Abstract
The free-radical copolymerization of 2-methyl-N-1,3-thiazole-2-ylacrylamide monomer (TMA) with glycidyl methacrylate (GMA) was carried out in 1,4-dioxane at 65 ± 1 °C using azobisisobutironitril (AIBN) as an initiator. The copolymers were characterized by FTIR, 13C-NMR and 1H-NMR spectroscopic methods. The copolymer compositions were determined by elemental analysis. The weight-average and number-average molecular weights of the copolymers were obtained by gel permeation chromatography (GPC). The polydispersity indices of the polymers, determined with gel permeation chromatography, suggested a strong tendency for chain termination by disproportionation. Thermal properties of the polymers were also studied by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). The monomer reactivity ratios were calculated according to the general copolymerization equation using Kelen–Tudos and Fineman–Ross linearization methods. The reactivity ratios indicated a tendency toward for alternation. The thermal decomposition activation energies of the polymers were evaluated by Ozawa method. The antibacterial and antifungal effects of the copolymers were also investigated on various bacteria and fungi. All the products showed moderate activity against different strains of bacteria and fungi.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyacrylamides are amorphous and water-soluble polymers with great industrial and agricultural interest [1–3] and their properties are attractive in fundamental research such as catalysis [4], polymer blends [5] and biomedical fields [6]. Their uses in the paper industry, water treatment, and mining are based on their ability to flocculate solids in aqueous suspensions [7–9]. Polymer supports based on glycidyl methacrylate are mainly used as excellent thermosetting adhesives that have gained popularity over the years because of their superior performance in many applications such as binding of drugs and biomolecules [10] and in electron industries as negative electron beam resists [11]. Copolymers of glycidyl methacrylate have received significant attention due to the pendent epoxide groups which enter into a large number of chemical reactions [12–14], thus offering the opportunity for chemical modification of the parent copolymers for various uses. Poly(TMA) has an secondary amide functional group (CONH–) in the side chain instead of the carbonyl unit present in GMA. The NH– group can interact with other functional groups such as ethers and esters in methacrylate through hydrogen bonding. Therefore, we expect that hydrogen bonding will exist in TM-co-GMA copolymers between the carbonyl groups of GMA units and the amide groups of TMA units.
The antimicrobial property of the polymers plays an important role in its many applications. Contamination of microorganisms is a great concern in several areas such as medical devices, health care products, water purification systems, hospital and dental equipments. One possible way to avoid the microbial contamination is to develop materials possessing antimicrobial activities. Consequently, biocidal polymers have received much attention in recent years [15]. New potential candidates are being designed and synthesized due to demand of new biomaterials for a variety of biomedical application. Various amide groups based on polymers and their copolymers forming in nature are being explored to deliver drugs to genetic materials. Thiazole and its derivatives have biological significance, for example it is found in the vitamin B1 molecule and in the coenzyme cocarboxylase [16]. The penicillin molecule also contains a thiazolidine ring. 2-Aminothiazoles are known mainly as biologically active compounds with a broad range of activity and as intermediates in the synthesis of antibiotics and dyes [17]. The homo and copolymerization behavior of methacrylic esters containing functional groups has been investigated in our laboratory [18–20]. To clarify the correlation between the structure and the reactivity of vinyl monomers in their radical polymerizations and copolymerization is a very important step toward control of monomer radical reactivity. The calculation of the monomer-reactivity ratios requires the mathematical treatment of experimental data on the composition of copolymers and monomer in feed mixtures. Copolymerization is the most successful and powerful method for effecting systematic changes in polymer properties [21]. The incorporation of two different monomers, having diverse physical and/or chemical properties, in the same polymer molecule in varying proportions leads to the formation of new materials with great scientific and commercial importance [22, 23]. Knowledge of the copolymer composition is an important step in the evaluation of its utility. Copolymer composition and its distribution are dependent on the reactivity ratios. The most common mathematical model of copolymerization is based on finding the relationship between the composition of copolymers and the composition of the monomer feed in which the monomer reactivity ratios are the parameters to be determined [24, 25]. The calculation of the monomer reactivity ratios requires the mathematical treatment of experimental data on the compositions of copolymers and monomer feed mixtures.
Thermogravimetric analysis (TGA) has been widely used to investigate the decomposition characteristics of many materials. Some methods have already been established to evaluate the kinetic parameters from thermogravimetric data.
