell density (i.e. 1 X 108 cells / mL).
Sterile, melted N-agar was initially inoculated with corrosponding cultures and poured into a sterile empty Petri plate and allowed to solidify. A ditch was prepared with the help of a sterile scalpel on opposite ends, with one for control (solvent without compound) and the other for the test sample. For finding the minimum inhibitory concentration, all the cultures were tested for different concentration of compound ranging from 50 – 1000 ppm. Then after, the plates were transferred to the refrigerator for 10 min to allow the sample diffuse out from the ditch and into the agar before organisms start growing followed by incubation at 37 °C for 24 h. Next day the distance in millimeter (mm), from the ditch was measured as a parameter of inhibition.
For the growth and test of bacteria and yeast, the N-broth and MGYP media were used. The composition used is as shown below.
-
N-broth Peptone 0.6 % (6.0 gm), NaCI 0.15 % (1.5 gm), Beef extract 0.15 % (1.5 gm) were dissolved in l L distilled water and pH was adjusted to 6.7–7.3.
-
MGYP Malt extract (3.0 gm); glucose (10.0 gm); yeast extract (3.0 gm) and peptone (5.0 gm) were dissolved in l L distilled water and pH was adjusted to 5.5.
Analytical procedures
Elemental analysis of carbon, hydrogen and nitrogen was carried out on a Coleman C, H, N analyzer (Table 1). The metal content was determined by complexometric titrations with standard Na2EDTA [17]. After decomposing the polymer-metal complexes with a mixture of concentrated hydrochloric, sulfuric and perchloric acids in a 5:2:3 ml ratio, respectively. Magnetic susceptibilities were measured using Gouy method at room temperature. The UV-VIS spectra of the polychelates have been recorded using UV-160AUV – Vis spectrophotometer meter, Shimadzu (Japan). FTIR spectra were recorded over the 4000–400 cm−1 range on a Perkin Elmer infrared spectrophotometer model 938 by using KBr pellets. 1H-NMR spectra were recorded on BRUCKER 400 MHz NMR spectrometer. Thermal measurements were carried out on a Du Pont thermal analyzer in nitrogen atmosphere at a heating rate of 10 °C min−1. Scanning Electron Microscope (SEM) studied was carried out by ESEM TMP + EDAX, PHILIPS.
Results and discussion
Chemistry
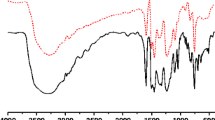
HEBP-1,3 BD resin was synthesized by polycondensation of 2-hydroxy-4-ethoxy benzophenone and 1,3 butane diol in acidic medium as shown in Scheme 1. The structures of the ligand and its polymer-metal complexes were determined by FTIR (Fig. 1), 1H-NMR and elemental analysis. The geometry of the central metal ion was conformed by electronic spectra (UV-visible) and magnetic susceptibility measurements. Micro-analytical data of the polymeric ligand and its polymer-metal complexes are presented in Table 1. The slight deviation in the elemental analysis results may be due to polymeric nature of the compounds, as the value of the end groups were not taken into account for the theoretical calculations. The analysis of the resin indicates that the molar ratio of 2-hydroxy-4-ethoxy benzophenone and 1,3 butane diol is 1:1. The micro-analytical data showed that the HEBP-1,3 BD and metal acetates were in a 1:2 ratio in all the polymer-metal complexes .The nature of the ligand, high thermal stability, metal –ligand ratio (1:2) and insolubility of polymer-metal complexes in common organic (chloroform, acetone, ethanol, benzene, toluene etc.) suggests their polymeric nature [18]. It was also observed from the analytical data that all the polymer-metal complexes were coordinated with two water molecules which is supported by the Thermo gravimetric analysis.
