Abstract
A new strategy was developed to prepare MgCl2-supported nickel-diimine catalysts for ethylene polymerization, in which an amino-functionalized nickel-diimine complex, bis(4-(4-amine-3,5-diisopropylbenzyl)-2,6-diisopropylphenylimino)acenaphtheneNiBr2 (abbreviaed as NiLBr2), was covalently supported on MgCl2/AlRn(OEt)3-n. The most significant feature of the supported catalyst is that it polymerizes ethylene with high activities in the presence of inexpensive general alkylaluminum compounds. According to the results of high temperature GPC, the number-average molecular weights of the polymers ranged from 0.35 × 106 to 1.08 × 106 and the polydispersities ranged from two to three. By modulating the polymerization temperatures, the products of different morphologies were obtained. The resultant PE was confirmed by 13C NMR to contain significant amounts of not only methyl but also ethyl, propyl, butyl, amyl, and long branches (longer than six carbons).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, more attention has been paid to late transition-metal catalysts for olefin polymerization since Brookhart and co-workers [1, 2] discovered highly active α-diimine nickel and palladium catalysts. These catalysts can produce branched polyethylene from ethylene alone. However, the homogeneous catalysts easily lead to an extremely exothermic polymerization process and result in serious fouling of the reactor in the slurry process of olefin polymerization. Thus the application of these catalysts in a continuous process has been difficult. In general, the way to solve these problems is to immobilize the catalysts on suitable carriers.
Most often, homogeneous catalysts have been supported on inorganic materials such as silica and on organic materials such as polystyrene [3–7]. For heterogeneous late transition-metal catalysts, silica (SiO2) is the commonly used support [8, 9].The supporting methods are classified mainly into two categories: the direct immobilization of the catalyst on silica and the immobilization of the catalyst on silica pretreated with aluminoxane or alkylaluminum compounds activator. Recently, a new strategy of covalently attaching (α-diimine) nickel catalysts to silica was demonstrated to be an effective method for ethylene polymerization, and the work reported by the group of Brookhart [8] is a successful example. However, as the most commonly used catalyst carrier, magnesium chloride (MgCl2) used to support late transition-metal catalysts is not widely reported in the literatures [10–12]. For the lack of hydroxyls on the surface of MgCl2, it is difficult to support late transition-metal catalysts or methylaluminoxane (MAO) on MgCl2 firmly. As far as we are concerned, the most commonly used MgCl2 support for the immobilization of these catalysts is MgCl2/AlRn(OEt)3-n obtained by the reaction of alkyl aluminums with adducts of magnesium chloride and ethanol under mild conditions.
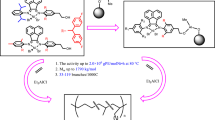
In our previous papers [13], the activity and property of unsupported, silica supported NiLBr2 for ethylene polymerization have been discussed. In this paper, we report a new strategy for immobilizing bis(4-(4-amine-3,5-diisopropylbenzyl)-2,6-diisopropylphenylimino)acenaphtheneNiBr2 catalyst on the MgCl2/AlRn(OEt)3-n support. The Ni (II) catalyst can easily be supported on MgCl2/AlRn(OEt)3-n through a covalent bonding with higher Ni loading of 2.6 mgNi/g cat. The high loading of catalyst is important for reducing the amount of inorganic impurities in the received polymers. After activation with AlEt2Cl (DEAC), the heterogeneous Ni (II) catalyst showed high activity. The influence of polymerization conditions on catalytic activity, properties of polyethylenes, and microstructures of the resulting branched polyethylenes are discussed.