With proper experimental procedures, information about the kinetics of decomposition can be obtained and the kinetic data obtained from thermogravimetric (TG) analyses and it may be used as criteria for the choice of a polymer. Thermal analysis methods have proved usefully not only in defining suitable processing conditions for polymers as well as useful service guidelines for their application, but also in drawing information on thermal properties–polymer chain structure relationships. Since thermal stability is connected to both the initial temperature and the rate of degradation of polymers, the determination of kinetic parameters associated with the degradation processes is an interesting topic of research.
No studies on reactivity ratios in the copolymerization of –2-methyl-N-1,3-thiazole-2-acrylamide (TMA) with any commercial monomer are found in the literature. TMA is a methacrylamide monomer having pendant thiazole group. In a previous studies [26, 27], the synthesis, characterizations and copolymerization behaviors of the similar methacrylate monomers were described. In this study, the synthesis, characterization, monomer reactivity ratios, thermal properties and antimicrobial properties of copolymers of 2-methyl-N-1,3-thiazole-2-acrylamide with glycidyl methacrylate were investigated.
Experimental
Materials
2-Aminothiazole, and methacryloyl chloride (Merck) were used as received. 1,4-Dioxane, potassium carbonate, acetonitrile, anhydrous magnesium sulphate (Aldrich) were used as received. All the other chemicals were analytical grade commercial products, and they were used without any further purification. Glycidyl methacrylate (GMA; Merck) was purified by distillation under reduced pressure. Benzoyl peroxide (BPO) was recrystallized from a chloroform–methanol (1:1) mixture.
Monomer synthesis
Synthesis of 2-methyl-N-1,3-thiazole-2-acrylamide was as follows: 2-Aminothiazole (1 mol) and K2CO3 (1 mol) were mixed in 20 ml of CH2Cl2 at 0 °C, and then methacryloyl chloride (1.1 mol) was added drop wise to the solution. The reaction mixture was stirred at room temperature for 24 h. The organic layer was washed several times with diethyl ether and dried over MgSO4. After removing diethyl ether, 2-methyl-N-1,3-thiazole-2-acrylamide (TMA) was crystallized from ethanol. The melting point of TMA was 80 °C, and yield was about 69%.
Elemental analysis (%): C = 49.94 (found), 49.98 (calcd) H = 4.75 (found), 4.79 (calcd) N = 16.59 (found), 16.65 (calcd) S = 19.11 (found), 19.06 (calcd).
IR (neat), cm−l: 1,675 (C=O for amide), 1,628 (CH2=C–), 1,600 (C=C), 3,200 (–NH–)
1H-NMR (δ, ppm from TMS in CDCl3): 7.2–7.5 (aromatic protons, 2H); 5.6 (CH2=, 1H); 6.2 (CH2=, 1H); 10.5 (–NH–, 1H); 1.9 (CH3–, 3H).
13C-NMR (δ, ppm from TMS in CDCl3): 170.0 (C=O of amide); 138.0 (=C); 124.1 (CH2=); 160 (–S–C=N– thiazole ring carbon); 138 (N–C= thiazole ring carbon), 115 (=C–S– thiazole ring carbon) 20.7 (CH3).
Homopolymerization
One gram of the monomer, TMA, and 50 mg of azobisizobutyronitrile (AIBN) were dissolved in 10 ml of 1,4-dioxane in a polymerization tube and oxygen-free nitrogen gas was purged through the solution for 10 min. Then the solution was kept in a thermostated water bath at 60 °C. After 18 h, the polymer was precipitated by adding the reaction mixture to n-hexane. The polymer was purified by repeated reprecipitation of a solution of the polymer in 1,4-dioxane from excess n-hexane. The polymer was filtered and then dried in vacuum at 50 °C. Yield was 72%.
Copolymerization
Copolymerizations of TMA with GMA using different proportions of TMA were carried out in glass ampoules under N2 atmosphere in 1,4-dioxane solution with BPO (1%, based on the total weight of monomers) as an initiator. The reacting components were degassed by threefold freezing–thawing cycle and then immersed in an oil bath at 65 ± 0.1 °C for a given reaction time. The reaction time was selected to give conversions less than 10% to satisfy the differential copolymerization equation [28]. After the desired time the copolymers were separated by precipitation in n-hexane and reprecipitation from CH2Cl2 solution. The polymers were purified by reprecipitation to avoid the formation of homopolymers. The polymers were finally dried over vacuum at 45 °C to constant weight. The amounts of monomeric units in the copolymers were determined by elemental analysis. The results are presented in Table 1.