FTIR and 1H NMR spectra
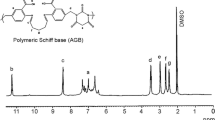
The important FTIR bands and proton signals of monomer, resin and its polymer-metal complexes with their assignments are discussed as under. The –C = O stretching frequency in the resin is observed around 1645–1650 cm−1, appearing at a lower frequency of 15 to 30 cm−l in all the polymer-metal complexes , which suggests −C = O→M coordination. In polymer-metal complexes the bands observed around 460–475 and 565 cm−1 indicates the M-O bond, suggesting that phenolic and carbonyl groups are involved in bond formation with the metal ion. The proposed structure of the polymeric ligand is shown in Scheme 3.
HEBP:
IR(KBr): 3200–3400, 2730, 1620, 1560, 1525, 1490, 1345, 1266, 890, 690 cm−1; 1H NMR (DMSO-d6): δ 12.73 (s, phenolic -OH), 4.11 (q, 2H, -CH2 of –OC2H5), 1.44 (t, 3H, -CH3 of –OC2H5), 6.53–7.66 (8H, Ar-H)
HEBP-1,3 BD:
IR(KBr): 3200–3400, 2950–2880, 2735, 1650, 1560, 1525, 1495, 1345, 1260,1020, 995, 890,690 cm−1; 1H NMR (DMSO-d6): δ 12.04 (s, phenolic -OH), 4.11 (q, 2H, -CH2 of –OC2H5), 1.36 (t, 3H, -CH3 of –OC2H5), 1.23 (t, 3H, bridge), 2.98 (t, 2H, bridge), 2.37 (q, 2H, bridge), 3.07 (t, 1H, bridge), 7.31-7.69 (6H, Ar-H).
M[(HEBP-1,3 BD)2.2H2O]n:
IR(KBr): 3100–3400, 2950–2880, 1610–1635, 1520–1480, 1440–1600, 1350–1340, 1265 ± 10, 960–1000,700–720, ,670–650, 565, 459–480 cm−1; 1H NMR (DMSO-d6): 4.09– 4.15 (q, 2H, -CH2 of –OC2H5 ), 1.36– 1.41 (t, 3H, -CH3 of –OC2H5),1.21– 1.27 (t, 3H, bridge), 2.94– 2.99 (t, 2H, bridge),2.34– 2.39 (q, 2H, bridge), 3.03– 3.10 (t, 1H, bridge), 7.27– 7.71 (6H, Ar-H).
5-(Ethoxycarbonyl) 4-phenyl-6-methyl-3-4-dihydropyrimidin-2 (1H)-one: IR(KBR):
3234, 3106, 2928, 1730, 1646, 1598, 1464, 1417, 1339, 1315, 1288 cm−1; 1H NMR (CDCl3 + DMSO-d6): δ = 1.17 (t, 3H, -CH3 of –OC2H5), 2.35 (s, 3H, -CH3), 4.07 (q, 2H, -CH2 of –OC2H5), 5.28 (d, 1H, -CH of hetero ring), 5.67 (s, 1H, -NH), 7.22–7.47 (m, 5H, Ar-H), 9.20 (s, 1H, -NH).
5-(Ethoxycarbonyl)-4-(4-Chlorophenyl)-6-methyl-3-4-dihydropyrimidin-2 (1H)-one:
IR (KBr): 3462, 3249, 3126, 2979, 2923, 1721, 1710, 1652, 1577, 1491, 1465, 1388 cm−1; 1H NMR (CDCl3 + DMSO-d6): δ =1.17 (t, 3H, -CH3 of –OC2H5), 2.32 (s, 3H, -CH3), 4.05 (q, 2H, -CH2 of –OC2H5) 5.46 (d, 1H, -CH of hetero ring ), 6.45 (s, 1H, -NH), 7.19–7.42 (m, 4H, Ar-H), 8.58 (s, 1H, -NH).
