Abstract
Thermal-induced gelation process of different polyacrylonitrile (PAN) solutions was investigated through dynamic rheological measurements. The rheological material parameters characterizing the gelation behavior of the PAN solutions were measured and the effects of such factors as water content, solvent type and concentration on the gelation process were analyzed. It is found that the gel point T gel of PAN/DMSO (dimethyl sulfoxide)–water solution increases with increased water content in the solution. Also, T gel becomes higher when the solvent has a lower solvency for PAN. A linear relationship between logG′ and logω, logG″ and logω indicates the beginning of gelation. The PAN concentration of the solution affects T gel in a way similar to that of the water content. The relaxation exponent n is found to relate to the fractal dimension of the polymer gel. For PAN/DMSO–water solution, this exponent n remains almost constant when the water content differs. The solvent type and concentration respectively influences n value in their own way. The PAN/DMSO–water solution systems in our work exhibit similar characteristics of gel structure, concluded from the n and d f values of these systems. A logarithmic plot between G′ and G″ can be used to evaluate the structural evolution of the solution, and a comparatively steep slope of the curve is an indication of gelation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that polymer gels, chemical or physical, are associated with the supermolecules, generally formed by crosslinking simpler linear polymer chains and their mechanical properties are closed related to the gel network structure formed. Many such chains are termed physically crosslinked where the crosslinks are usually of low and finite energy and of finite lifetime. Such crosslinks are not limited to single points on the chains but correspond to more or less extended junction zones [1]. The term “physical gel” introduced by De Gennes [2] is often used to imply the thermoreversibility, the case with many such systems.
Gelation may be defined as a process or a series of processes which leads to the formation of a gel. Most commonly, physical gelation of a polymer solution is resulted from its reduced solvency, caused by temperature changes or by addition of a poor solvent into an otherwise well dissolved solution. Such gels are often thermoreversible (i.e., heating causes the crystallite junctions of gel to dissolve) [3], and the mechanisms of such physical gelation include phase separation due to crystallization, vitrification and liquid–liquid phase separation, to the best of our knowledge.
The critical gel point is considered as the turning point where gelation process starts. Detection of the gel point has often relied on very simple criteria based on rheological methods. For example, it may be determined when G′ of the sample becomes greater than a pre-assigned threshold value. Winter and co-workers [4, 5] assumed (this assumption seems to neglect entanglement effect) that at the gel point all relaxation modes should be present in the relaxation spectrum, i.e. all possible path lengths through the gel exhibited. The stress relaxation modulus could be related to t by a power law \( G{\left( t \right)} \sim t^{{ - n}} {\left( {0 < n < 1} \right)} \), which characterizes the gel point in an intermediate state between a liquid and a solid. In the linear response domain the storage (elastic) modulus G′ and the loss (viscous) modulus G″ show at the gel point, a power-law dependence of n of the relaxation exponent on frequency ω and, therefore, the loss tangent (in Eq. 3) does not depend on ω [6, 7], i.e.,
Winter and Chambon [4] and Madbouly and Ougizawa [8] both showed that the relevant proportionality constants (k 1 and k 2) in Eqs. (1) and (2) are not in general the same so the gel point does not necessarily correspond to the crossover point of G′ and G″.
At very low frequencies, polymer solutions flow as viscous solutions and the power law relationships for G′ and G″ are \(G\prime \left( \omega \right) \sim \omega ^2 \) and \(G\prime \prime \left( \omega \right) \sim \omega ^1 \), respectively. In contrast at extremely high frequencies, the polymer solutions behave more like solids that G′ and G″ become more or less frequency insensitive, i.e. \(G\prime ,G\prime \prime \sim \omega ^0 \). Given appropriate time scales, the same phenomena can also be observed at by changing the temperature, where high temperature leading to a viscous sol state and low temperature causing the gel network to firm up. It is not difficult to estimate that the exponent n related to the critical gel point should possess values between 0 and 1 for G′ and G″. Some researchers reported the critical value of n was in the 0.5–0.75 range [9–11] and even 0.13–0.92 [12], depending on stoichiometry, concentration and polymer molecular weight. On many occasions, n is found to be 0.5–0.8.