Characterization techniques
Infra-red spectra were measured on a Perkin Elmer Spectrum BXII FT-IR spectrometer. 1H- and 13C-NMR spectra were recorded in CDCl3 with tetramethylsilane as the internal standart using Gemini Varian 200 MHz spectrometers. The glass transition (Tg) temperatures were determined using a Setaram 131 DSC apparatus. Samples of about 5–8 mg held in sealed aluminum crucibles and the heating rate of 20 °C/min under a dynamic nitrogen flow (5 l h−1) were used for the measurements. The number and weight average molecular weights \(\left( {\overline {M_{\text{w}} } ,\overline {M_{\text{n}} } } \right)\) and the polydispersity index (PDIs) of homopolymers as well as copolymer samples were determined by GPC with polystyrene and tetrahydrofuran as the standard and solvent, respectively.
Determination of the copolymer composition and monomer reactivity ratios
The compositions of the poly(TMA-co-GMA) samples were determined by elemental analysis. The copolymerization behavior of the comonomers can be better understood by the determination of reactivity ratios. The reactivity ratios of TMA with GMA were determined by the application of Fineman–Ross (FR), and Kelen–Tüdös (KT) linearization methods from the monomer feed ratios and the copolymer compositions [31–33]. According to the FR method the monomer reactivity ratios can be obtained by the equation:
where the reactivity ratios, r 1 and r 2 are correspond to the TMA with GMA monomers respectively. The parameters G and H are defined as follows:
with
Where M 1 and M 2 are the monomer molar compositions in feed and m 1 and m 2 are the copolymer molar compositions.
The reactivity ratios can also be obtained using the KT method which is based on the equation:
where η and ξ are functions of the parameters G and H
and α is a constant which equals to (H max × H min)1/2, H max, H min being the maximum and the minimum H values, respectively, from the series of measurements. From a linear plot of η as a function of ξ, the values of η for ξ = 0 and η = 1 can be used to calculate the reactivity ratios according to following equations:
Thermal stability of the polymers
The thermal stabilities of the polymers were investigated by thermogravimetric analysis (TGA) in a nitrogen stream at a heating rate of 10 °C min−1. For the study on the kinetics of thermal degradation of polymers, we can select the isothermal thermogravimetry (ITG) or the thermogravimetry (TG) at various heating rates. ITG is superior for obtaining accurate activation energy for thermal degradation although it is time-consuming. Therefore, in the present work TG curves at various heating rates were obtained and the activation energies (ΔE d) for thermal degradation of polymers were calculated by Ozawa’s plot which is a widely used method. Degradations were performed in the scanning mode from 35 up to 500 °C under nitrogen flow (20 ml min−1) at various heating rates (β: 2.0, 4.0, 7.0, 10.0, 12.5 and 15.0 °C min−1). Samples of 8–12 mg held in alumina open crucibles were used and their weights were measured as a function of temperature and stored in the list of data of the appropriate built-in program of the processor. The TGA curves were immediately printed at the end of each experiment and the weights of the sample were then transferred to a PC at various temperatures.
Antimicrobial activity
The biological activities of the monomers and their homopolymers and copolymers were tested against different microorganisms with DMSO as the solvent. The sample concentrations were 50 and 100 μg. All microorganism strains were obtained from the Culture Collection of Microbiology Laboratory of Afyonkarahisar Kocatepe University (Afyonkarahisar, Turkey). In this study, Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 25922 and Pseudomonas aeruginasa ATCC 27853 were used as bacteria. Candida albicans CCM 31 was a fungus. YEPD medium cell culture was prepared as described by Connerton [40]. Ten milliliters of YEPD medium were inoculated with each cell from plate cultures. Yeast extract 1% (w/v), bactopeptone 2% (w/v), and glucose 2% (w/v) were obtained from Difco. Microorganisms were incubated at 35 °C for 24 h. About 1.5 ml of these overnight stationary phase cultures were inoculated onto 250 ml of YEPD and incubated at 35 °C until OD600 reached 0.5.