Experimental
Materials
Polymerization-grade ethylene was provided by Shanghai Jinshan Petroleum Co., and was further purified before charging into the reactor through a DC-IB gas purification instrument. DEAC and AlEt3 (TEA) were purchased from Shanghai Petrochemical Co. (China) and used as a solution in n-heptane of 400 g/L, respectively. The catalyst complex of NiLBr2 (Scheme 1) was synthesized according to a previously reported procedure [12]. Solid MAO was prepared by the reaction of trimethylaluminum (TMA) with water in Al2(SO4)3·18H2O dispersing in toluene, and then the received product was filtered and evaporated in vacuum. The TMA content retained in MAO was about 26.8 mol% as determined by 1H-NMR. Toluene was purchased from Guangzhou Chemical Co. (China), and dried over sodium under reflux for 24 h. Anhydrous MgCl2 was provided by HuShun aluminum plant. All the other chemicals were purchased commercially, and used without further purification. MgCl2/AlRn(OEt)3-n was prepared according to the literature [14].
Preparation of supported catalyst
MgCl2/AlRn(OEt)3-n support (0.3 g) was mixed with a solution of NiLBr2 (0.0714 g) in CH2Cl2 (50 ml). After 24 h, the solid support was isolated by filtration, washed three times with CH2Cl2 and dried in vacuum at 40 °C. The supported catalyst was prepared as shown in Scheme 2.
Polymerization of ethylene
The polymerization of ethylene was carried out in a 250 ml Schlenk flask equipped with a mechanical stirrer. The flask was dried by heating at 80 °C under vacuum for 1 h and swept with dry N2 for three times. After the flask was dried completely and cooled down to room temperature, the flask was charged into n-heptane (50 ml) and the solvent was saturated with ethylene monomer at 1.5 atm. Alkylaluminum compounds co-catalysts dissolved in n-pentane was injected into the reaction solution. The heterogeneous catalyst (0.04 g) was then added and the reactor was heated to the polymerization temperature within 2 min. After an hour, the polymerization was stopped by addition of ethanol. The reaction solution was poured into the large amount of 10% HCl–EtOH solution, affording polyethylene as a suspended precipitate. The polymer was filtered and washed with ethanol several times, and dried in vacuum at 40 °C for 20 h.
Characterizations of supported catalysts and polymers
The specific surface area and the pore size distribution of the support were measured by N2-BET method with CE SORPTOMATIC 1990. The Ni content in the supported catalyst was determined by an Inductively Coupled Plasma-atomic Emission Spectrometry (TJA Co., USA). 1H-NMR spectra were recorded on a Bruker-500 spectrometer. Molecular weights were determined by high-temperature GPC in 1, 2, 4-trichlorobenzene. The 13C NMR spectroscopic data for polyethylene were obtained using o-dichlorobenzene as the solvent with a Varian INOVA-500 NMR spectrometer at 130 °C. Melting points were determined by differential scanning calorimetry (DSC) with a Perkin-Elmer 7 Series Thermal Analysis System.
Results and discussion
Influence of the cocatalyst on catalyst activity
The objective of this work is to study the influence of the cocatalyst on catalyst performance with regard to activity. Therefore, ethylene polymerization using homogeneous (abbreviated as H-1) and heterogeneous nickel-diimine catalysts (abbreviated as SC-1) were performed under identical reaction conditions. As the NiLBr2/ MgCl2/AlRn(OEt)3-n supported catalyst was concerned, the effect of co-catalysts (DEAC, TEA and MAO) on ethylene polymerization at 50 °C and Al/Ni ratio of 800 were investigated (as shown in Table 2). As expected, no catalytic activity was observed for the complex in the presence of TEA in the homogeneous and heterogeneous polymerization. Theoretical and mechanistic studies suggest that DEAC and MAO are good co-catalysts for homogeneous (α-diimine) nickel complex in the ethylene polymerization. According to our experiment results, the activities obtained by the supported (α-diimine) nickel complex/DEAC system is high, however, the complex with MAO system display no activities under the same polymerization condition. These facts indicate that the complex NiLBr2 mainly exists in the interior of the support, but not on the surface of the support. Table 1 presents the N2-BET surface areas information of the supported catalyst. It is revealed that the MgCl2/AlRn(OEt)3-n support with high surface area and high porosity, may be suitable for