The main characteristic of PAN is the presence of a permanent dipole in the monomer unit caused by the bulky CN group with strong polarity. Labudzinska and Ziabicki concluded that thus resulted strong intermolecular interactions between the macromolecules in PAN-DMF (dimethyl formamide) solution give rise to formation of somewhat stable junction points and thus to gelation [13]. Another publication, however, reported that the junction points of PAN gel network were generated by crystallization [14]. Despite the gelation mechanism, it is always possible to determine the gel point and to assess the influences of all the related variables such as the solvent type, the polymer concentration, the system temperature, and the addition of nonsolvent etc., since there are definitive relationships between the structure of gels, their rheological behaviors and these system parameters. It was found that decrease of temperature turns a PAN solution into a gel, whereas introduction of water into the PAN solution can lead to turbid gels even at a higher temperature [3].
The attempt of the present article was to study the thermal-induced gelation process, or alternatively, sol–gel transition of a concentrated PAN solution by examining its rheological behavior in given dynamic conditions. More specifically, we first examined the change in the loss tangent tanδ and the complex viscosity η * of PAN solutions and established connections to gelation process in rheological terms. Next the effects of temperature, solvent type, water (nonsolvent) content and concentration on the gelation process of the PAN solution were investigated. Then by conducting temperature scanning tests, the storage (elastic) modulus G′ and loss (viscous) modulus G″ of the PAN solution during the gelation process were traced and the logarithmic relationship between them was derived and investigated.
Experimental
Materials
PAN copolymers (Acrylonitrile:Itaconic acid = 98:2) were provided by Shanghai Institute of Synthetic Fiber with a viscosity-average molecular weight \(M_\eta = 7.8 \times 10^4 {\text{g}} \cdot {\text{mol}}^{ - 1} \). DMSO (analytically pure) was purchased from Shanghai Wulian Chemical Industry Co. Ltd., and DMAc (analytically pure) from Shanghai chemical reagent Co. Ltd. Deionized water was used.
Preparation of PAN solution
A certain amount of PAN copolymers were dispersed in various mixtures of DMSO, water and dimethyl acetamide (DMAc) in a three-neck bottle. The resulting slurry was left swollen at 50 °C for 2 h first, and at 60 °C for another 2 h, stirred constantly by an electric paddle stirrer. Subsequently, it was stirred again at 70 °C for 6 h to produce a homogeneous viscous solution with 20 wt.% PAN. Four such PAN solutions having water content of 0 wt.%, 2 wt.%, 4 wt.%, 6 wt.% were used. Other PAN solutions including 17 wt.% PAN/DMSO, 23 wt.% PAN/DMSO and 15 wt.% PAN/DMAc were prepared in the same way. The solutions were deaerated in a vacuum drying oven at 70 °C and then kept at this temperature for 18 h before the rheological analyses.
Rheological measurements
The dynamic rheological measurements were conducted using an advanced Solution and Melt Rotation Rheometer (ARES–RFS, made by TA, USA) equipped with two parallel plates. The temperature control was done with a thermostatic bath within ±0.1 °C of the preset value. The parallel plate on which samples (PAN solutions) were placed was 25 mm in diameter and the sample thickness was 2 mm. A thin layer of paraffin oil was applied to protect the sample from dehydration or evaporation and thus minimize errors. The details of the rheological experiments were as follows:
-
1.
Strain scanning at different temperatures in both sol and gel states of the samples and over the oscillation frequencies from 0.1 to 10 Hz, to detect the linear viscoelastic region of the samples. It was found that all the samples demonstrated certain linear viscoelastic behavior for the strain range of 0.5% to 10% in all cases, therefore 1% was chosen as the set strain amplitude in the following tests.
-
2.
Frequency scanning from 100 rad/s to 0.5 rad/s at eight given temperatures (70–42 °C, with interval of 4 °C) to obtain the loss tangent tanδ and the complex viscosity η *. Before the test at each temperature, all the samples were kept at that temperature for 3 min to eliminate the effect of the thermal history.
-
3.
Frequency scanning for the 15 wt.% PAN/DMAc solution and 20 wt.% PAN/DMSO–water solutions with and without 6 wt.% water at the temperatures near their own gel point T gel to obtain the dynamic shear moduli, i.e. storage modulus G′ and loss modulus G″ when the gelation happened. Before the measurement, the solutions had been kept at their own T gel for 3 min.