5-(Ethoxycarbonyl)-4-(4-Hydroxyphenyl)-6-methyl-3-4- dihydropyrimidin-2 (1H)-one:
IR (KBr): 3444, 3291, 3126, 2919, 1702, 1651, 1619, 1491, 1425, 1388, 1364, 1314, 1262, 1237, 1142, 1092, 1015, 967, 837 cm−1; 1H NMR (CDCl3 + DMSO-d6): δ = 1.09 (t, 3H, -CH3 of –OC2H5), 2.32 (s, 3H, -CH3), 4.05 (q, 2H, -CH2 of –OC2H5), 5.46 (d, 1H, -CH of hetero ring), 8.29 (s,1H phenolic -OH), 6.29 (s, 1H, -NH), 6.81–7.29 (m, 4H, Ar-H), 8.19 (s, 1H, -NH).
5-(Ethoxycarbonyl)-4-(4-Hydroxy-3-Methoxphenyl)-6-methyl-3-4-dihydropyrimidin-2 (1H)-one:
IR (KBr): 3444, 3290, 3126, 2922, 1704, 1641, 1621, 1491, 1425 cm−1; 1H NMR (CDCl3): δ = 1.1 (t, 3H, -CH3 of –OC2H5), 3.98 (q, 2H, -CH2 of –OC2H5), 2.27 (s, 3H, -CH3), 3.77 (s, 3H, -OCH3), 5.20 (d, 1H, -CH of hetero ring), 6.86 – 7.11 (m, 3H, Ar-H), 7.59 (s, 1H, -NH), 9.46 (s, 1H, -NH), 8.38 (s, 1H phenolic -OH).
5-Acetyl-4-phenyl-6-methyl-3-4-dihydropyrimidin-2(1H)-one:
IR(KBR): 3234, 3106, 2928, 1730, 1646, 1598, 1464, 1417, 1339, 1315, 1288 cm−1; 1H NMR (CDCl3 + DMSO-d6): δ = 2.17 (t, 3H, -COCH3), 2.35 (s, 3H, -CH3), 5.28 (d, 1H, -CH of hetero ring), 5.67 (s, 1H, -NH), 7.22-7.47 (m, 5H, Ar-H), 9.20 (s, 1H, -NH).
5-Acetyl-4-(4-Chlorophenyl)-6-methyl-3-4-dihydropyrimidin-2 (1H)-one:
IR (KBr): 3442, 3289, 3126, 2919, 1702, 1641.5, 1619, 1491, 1425, 1388, 1364, 1314, 1262, 1237, 1142, 1092, 1015, 967, 837 cm−1; 1H NMR (CDCl3): δ = 2.26 (s, 3H, -COCH3), 2.32 (s, 3H, -CH3), 5.05 (d, 1H, -CH of hetero ring), 7.38 (s, 1H, -NH), 7.26–7.6 (m, 4H, Ar-H), 9.28 (s, 1H, -NH).
5-Acetyl-4-(4-Hydroxyphenyl)-6-methyl-3-4-dihydropyrimidin-2 (1H)-one:
IR (KBr): 3373, 3256, 3116, 2965, 2920, 1702, 1681, 1669, 1609, 1449, 1425, 1388, 1364, 1314, 1262 cm−1; 1H NMR (CDCl3 + DMSO-d6): δ = 2.19 (s, 3H, -COCH3), 2.32 (s, 3H, -CH3), 5.03 (d, 1H, -CH of hetero ring), 7.39 (s, 1H, -NH), 7.06–7.38 (m, 4H, Ar-H), 9.26 (s, 1H, -NH), 8.62 (s, 1H, -OH).
5-Acetyl-4-(4-Hydroxy-3-Methoxphenyl)-6-methyl-3-4-dihydropyrimidin-2(1H)-one:
IR (KBr): 3444, 3290, 3126, 2922, 1704, 1641, 1621, 1491, 1425 cm−1; 1H NMR (CDCl3): δ = 2.08 (s, 3H, -COCH3), 2.35 (s, 3H, -CH3), 3.83 (s, 3H, -OCH3), 5.23 (d, 1H, -CH of hetero ring), 6.69 – 6.85 (m, 3H, Ar-H), 8.69 (s, 1H, -NH), 7.26 (s, 1H, -NH), 8.94 (s, 1H, -OH).