supporting late-transition metal catalysts. Studies on the structure of MAO, [–Al(Me)–O–]n suggested that the MAO molecules could be one-dimensional linear chains or cyclic ring three-dimensional cage structure with n = 5–20 [15]. Based on the work by Sano and co-workers on the adsorptive separation of MAO by MCM-41 [16], the molecular diameter of MAO was estimated to be approximately 2.5 nm. According to the reported crystallographic studies on the molecular structures of some Ni-diimine complexes [17, 18], the molecular diameter of NiLBr2 was about 1.5 nm. For the NiLBr2 molecules ranging from 1.5 to 2.5 nm, the effective diffuse of the NiLBr2 molecules into mesopores are easier. In order to further explore this, a two-stage experiment was carried out to evaluate the performance of the supported catalyst according to above polymerization procedure. In the first stage, MAO co-catalysts dissolved in toluene was injected into the reaction solution. The heterogeneous catalyst (0.04 g) was then added and the reactor was heated to the polymerization temperature within 2 min. After 10 min, the polymerization was stopped by turning the ethylene off and relieving the pressure. Subsequently, AlEt2Cl co-catalysts dissolved in n-heptane was injected into the reaction solution, ethylene was introduced into the reactor again, and the pressure was maintained at 1.5 atm. The result was listed in Table 2. In run 9d,MAO was unable to initiate the polymerization of ethylene in the first 10 min. After addition of AlEt2Cl, the polymerization started immediately and the polymerization activity reached 3.17 × 106 g of PE/mol of Ni h.
Apparently, the porous surface structure of MgCl2 support allows complete penetration of NiLBr2 (about 1.5 nm diameter) into its internal pores (about 2.0 nm in diameter) to generate active center for olefin polymerization. Moreover, the pore size of MgCl2 support limits larger co-catalyst complexes of MAO (2.5 nm diameter) into internal pores,but allows diffusion of less size co-catalyst complexes DEAC into their internal pores and then reacted with (α-diimine) nickel complex to effectively form active centers.
Table 2 summarizes the ethylene polymerization activity of the supported catalysts. The results show that the MgCl2-supported catalysts were even more active than the corresponding silica-supported [13] or homogeneous counterparts. MgCl2 may function as a Lewis acid to generate a cationic active species from an alkylated complex [19]. The polyethylene molecular weight data shown in Table 3 reveals narrow polydispersities, indicating that the single-site characteristics of the catalysts are less affected by immobilization on the support. The polydispersities are narrower than those of polymers prepared by using the silica-supported analogous nickel diimine catalysts by our group [13]. The polymer with the highest molecular weights was obtained with the complex at 0 °C, and increase of temperature leads to the increase of chain transfer rate relative to propagation rate.
Effects of polymerization conditions
Table 3 shows the effects of the Al/Ni ratio on catalytic activity of ethylene polymerization and properties of polyethylenes. The activity increases to 4.01 × 106 g PE/ (mol of Ni h) at Al/Ni ratio of 800 as the Al/Ni ratio increasing, however, further increase of Al/Ni ratio leads to the decrease of activity. For late transition metal catalysts, it is believed that aluminum alkyl cocatalysts are responsible for activation [1]. The suitable amount of AlEt2Cl make nickel α-diimine complex to form cationic nickel species, and thus initiate the polymerization of ethylene. The concentration of cationic nickel species increases with Al/Ni ratio, and reaches the highest point at 800. Molecular weights and molecular weight distributions of all the polyethylenes were determined by high-temperature GPC. As shown in Table 3, the polymers exhibit high molecular weight and narrow molecular weight distribution ranging from 2.37 to 2.87. The molecular weight of polyethylenes decreases with the increase of Al/Ni ratio. For example, the weight average molecular weight of the polymer is 0.68 × 106 as the Al/Ni ratio is at 400, and it decreases to 0.48 × 106 as Al/Ni ratio increases to 800. AlEt2Cl is regarded as a chain transfer reagent in ethylene polymerization. Therefore, chain transfer rate will increase with the Al/Ni ratio increasing and thus lead to the decrease in the molecular weight of polyethylenes.