-
4.
Temperature scanning from 70 °C to 40 °C with cooling rate of 2 °C/min for all the samples to trace G′ and G″ against temperature. The oscillation frequency was fixed at 6.3 rad/s during each temperature scan test.
A new solution was used for each new test in order to avoid the memory effects.
Results and discussion
Water effect on the gelation behavior of PAN solution
For thermoreversible gels, thermal-induced gelation is the most common gelation mechanism. It has been stated that the results of the gelation temperature T gel determined from the crossover point of G′ and G″ are frequency dependent for many systems. For instance the T gel of thermally crosslinked PVME obtained at 1 rad/s was found to be around 20 °C lower than that determined at 100 rad/s [15]. Another technique based on the temperature dependence of the loss tangent tanδ at different oscillation frequencies has been proved more feasible, i.e., T gel is the temperature at which tanδ becomes frequency independent and all curves at different oscillation frequencies converge at the point [16].
Water is a strong cohesive solvent due to the strong intermolecular forces caused by hydrogen bonds. It promotes the aggregation of PAN [17] as it weakens the interactions between PAN molecular chains and the solvent, through forming hydrogen bonds with the solvent molecules. The aggregation actually produces or causes physical cross-linking initiation points for the system to gel. Water on the other hand can interact with the lone pair orbital of nitrogen in a manner similar to hydrogen bonding, and thus decouple the nitrile–nitrile association so that the PAN molecules can move more freely away from gel with each other. In PAN solution, water mainly interacts with DMSO and PAN molecules tend to aggregate rather than move away from each other. Therefore, the gel-promoting effect of water predominates in PAN solution.
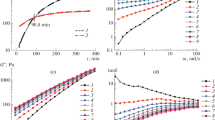
Frequency scanning tests for the 20 wt.% PAN/DMSO–water solutions with different water contents were carried out and the results are shown in Figs. 1 and 2. We can see in Fig. 1 that the five curves of tanδ at different frequencies cross over at a point, which is considered in this technique to be the critical gel point T gel. It is noticeable that the gel point T gel increases and the loss tangent tanδ decreases with the increased water content (Table 1) for all the PAN solutions. Water favors the aggregation of PAN molecules in solution so as to promote the thermal-induced gelation of PAN solution at a higher temperature. Alternatively, the addition of water worsened the solvency of DMSO such that the PAN molecular chains tend to aggregate and form a network structure more easily. tanδ is a measure of the viscous/elastic ratio for the solution. The PAN solution with more water exhibits a more gel-like (elastic) behavior and the tanδ is therefore depressed.
In addition, the critical value of tanδ exceeds 1 and n is thus over 0.5 for each solution, as can be seen from Table 1. Michon et al. reported that n depends on the concentration and the solution thermal history [1]. In general, n value is not universal and varies with the gelling systems [18]. A lower value of n implies the formation of a more elastic gel [19]. Also, low relaxation exponents n (between 0.1 and 0.25) are characteristic for crystallizing polymers [20].The rising cross-link density of polymers leads to a reduction of n for chemical gels [21]. Winter et al. found that the relaxation exponent n is related to the fractal dimension of the polymer gel, which reflects the degree of compactness of the gel network [18, 20, 22, 23]:
where d f is the fractal dimension of the polymer. In this study we maintained the same concentration and thermal history for all the samples, so the only difference among the solutions is the level of water. Although the gel point T gel varies with the water content in the solution, n only slightly changes, implying a unique feature of this system. The n values of our finding (0.531–0.551) suggest a moderate elastic nature of the solution. PAN is a type of paracrystalline polymer and is difficult to crystallize in solvents such as dimethyl formamide (DMF), DMSO, etc. [3, 24]. Besides, the not-very-strong dipole–dipole interaction between the nitrile groups gives rise to the crosslinking of PAN molecules and the density of the cross-links in PAN gels is medium. The values of d f listed in Table 1 indicate that the fractal structure of a PAN gel possesses similar fractal dimensions when containing different amount of water.