The antibiotic sensitivity of the polymers was tested with the antibiotic disk assay as described [41]. Nutrient Agar (NA) was purchased from Merck. About 1.5 ml of each prepared different cell culture were transferred into 20 ml of NA and mixed gently. The mixture was inoculated in to the plate (9 cm, diameter). The plates were rotated firmly and allowed to dry at room temperature for 10 min. The prepared antibiotic discs were placed on the surface of the agar medium [42]. The plates were kept at 5 °C for 30 min and then incubated at 35 °C for 2 days. The antimicrobial concentration of this study is 108 cfu/ml. If a toxic compound leached out from the disc, it means that the microbial growth is inhibited around the sample. The width of this area expressed the antibacterial or antifungal activity by diffusion. The zones of inhibition of microorganism growth of the standard samples monomers, homopolymers and copolymers were measured with a millimeter ruler at the end of the incubation period.
Results and discussion
As shown in Scheme 1, according to the usual method the preparation of TMA monomer was synthesized from 2-amino thiazole with methacryloyl chloride. The monomer was polymerized in 1,4-dioxane solution using AIBN as initiator.
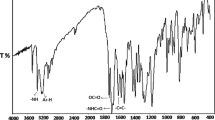
The 13C-NMR and 1H-NMR spectra of poly(TMA) and their attributions are shown in Fig. 1a and b, respectively. The structure of poly(TMA) was confirmed by 1H- and 13C-NMR spectral data.
Structural characterization of the copolymers
Six new copolymers of TMA-co-GMA having different copolymer composition were prepared according to the experimental details given in Table 1 by using BPO as initiator in 1,4-dioxane solution under nitrogen atmosphere. The formula of poly(TMA-co-GMA) is illustrated in Scheme 2.
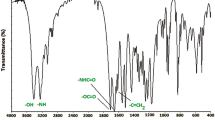
The FT IR spectrum of poly(TMA-co-GMA) [47:53] is shown in Fig. 2. It shows a peak at 3,058 cm−1 corresponding to the aromatic C–H stretching. The asymmetrical and symmetrical stretching due to the methyl and methylene groups are observed at 2,995, 2,948 and 2,892 cm−1. The shoulder at 1,728 cm−1 and peak at 1,680 cm−1 are attributed to the ester and amide carbonyl stretching of both GMA and TMA units. The asymmetrical and symmetrical bending vibrations of methyl groups are seen at 1,453 and 1,380 cm−1, respectively. The symmetrical stretching of the epoxy group is observed at 1,225 cm−1. Another band seen at 908 cm−1 is due to the asymmetric stretching of the epoxy group. The C–O stretching is observed at 1,161 cm−1.
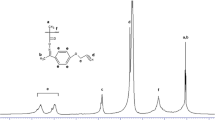
The 1H-NMR spectrum of poly(TMA-co-GMA) [47:53] (Fig. 3) is consistent with its chemical structure. The aromatic protons show signals between 7.1 and 7.8 ppm. The spectrum shows two signals at 4.41 and 3.79 ppm, which are due to –COOCH2– group of GMA units. The peak at 3.16 ppm is due to the methyne proton of the epoxy group. The methylene protons of the epoxy group show signals at 2.78 and 2.51 ppm. The methylene group of the polymer backbone shows a broad signal between 2.25 and 1.55 ppm due to the tacticity. The α-methyl protons of both the monomeric units show signals between 1.55 and 0.82 ppm.
In the 13C-NMR spectrum of copoly(TMA-co-GMA), the resonance signals at 172.1 and 168.4 ppm are due to the ester carbonyl and amide carbonyl carbons, respectively. The signal at 160 ppm assigned to the aromatic carbons attached to the –NH group. The signals at 115.5 and 137.7 ppm arise from two aromatic carbons in the TMA unit. The methyleneoxy group between the carbonyl group and the epoxy group in the GMA unit shows signals at 66.58 ppm. The methyne and methylene carbons of the epoxy group gave signals at 54.91 and 49.23 ppm, respectively. The backbone methylene carbons of the two comonomer units are observed at 46.6 ppm. The signal at 23.2 ppm corresponds to the α-methyl carbons of both types of monomer units.
Molecular weights of the polymers
The PDIs of the polymers are close to 1.6. The theoretical values of PDI for polymers via radical recombination and disproportionation are 1.5 and 2.0, respectively [29, 30]. This suggests that polymers were produced mainly via termination of growing chain by recombination. The results are given in Table 2.