A series of experiments were undertaken to determine the effect of temperature on SC-1 performance and properties of resultant polyethylene. Table 4 shows the results of polymerizations performed at four different temperatures (0, 20, 40 and 60 °C). First, the catalysts show the highest activity of 4.01 × 106 g PE/ (mol of Ni h) when polymerized at 40 °C. Second, the molecular weight of polyethylenes decreases with temperature increasing. The Mw of the polymer is 1.08 × 106 as the temperature is at 0 °C, and it decreases to 0.48 × 105 as temperature reaches to 50 °C. Third, the melting temperature (Tm) of polyethylene significantly decreases with the increase of temperature due to the increase of chain-transfer rate. The results are associated with those observed by Brookhart et al [1].
Microstructures of the resulting branched polyethylenes
Different from the conventional Ziegler–Natta catalysts, nickel α-diimine catalyst can produce polyethylene with branch structures without the use of α-olefins comonomers. Branching of polyethylene products is believed to exist according to the mechanism of ‘chain walking’ [1]. However, the results indicate that the support system showed no serious effect on the basic modes of chain growth and branching. Similar to the homogeneous catalysts, the melting points of the polymers catalyzed by the supported systems reach to higher temperatures with the decrease of reaction temperature due to the competition of propagation versus chain walking. DSC results indicate that some polyethylene samples exhibit no melting peaks while the others show broad melting peaks. It is suggested that the polyethylenes obtained in our experiment are branching polyethylenes. The DSC curves of polyethylenes of different branching degrees prepared by the support system are shown in Fig. 1. Tm decreased with an increase of branching degree of polyethylene and gave rise to linear semi-crystalline and totally amorphous polymers. The polyethylenes prepared at 0, 20 and 40 °C had a Tm value of 120.2 °C (as shown in Fig. 1a), 118.2 °C (as shown in Fig. 1b) and 113.6 °C (as shown in Fig. 1c), respectively. The highly branched polyethylenes prepared at 60 °C showed no melting peaks (as shown in Fig. 1d). The melting peaks also became broader as the branching degree increased. The melting behavior of polyethylene is mainly related to the short chain branching density. Increasing short chain branching density decreases lamellar thickness of crystal structure and thus lowers melting temperature of the polymer [20].
The microstructures of the branched polyethylenes obtained by the supported (α-diimine)nickel catalyst are analyzed by the high-temperature 13C-NMR spectroscopy. The analysis indicates that some polyethylenes are extensively branching and the branched structures are mainly consisted of methyl branches. A representative 13C-NMR spectrum of polyethylene prepared by the support catalyst at 0, 20, 40 and 60 °C, respectively, is shown in Fig. 2. Based on chemical shift calculations performed by the method of Linderman and Adams [21], each resonance peak was assigned. Branches are named by xBn, where B designates a branch chain, n is the length of the branch, x is the carbon number starting with the methyl group of the branch chain and the end methyl noted as n = 1 [22]. The corresponding branch resonance peaks can be found in our polymer spectrum. The characteristic chemical shifts at 19.74 (1B1), 32.99 (brB1), 10.95(1B2), 39.42 (brB2), 14.38 (1B3), 23.14 (2B4), 37.94 (brB4), 22.63 (2B5), 31.94 (3Bn) and 29.34 (4Bn) for the supported catalyst. However, a linear semi-crystalline polyethylene is prepared at 0 and 20 °C, for only the signals of methyl branches (Fig. 2a) in the 13C-NMR spectrum are observed. Some small resonances attributed to ethyl, propyl, butyl and pentyl branches can be observed in Fig. 2d for branched polyethylene polymerized at 60 °C. It is surprising that long branches (longer than six carbons) are confirmed by the presence of the 3Bn and 4Bn carbon resonances at 31.95 and 29.34 ppm, and a pair of resonances attributable to a branch terminated with a sec-butyl group are also found in Fig. 2d. This is the smallest branch-on-branch possible in an ethylene