The complex viscosity η * behaves very differently with the frequency in the same temperature range for the four different solutions as shown in Fig. 2. For the solution without water, η * changes only slightly with frequency at T > 50 °C. At T < 50 °C, however, η * becomes frequency dependent. This unique behavior of η * is attributed to the fact that, at high temperature (i.e. T > 50 °C), the PAN solution exhibits almost frequency independent viscous behavior over the selected range of frequency. At T gel, the PAN solution changes from liquid-like to solid-like behaviors and consequently becomes frequency dependent as the gelation process evolves to form a fractal polymer gel. For the PAN solution with 6 wt.% water, η * starts to be frequency dependent at about 66 °C, and the turning temperature for the other two solutions are around 58 °C and 62 °C. The different behavior of η * with frequency for the three solutions is in good agreement with the results talked before.
The complex viscosity η * decreases with the increased oscillation frequency ω for all the solutions. Muthukumar and Winter [25] investigated the behavior of polymeric systems following Rouse chain dynamics, i.e. both the hydrodynamic and excluded volume effects are fully screened. The frequency dependence of the viscosity is shown to be
According to Eq. (5), η * is inversely proportional to ω as d f is always positive. Furthermore, for many polymer solutions, the frequency dependence of η * measured in oscillatory shear and the steady-state shear rate dependence of apparent viscosity η a are observed to be closely superposable, when the numerical values of ω and \(\dot \gamma \) are comparable. This empirical correlation, often called the Cox–Merz rule, has been observed for a great number of polymer solutions. PAN solution is a typical non-Newtonian fluid and it exhibits pronounced pseudoplastic behavior under high but constant shear rate of steady-state measurements. Therefore, the shear thinning behavior of η * is not surprising.
Figure 3 shows the frequency dependence of G′ and G″ for the PAN solutions at water content =0 and =6 wt.% respectively at the temperatures very close to their gel points of different values. For both solutions, the logG′ and logG″ versus log ω curves are linear within the error limit, signifying the occurrence of gelation. Limitations of the experiment at extremely low and high frequencies arise respectively from the long experimental times (the sample structure may change) and inertial effects. Besides, both G′ and G″ of the solution of water content = 0 and T gel = 46 °C are larger than those of the solution with 6 wt.% water and T gel = 67 °C.
Effects of solvent type and concentration on the gelation behavior
DMAc is another popular solvent for PAN. Compared with DMSO, lower concentration is required for a PAN/DMAc solution to reach similar rheological behavior, for DMSO is a stronger solvent for PAN than DMAc. In our work, a 15 wt.% PAN/DMAc solution without water was used and the results of frequency scanning tests are demonstrated in Figs. 4 and 5. The tanδ versus temperature curves in Fig. 4a intersect at a single point from which we obtain the gel point T gel = 63.8 °C, much higher than that in Table 1 of the 20 wt.% solution without water, yet between those of the 20 wt.% solutions with 4 wt.% and 6 wt.% water, respectively. The possible explanation is that DMAc is a weaker solvent for PAN so that it cannot separate PAN molecular chains as far apart as DMSO, and the polymer chains are closer to each other in PAN/DMAc solution, giving rise to more aggregation, lower T gel, at the same water content. The relaxation exponent n characterizing the fractal dimension of the gel structure is 0.504, close to those of the PAN/DMSO–water solutions, signifying similar number of cross-links in the case of PAN/DMAc system. As for the η * versus temperature curves in Fig. 4b, the turning temperature below which the η * becomes more significantly frequency dependent is 62 °C and the shear-thinning behavior is also observed. At 64 °C near the gel point T gel, the logG′ and logG″ versus logω curve in Fig. 5 is approximately linear, similar to the cases in Fig. 3.
To investigate the concentration effect of the PAN/DMSO–water solution on the gelation process, frequency scanning for both 17 wt.% and 23 wt.% PAN/DMSO solutions without water was performed (Fig. 6). The gel point T gel of the two solutions is 43.9 °C and 53.3 °C, respectively, sandwiching the 46.2 °C in Table 1 for the 20 wt.% PAN/DMSO solution without water, as expected. Furthermore, the relaxation exponent n of the three solutions is 1.162 (17 wt.%), 1.167 (20 wt.%) and 1.163 (23 wt.%), indicating PAN concentration has little influence on the fractal dimension of the PAN gel structure in the data range examined.