Vapor pressure osmometry
The Number Average Molecular Weight (\( \overline{\text{M}} {\text{n}} \)) of the polymeric ligand (HEBP-1,3 BD) samples were estimated by Vapour Pressure Osmometry [19]. Dilute solutions of polymer samples were prepared to determine \( \overline{\text{M}} {\text{n}} \). Four concentrations 2.21, 4.42, 6.63 and 8.84 g.kg−1 were prepared in DMF. The VPO experiment was carried out for each concentration and the corresponding bridge output reading in millivolts was noted 21.00, 40.00, 61.00 and 82.00 respectively. The plot of millivolts Vs concentration was drawn. With the help of the slope (8.93) and the VPO constant K (1.15 × 104), the \( \overline{\text{M}} {\text{n}} \)= 1287 g.mole−1 value of the polymer was calculated.
Thermogravimetric analyses
The % weight loss at various temperatures and the characteristic % weight left at 700 °C for HEBP-1,3 BD resin and its metal polymer-metal complexes were studied. In the present study no sharp weight loss was observed in the TG curve of the polymer-metal complexes, indicating their polymeric nature. After 1–2 % weight loss of lattice / absorbed water / solvent molecules, the polymer-metal complexes gradually degraded. In case of two step degradation, the first step was rapid than the second step. This may be due to the fact that the non coordinate part of the ligand decomposes first, while the coordinated part decomposes later [20]. In case of all the polymer-metal complexes, the curve showed a 9–11 % weight loss corresponding to two coordinated water molecules in the temperature range of 150 – 200 °C. According to Nikolaev et al. [21] water eliminated above 150 °C may be due to coordination with the metal ion. The nature of the water molecules observed in polymer-metal complexes is water of coordination (Table 2). The presence of water molecules in the polymer-metal complexes have also been supported by IR studies. For the resin, a steady and regular loss of weight was observed and at 310 °C the weight loss was about 19 %. The rate of decomposition was then quite rapid between 410 and 630 °C. And maximum decomposition of the resin has been observed at 780 °C. While in case of polymer-metal complexes, the rate of decomposition of the polymer-metal complexe is higher than that of the parent resin, suggesting that there may be strong intramolecular hydrogen bonding. Absence of such hydrogen bonding in polymer-metal complexes favors the reduction in the thermal stability of polymer-metal complexes compared to the parent resin [22–24]. It seems that metal ions accelerate the decomposition of polymer-metal complexes. The absence of such hydrogen bonding in polymer-metal complex favors the reduction in thermal stability of polymer-metal complexes compared to the parent resin. The thermal stability of the ligand and polymer-metal complex is in the order: ligand > polymer-metal complex. This result revealed that the resin shows better heat resistant characteristic than all the polymer-metal complexes.
SEM of HEBP-1,3 BD resin and its Sm (III) polymer-metal complex
Scanning electron microscope (SEM) of pure resin and polymer-metal complex was carried out to understand the inner morphology and pore structure. The morphology of fracture surface for the resin is quite different from polymer-metal complex. It is clear from the SEM of resin (Fig. 2a) is porous in nature. The color of the resin appeared to be black. The morphology of the resin shows a fringed model of the crystalline-amorphous structure. The fringes represent transition state between the crystalline and amorphous phase [25]. The resin exhibits more amorphous character with closed packed surface having deep pits, therefore, it is act as an efficient ion exchange material.
The pores of the resin samples are filled after interaction with metal ion with polymeric ligand. This is clear from the SEM of polymer-metal complex (Fig. 2b). The nature of the resin and polymer-metal complex is quite different; this is due to chelation of metal ion in polymeric backbone. The polymer-metal complex is found to be crystalline in nature. Thus incorporation of metal ions significantly enhances the crystallinity of the polymers [26]. Thus physical and chemical properties of resin are changed after chelation.