polymerization. Table 5 shows the effect of different temperatures on degree of branching and branching distribution of polyethylenes. The degree of branching at 0 °C is low and almost all the methyl branches are incorporated into the polyethylene backbone, and the incorporation of long branches cannot be observed in the 13C NMR spectrum. As the temperature increases, the content of methyl branching decreases, and the long branches and degree of branching increases. At 60 °C, the methyl branches of 61.23% and long branches of 9.50% are incorporated into the polyethylene backbone. It can also be noted that the chain walking mechanism appear to be pronounced in the supported catalyst system. An increase of polymerization temperature improves the rate of chain walking and results in the formation of more branching polyethylene of low melting point. The 13C-NMR (Table 5) results of the polyethylene produced by these catalytic systems are consistent with the results reported by Brookhart and his co-workers [1].
Scanning electron microscope was also used to examine the morphology of PE particles produced by supported catalysts. The polymer particles revealed in Fig. 3 show retention and replication of the spherical morphology of the original support during catalyst immobilization and polymerization. Replication of the support morphology for the polymers indicates that the supported catalyst has a uniform distribution of active sites and high porosity. The received polymers were free-flowing powders and show no evidence of reactor fouling.
Scanning electron microscopic images of supported catalyst, PE particles produced by homogeneous catalyst and PE particles produced by supported catalyst. a 1,500-fold magnification (supported catalyst), b 200-fold magnification (PE particles produced by homogeneous catalyst), c 1,000-fold modification (PE particles produced by supported
Stability of the supported catalyst
The complexes formed according to Scheme 3 are rather firmly fixed on the MgCl2 support, since a further treatment of the “active” MgCl2 with AlEt3 or washing with hexane does not remove the surface organoaluminium compound. Moreover, as can be seen later, a treatment of MgCl2 even with (α-diimine) nickel complex does not remove the aluminium.
As shown in Scheme 2, the supporting mechanism is suggested to be based on the reaction of NH2 in NiLBr2 with Al–Et on the activated MgCl2 support to form N–Al bond. The immobilized Ni complex will not be removed from the support by washing with solvent, and the covalent bond must be formed between the Et3Al-modified support surface and nickel α-diimine catalyst. It is suggested that the washed supported catalyst show no difference with the original hetergeneous catalyst in polymerization, and the Al-N bond is believed to form. Thus, the following experiment was designed. In a flame-dried Schlenk-type apparatus, 0.04 g supported catalyst was dissolved in 50 mL of toluene under N2 atmosphere. After vigorously stirring at 50 °C for about 30 min, the mixture was filtered under N2 atmosphere. The solid and the liquid were used to catalyze the ethylene polymerization in the usual procedure, respectively. After polymerization for about 30 min, the solid-catalyzed polymerization yielded polyethylenes, while for the liquid only a trace of polyethylene was produced. Therefore, it is concluded from the result that the synthesized supported catalyst was anchored on the support.
Conclusion
In the ethylene polymerization, MgCl2 supports were modified with AlEt3, applied to heterogenisation of the (α-diimine) nickel catalyst, and activated by the alkylaluminium compounds (MAO, AlEt3, AlEt2Cl, respectively). The results showed that the active sites mainly formed inside, but not on the outer surface of the particle. The influence of polymerization conditions on catalytic activity and properties and microstructures of the resulting branching polyethylenes were discussed. The catalyst systems exhibit good activity after activation by AlEt2Cl as compared to the homogeneous catalysts and SiO2 supported catalyst. The molecular weight distributions of the polyethylenes are narrow. Replication of supported catalyst morphology was found in the polymers produced at some conditions.