logG′ versus logG″ during the gelation process of PAN solution
Logarithmic plots of G′ versus G″ have also been widely applied to interpret the rheological behavior with respect to the miscibility of the polymer blends [26–28]. According to the Doi–Edwards theory, the shear storage modulus G′ and loss modulus G″ satisfy the following equations:
where c is a constant, T the absolute temperature, M c the average molecular weight between the entanglement points, ω the oscillation frequency and τ the disentanglement time. When ω is low and there is long enough time for disentanglement, i.e. ωτ << 1, we obtain
That is,
Han and Jhon considered the slope values of logG′ versus logG″ curve exceeding 2 to be evidence of polydispersity [24]. As for polymer solution, the greater slope in the sol–gel transition state may result from the higher degree of inhomogeneity or polydispersity, originated from the coexistence of the gel and solution.
This method was also employed in our work to study the miscibility or polydispersity of PAN solution during the gelation process. Figures 7, 8, and 9 represent the results of the temperature scanning tests for the three 20 wt.% PAN/DMSO–water solutions. Plot (a) in each figure shows the temperature dependence of G′ and G″ in the cooling process. G′ and G″ both increase with the decrease of temperature, and the G′ grows more rapidly than G″, showing a gradually evolving gel structure.
Plot (b) demonstrates the logarithmic relationship between G′ and G″ based on plot (a). For the 20 wt.% PAN/DMSO solution without water, logG′ versus logG″ curve appears to consist of three parts with different slopes. At high temperatures before gelation, i.e., both G′ and G″ are small, the solution is homogeneous and the slope of the curve is relatively small. When the temperature decreases, the curve becomes steeper, indicating the occurrence of gelation. The third part of the curve whose slope decreases corresponds to the gel state of the solution. More careful observation reveals that the second part of the logG′ versus logG″ curve starts at the temperature much higher than the gel point T gel, likely indicating the occurrence of micro-phase separation in the solution before the gelation process starts.
The curve of the solution with 4 wt.% water in Fig. 8 is similar to that of the solution without water, except that the second part in Fig. 8b with a large slope begins at higher temperature near its T gel. As for the solution with 6 wt.% water in Fig. 9b, the first part of the curve disappears, ascribed to the higher gelation temperature of the solution.
We can see in Table 2 that the slope value of the second part of the curve is larger than 2 in each case, representing the gelation process in light of Han’s theory. And the slope value increases when the water content in the solution rises, though the trend becomes less obvious when the water level is higher. In the case of the first and third part, the slopes are below 2, explained by the homogeneity of the system when only solution or gel is present. The same trend is observed in terms of the changes of the slope with the water level. Besides, the slope of the third part is smaller than that of the first part. This means, the formed PAN gels in our study are highly homogeneous as the PAN/DMSO–water solutions are homogeneous.
Conclusions
The rheological properties of different PAN solutions were investigated through dynamic rheological measurements. The rheological material parameters (tanδ, η *, G′ and G″) of the PAN solutions are very sensitive as expected to thermal induced gelation whether in the frequency scanning or temperature scanning tests. When the loss tangent tanδ is examined, the gelation process initiates at the temperature where tanδ becomes frequency-independent. This temperature is termed as the gel point T gel, which is greatly influenced by the solvent type and the water content in the solution. T gel rises when the water content in PAN/DMSO solution increases as well as when the solvent used (DMAc) has poorer solvency for PAN. These are because the PAN molecules tend to aggregate when the solvent cannot separate them fully apart. The effect of concentration on T gel of the PAN solution can also be ascribed to the enhanced aggregation of PAN molecules when the concentration increases. In the frequency scanning tests, the linear curves of logG′ and logG″ versus logω obtained at the temperature near T gel highlight the characteristics of gelation behavior of the PAN solutions.
The relaxation exponent n can be used to characterize the fractal dimension of the PAN gel. For the PAN/DMSO solutions with varied water contents, similar values of n and d f are observed, indicating a unique gel structure. The 15 wt.% PAN/DMAc solution has a relatively smaller n than all the PAN/DMSO solution, yet the gel structure of this system resembles that of the PAN/DMSO solution. The concentration of PAN/DMSO solutions exerts little influence on the value of n over the data range of this work, further verifying the unique nature of the PAN gel.