Electronic spectra and magnetic measurements
The experiments were performed by maintaining a 5 μM concentration of polychelates solution in DMF. The electronic spectra of all the polymer-metal complexes exhibited two additional bands in the region 260–300 nm and 445–465 nm. The first band occurs in the spectra of the polymeric ligand, is assigned to the type π → π 1* and π → π 2* [27]. The second band is assigned to the polymeric ligand → Ln (III) transitions in all the polymer-metal complexes. The La (III) polymer-metal complexes were found diamagnetic in nature as expected for six coordinated octahedral geometry. The electronic spectra of Pr(III), f3, polymer-metal complexes exhibits absorption at 21,324, 20,865, 19,458 and 17,715 cm−1, assigned to 3H4 → 3p2, 3H4 → 3P1, 3H4 → 3po and 3H4 → 1D2 transitions of Pr(III) in a octahedral environment, due to large crystal field with magnetic moment 3.70 BM. The Nd (III) polymer-metal complexes are paramagnetic as expected for f4 system. Bands were obtained at 18,970, 17,645, 14,273 and 9,885 cm−l for 4I9/2 → 2G9/2, 4I9/2 → 4G5/2, 4I9/2 → 2 S3/2, and 4I9/2 → 4F5/2 transitions of Nd(III) in octahedral geometry. In addition the bands at 23,168, 22,920 and 23,880 cm−1 for polymer-metal complexes are assigned to 4H5/2 → 4F9/2, 4H5/2 → 6p5 and 4H5/2 → 4I11/2 transitions of Sm(III) in octahedral geometry due to large crystal field splitting and all the polymer-metal complexes are paramagnetic in nature. The magnetic moment 1.74 B.M. is obtained as expected. The Gd (III) and Tb (III) polymer-metal complexes were found paramagnetic in nature 7.84 B.M. and 9.46 B.M. as expected for six coordinated octahedral polymer-metal complexes. The electronic spectra of Dy (III) f10 polymer-metal complexes exhibits absorption at 27,750 cm−l assigned to 6H15/2 → 6H13/2 transition of Dy (III) in octahedral geometry due to large crystal field splitting. The proposed structure of polymer-metal complex and its cluster are as shown in the Scheme 4a and b.where, M = La (III), Pr (III), Nd (III), Sm (III), Gd (III), Tb (III) and Dy (III)
Catalytic study
3,4-dihydropyrimidin-2(1H)-ones and their derivatives are important class of compounds due to their diverse therapeutic and pharmacological applications. Many functionalized derivatives of 3,4-dihydropyrimidin-2(1H)-ones are known to act as anti-hypertensive agents, alpha-laantagonists, nueropeptide-Y (NPY) antagonists and also serve as integral backbones of several calcium channel blockers [28]. Several marine alkaloids containing the 3,4-dihydropyrimidin-2(1H)-ones units are found to exhibit diverse biological activities as anti-viral, anti-bacterial, anti-tumor and anti-inflammatory agents [29]. Therefore the family of these compounds received a great deal of attention towards their synthesis. This reaction was first reported by Biginelli in 1893, but suffers from low product yield especially with substituted aromatic aldehydes [30]. Subsequently several modifications were reported for the Biginelli condensation reaction to improve the yields. The Biginelli reaction was carried out employing many reagents such as lanthanum chloride, manganese acetate, montmorillonite KSF, silica-supported sulfuric acid and metal triflates [31, 32]. Most of these reported catalysts suffer from various drawbacks such as stringent reaction conditions; tedious work-up procedure, long reaction time, use of stoichiometric amounts of catalysts and some of the catalysts employed are expensive.
In recent years, the developments of more economical and environmental friendly conversion processes are gaining interest in the industry and chemical technology. In continuation of our interest to develop metal based catalysts, we intended to find efficient, practically viable, environmentally benign and high yielding method for the Biginelli three component, one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using selected Nd (III) and Sm (III) polymer-metal complexes.
To study the efficiency and effectiveness of the catalyst, selected reactions were studied using Nd (III) and Sm (III) polymer-metal complexes under similar conditions to synthesize the 3,4-dihydropyrimidin-2(1H)-ones and results are summarized in Tables 3 and 4. It is found that Nd (III) and Sm (III) polymer-metal complexes are giving excellent results for synthetic studies. The yield is obtained nearly or more than 90 %, time required for completion of the reaction is less and m.p. of the product is same or very close to reported values, which is acceptable as per the present standard.