References
Johnson LK, Killian CM, Brookhart M et al (1995) J Am Chem Soc 117:6414 doi:10.1021/ja00128a054
Johnson LK, Mecking S, Brookhart M et al (1996) J Am Chem Soc 118:267 doi:10.1021/ja953247i
Vaughan GA, Canich JM, Matsunaga PT, Dindelberger DE, Squire KR (1998) WO 9748736 Dec1996
MacKenzie PB, Moody LS, Killian CM, Lavoie GG (1998) WO9962968 Dec1998
Simon LC, Patel H, Soares JBP, de Souza RF et al (2001) Macromol Chem Phys 202:3237 doi:10.1002/1521-3935(20011101)202:17<3237::AID-MACP3237>3.0.CO;2-T
Bennett AMA, Mclain SJ (1999) WO 9856832 Dec1997
Reddy CS, Das CK et al (2007) J Polym Res 14(2):129 doi:10.1007/s10965-006-9092-4
Pflugl PP, Brookhart M et al (2002) Macromolecules 35:6074 doi:10.1021/ma020230t
Ye Z, Alsyouri H, Zhu S, Lin YS et al (2003) Polymer (Guildf) 44:969 doi:10.1016/S0032-3861(02)00877-7
Guan Z, Zheng Y, Jiao S-K et al (2002) J Mol Catal Chem 188:123 doi:10.1016/S1381-1169(01)00437-X
Severn JR, Chadwick JC, Van Axel Castelli V et al (2004) Macromolecules 37:6258 doi:10.1021/ma049247x
Huang R-B, Liu D-B, Wang S-B, Mao B-Q et al (2005) J Mol Catal Chem 233:91 doi:10.1016/j.molcata.2005.02.004
Jiang H-L, Qing Wu, Zhu F-M, Wang H-H et al (2007) Journal of Applied Polymer Science 103(3):1483 doi:10.1002/app.24972
Pakkanen TT, Pakkanen TA, Iiskola E, Sormunen P et al (1990) J Catal 121:248 doi:10.1016/0021-9517(90)90235-C
Chen EYX, Marks TJ et al (2000) Chem Rev 100:1391 doi:10.1021/cr980462j
Sano T, Doi K, Hagimoto H, Wang Z, Toshiya U, Soga K et al (1999) Chem Commun (8)733 doi:10.1039/a900030e
Gates DP, Svejda SA, Onate E, Killian CM, Johnson LK, White PS, Brookhart M et al (2000) Macromolecules 33:2320 doi:10.1021/ma991234+
Liimatta JO, Lo’fgren B, Miettinen M, Ahlgren M, Haukka M, Pakkanen TT et al (2001) J Polym Sci Part Polym Chem 39:1426 doi:10.1002/pola.1119
Hedden D, Mark TJS et al (1988) J Am Chem Soc 110:1647 doi:10.1021/ja00213a061
Alobaidi F, Ye Z, Zhu S et al (2004) Polymer (Guildf) 45:6823 doi:10.1016/j.polymer.2004.08.018
Linderman LP, Adams NO et al (1971) Anal Chem 43:1245 doi:10.1021/ac60304a002
Usami T, Takayama S et al (1984) Macromolecules 17:756 doi:10.1021/ma00139a022
Acknowledgment
The work was supported by NSFC and SINOPEC (Joint-Project 20334030), Foudation of Nanchang Hangkong University (20060188).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, H., He, F. & Wang, H. A new strategy to prepare branching polyethylene by using an α-diimine nickel (II) complex covalently supported on MgCl2/AlRn(OEt)3-n . J Polym Res 16, 183–189 (2009). https://doi.org/10.1007/s10965-008-9216-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-008-9216-0