The complex viscosity η * obtained at different frequencies diverge at a certain temperature for each PAN solution. This temperature is in the vicinity of T gel and increases with the water content. η * decreases with the increase of oscillation frequency, showing a shear-thinning behavior of PAN solution. The slope value of the logG′ versus logG″ curve is greater (above 2) when the solution becomes more inhomogeneous, i.e. both the gel and solution exist. The slope is smaller than 2 before and after the gelation occurs. The slope value grows with increased water content in the solution for each part of the curve. Although the critical temperature where the slope changes differs more or less from the gel point T gel, it increases with the water content just as T gel.
References
Michon C, Cuvelier G, Launay B (1993) Rheol. Acta 32:94–103 doi:10.1007/BF00396681
De Gennes PG (1985) Scaling concepts in polymer physics. Cornell University Press, New York
Bashier Z (1992) J Polym Sci Polym Phys 30:1299–1304 doi:10.1002/polb.1992.090301115
Winter HH, Chambon FJ (1986) Rheol 30(2):367–382 doi:10.1122/1.549853
Te Nijenhuis K, Winter HH (1989) Macromolecules 22:411–414 doi:10.1021/ma00191a074
Chambon F, Winter HHJ (1987) Rheol 31(8):683–697 doi:10.1122/1.549955
Cuvelier G, Launay B (1990) Makromol Chem Macromol Symp 40:23–31
Madbouly SA, Ougizawa T (2004) J Macromol Sci Phys B 43:471
Lairez D, Adam M, Emery JR et al (1992) Macromolecules 25:286–289 doi:10.1021/ma00027a046
Lin YG, Mallin DT, Chien JCW (1991) Macromolecules 24:850–854 doi:10.1021/ma00004a006
Masataka S, Hirokazu H, Takashi T (2007) Rheol Acta 46:957–964 doi:10.1007/s00397-007-0178-7
Scalan JC, Winter HH (1991) Macromolecules 24:47–54 doi:10.1021/ma00001a008
Labudzinska A, Ziabicki A (1971) Kolloid Z 243:21 doi:10.1007/BF01500610
Flodin P (1988) Macromol Chem Macromol Symp 22:253
Madbouly SA, Otaigbe JU, Nanda AK et al (2005) Macromolecules 38:4014 doi:10.1021/ma050453u
Winter HH, Morganelli P, Chambon F (1988) Macromolecules 21:532 doi:10.1021/ma00180a048
Piculell L, Thuresson K, Lindman B (2001) Polym Adv Technol 12:44–69 doi:10.1002/1099-1581(200101/02)12:1/2<44::AID-PAT944>3.0.CO;2-O
Lue A, Zhang L (2008) J Phys Chem B 112:4488–4495 doi:10.1021/jp077685a
Gao S, Nishinari K (2004) Biomacromolecules 5:175–185 doi:10.1021/bm034302f
Winter HH, Mours M (1997) Adv Polym Sci 134:165–234 doi:10.1007/3-540-68449-2_3
Scanlan JC, Winter HH (1991) Macromolecules 24:47–54 doi:10.1021/ma00001a008
Hess W, Vilgis TA, Winter HH (1988) Macromolecules 21:2536 doi:10.1021/ma00186a037
Muthukumar M (1989) Macromolecules 22:4656 doi:10.1021/ma00202a050
Han CD, Jhon MS (1986) J Appl Polym Sci 32:3809–3840 doi:10.1002/app.1986.070320302
Muthukumar M, Winter HH (1986) Macromolecules 19:1284–1285 doi:10.1021/ma00158a064
Kim JK, Son HW, Lee Y et al (1999) J Polym Sci Polym Phys 37:899–906
Ohta Y, Murase H, Sugiyama H et al (2000) J Appl Polym Sci 40(11):2414–2422
Chuang HK, Han CD (1984) J Appl Polym Sci 29:2205–2229 doi:10.1002/app.1984.070290625
Acknowledgements
This work was supported by grants from the National Basic Research Program (973 Program) (2006CB606505), the Shanghai Fundamental Theory Program (07DJ14002) and the Shanghai Leading Academic Discipline Project (B603).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, L., Pan, D. & Pan, N. Rheological study on thermal-induced gelation behavior of polyacrylonitrile solution. J Polym Res 16, 341–350 (2009). https://doi.org/10.1007/s10965-008-9234-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-008-9234-y