Antimicrobial activity of resin and polymer-metal complexes
The polymeric ligand and their metal complexes were studied for their antimicrobial activity against standard bacterial strains of Escherichia coli, Bacillus subtilis, Staphylococcus aureus (bacteria) and Saccharomyces cerevisiae (yeast). The compounds were tested at different concentrations ranging from 50 ppm-l000 ppm to find out the minimum concentration of the ligand and the polymer-metal complexes, which inhibits the microbial growth. The minimum concentration 500 ppm was found. The inhibition of growth from the ditch was measured in millimeter (mm) and the results are shown in Table 5. The polymeric ligand was found biologically active and their polymer-metal complexes showed significantly enhanced antibacterial activity against one or more bacterial species, with compared to the uncomplexed polymeric ligand. It is known that chelation tends to make the ligands act as more potent bactericidal agents, than the parent ligand. The antimicrobial activities of the compounds increase after metal chelation. Chelation reduces the polarity of the central metal ion by partial sharing of its positive charge with the donor groups [33] increasing lipophilic nature of the central metal ion, which in turn favors its permeation to the lipid layer of the membrane. Other factors, viz., stability constant, molar conductivity, solubility and magnetic moment, are also responsible for increasing the anti-microbial activity of the complexes [34].
Conclusion
2-hydroxy-4-ethoxybenzophenone-1,3 butylene (HEBP-1,3 BD) resin was synthesized by the condensation of 2-hydroxy-4-ethoxybenzophenone and 1,3 butane diol in acidic medium, and its polychelates were synthesized with hydrated metal acetates in good yield and characterized by various physicochemical methods. Resin and polychelates were soluble in DMF and DMSO but insoluble in chloroform, acetone, ethanol and benzene. It was observed that the attachment of the metal ion to the polymeric backbone decreases the thermal stability because of breaking of an intramolecular hydrogen bond present in the polymeric ligand. Thus an intramolecular hydrogen bond plays an important role in thermal stability. Insertion of metal ion enhances the antimicrobial activity significantly. Thus, polymer-metal complexes can be used as antifungal and antifouling coatings in various applications. Polymer-metal complexes are found as an efficient, effective and economical catalyst in Biginelli condensation reaction. The main advantage of these catalysts is that, it can be reused by giving simple water treatment and drying.
References
Carlucci L, Ciani G, Proserpio DM (2003) Coord Chem Rev 246(1–2):247. doi:10.1016/S0010-8545(03)00126-7
Manners I (1999) Pure Appl Chem 71(8):1471. doi:10.1351/pac199971081471
Manners I (2002) J Polym Sci Part Polym Chem 40(2):179. doi:10.1002/pola.10069
Cyr PW, Tzolov M, Manners I, Sargent EH (2003) Macromol Chem Phys 204(7):915. doi:10.1002/macp.200390061
Kaya I, Cihangiroglu N (2004) J Polym Res 11:37. doi:10.1023/B:JPOL.0000021746.50347.34
Nair VA, Mustafa SM, Krishnapillai S (2003) J Polym Res 10:267. doi:10.1023/B:JPOL.0000004629.53837.b3
Eddaoudi M, Li H, Yaghi OM (2000) J Am Chem Soc 122(7):1391. doi:10.1021/ja9933386
Guo X, Chen M, Tianhong Lu T, Huang X (2008) J Polym Res 15:141. doi:10.1007/s10965-007-9153-3
Parac-Vogt TN, Binnemans K (2004) Tetrahedron Lett 45(15):3137. doi:10.1016/j.tetlet.2004.02.084
Gaona-Tiburcio C, Almeraya-Caldero’n F, Chacon-Nava JG, Matutes-Aquino JA, Martinez-Villafan’e A (2004) J Alloy Comp 369(1–2):78. doi:10.1016/j.jallcom.2003.09.050
Cao RD, Sun F, Liang YC Y, Hong MC, Tatsumi K, Shi Q (2002) Inorg Chem 41:2087. doi:10.1021/ic0110124
Lin HM, Qing CX, Qian M, Ping XH (2005) Russ J Coord Chem 31(5):368. doi:10.1007/s11173-005-0106-2
Evans CH (1992) Biochemistry of the Lanthanides, vol 8. Plenum press, New York and London
Shah TB, Patel HS, Dixit RB (2001) Int J Polymeric Mater 49(3):271. doi:10.1080/00914030108039779
Patel MM, Kapadia MA, Patel GP, Joshi JD (2007) J Appl Polym Sci 106(2):1307. doi:10.1002/app.26711
Stanier RY (1986) Introduction to the Microbial World, 5th edn. Prentice Hall Inc, NJ, p 16
Vogel AI (1978) A Text Book of Quantitative Inorganic Analysis, 4th edn. Longmans Green and Co. Ltd., London
Dwivedi DK, Shukla RK, Shukla BK (1991) Acta Cienc Indica Chem 17c (14): 383, Chem Abstr 117:14241n, (1992)
Ursu M, Frey H, Neuner I, Thomann R, Rusu M (2004) Rep Romanian Phys 56(3):445
Ahamad T, Kumar V, Nishat N (2006) Polym Int 55(12):1398. doi:10.1002/pi.1999
Nikolaev AV, Logvinenko VA, Myachina LT (1969) Thermal Analysis. Academic Press, New York, p 79
Pittman CW Jr, Voes RL, Elder J (1971) Macromolecules 5:302. doi:10.1021/ma60021a008
Upadhyaya HD, Patel PP, Patel MM (1990) Synth React Inorg Met-Org Chem 20:1153. doi:10.1080/00945719008048625
Patel MM, Kapadia MA, Patel GP, Joshi JD (2007) J Appl Polym Sci 106(2):1307. doi:10.1002/app.26711
Shah BA, Shah AV, Shah PM (2006) Iran. Polym J 15(10):809
Samal S, Das RR, Dey RK, Acharya S (2000) J Appl Polym Sci 77(5):967. doi:10.1002/1097-4628(20000801)77:5<967::AID-APP3>3.0.CO;2-5
Gudasi KB, Havanur VC, Patil SA, Patil BR (2007) Metal-Based Drugs . doi:10.1155/2007/37348
Kappe CO (2000) Eur J Med Chem 35:1043. doi:10.1016/S0223-5234(00)01189-2
Overman LE, Rabiniwitz MH, Renhowe PA (1995) J Am Chem Soc 117(9):2657. doi:10.1021/ja00114a034
Partha P, Baruah PP, Gadhwal S, Prajapati D, Sandhu JS (2002) Chem Lett 31(10):1038
Sabita G, Reddy KK, Reddy KB, Yadav JS (2003) Tetrahedron Lett 44(34):6497. doi:10.1016/S0040-4039(03)01564-8
Paraskar AS, Dewkar GK, Sudalai A (2003) Tetrahedron Lett 44(16):3305. doi:10.1016/S0040-4039(03)00619-1
Nishat N, Ahmad S, Ahamad RT (2006) J Appl Polym Sci 100(2):928. doi:10.1002/app.23064
Nishat N, Ahmad S, Ahamad RT (2006) J Appl Polym Sci 101(3):1347. doi:10.1002/app.23036
Acknowledgement
The authors thank the Heads of the, Department of Chemistry and Bioscience, Sardar Patel University, Vallabh Vidyanagar, for providing laboratory facilities. One of the authors (MMP) is thankful to UGC, New Delhi, for the award of a research fellowship for meritorious students (RFSMS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, M., Kapadia, M. & Joshi, J. Polymer-metal complexes of phenolic resin with Ln (III): thermal, catalytic and antimicrobial aspects. J Polym Res 16, 755–765 (2009). https://doi.org/10.1007/s10965-009-9282-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-009-9282-y