Monomer reactivity ratios
Kelen–Tudos and Fineman–Ross parameters were calculated from the above equations. Typical plots are shown in Figs. 4 and 5, respectively. The following values were found: r 1 = 0.31 ± 0.070, r 2 = 0.27 ± 0.025 (Fineman–Ross); r 1 = 0.360 ± 0.002, r 2 = 0.32 ± 0.065 (Kelen–Tudos). The monomer reactivity ratios (r 1 and r 2) of poly(TMA-co-GMA) are less than 1. This indicates that the system copolymerizes statistically. Comparing TMA to GMA, it should be stated that two monomers show similar copolymerization behavior. The reactivates of these monomers are close together. The product r 1 × r 2 is very much less than 1 suggesting that the system shows strong alternating tendency. Thus, the factors which are general reactivity and alternating tendency are predominant in determining the behavior of monomers in copolymerization.
Glass transition temperatures
The pure poly(TMA) and poly(GMA) show single glass-transition temperatures at about 139 and 74 °C, respectively. In comparison to that of poly(GMA), the shift to higher temperature is also noted for all the copolymers studied and its magnitude is dependent on the increasing in TMA molar fraction in the copolymer chain. This result arises from two factors: (1) the value of Tg of pure poly(GMA) (74 °C) is lower than that of poly(TMA) and (2) the GMA monomer plays a role strictly as an inert diluents segment on the poly(TMA) polymer chain to reduce the strength of its hydrogen bonding. The results clearly indicate that Tg values of copolymers depend on the composition of comonomers and increase with increasing TMA content in the polymer chain. This means that the free volume in poly(GMA) was higher than that in poly(TMA). The thiazole groups are capable of making hydrogen-bonding interactions because of the –NH– unit. The hydrogen bonding causes reducing of flexibility of the chain and the free volume, thus Tg increases. The Tg values of copolymers are between those of the corresponding homopolymers. These values are indicated in Table 2.
Decomposition kinetics
In Fig. 6, the TGA thermograms of polymers are shown. It is clear that two degradation stages for poly(TMA) are observed. The initial decomposition temperatures of copolymers are in the range of 200–231 °C depending on the monomer composition in the feed. As the poly(TMA) mole fraction in the copolymer composition increases, the thermal stability of the copolymers increases, too. The increment in the initial degradation temperature and final degradation temperature with the increase of poly(TMA) mole fraction in the copolymer is due to the increase of thiazole group content in the copolymer. Some degradation characteristics of the copolymers are given in Table 3 by comparison with those of the given homopolymers. The thermal degradation of poly(-N-alkyl methacrylamide)s begins with liberation of ammonia and then the imide group occurs. Grassie et al. [34] reported that ammonia and water are the only volatile products below 340 °C for polyacrylamide. Minsk et al. [35] showed that dry polyacrylamide is stable up to 285 °C, and decomposes above this temperature with liberation of ammonia and formation of an imide group. In this study, the volatiles collected during thermogravimetric runs were analyzed by gas chromatography and the presence of ammonia was verified [36, 37].
According to the method of Ozawa [38], the apparent thermal decomposition activation energy, E d, can be determined from the TGA thermograms under various heating rates such as in Fig. 7, and the following equation:
where R is the gas constant; b, a constant (0.4567); and β, the heating rate (°C/min). According to Eq. 7, the activation energy of degradation can be determined from the slope of the linear relationship between log β and 1/T as shown in Fig. 8 for poly(TMA-co-GMA) and also the ΔE d values for polymers are given in Table 4. E d calculated from the Ozawa method is superior to other methods for complex degradation, since it does not use the reaction order in the calculation of the decomposition activation energy [39].
Antimicrobial effects of the monomers and polymers
The results show that the investigated polymers [except poly(GMA)] have good biological activity comparable to that of standard drugs such as Penicillin G and Teicoplanin. However, the inhibition zone has been exhibited by the polymers either increase or stay constant with the TMA content. Antibiotic discs of the polymers in the culture depend on the disc concentration. This supports the argument that some type of bimolecular binding most probably occurs to the thiazole groups causing the inhibition of biological synthesis and preventing the organisms from reproducing. The result suggests that the TMA monomer, its homo- and copolymers with GMA have more effect on the Escherichia coli microorganism in comparison than standard drugs. In the literature reported that some new methacrylate monomers, polymers and copolymers show good biological activity [43]. The data reported in Tables 5 and 6 represent the average of three experiments.
Conclusions
The synthesis of new methacrylamide monomers (TMA) having pendant thiazole moieties have been reported for the first time. The structure of monomer and their polymer was characterized by spectroscopic methods. Copolymers of this monomer with GMA having different copolymer compositions were prepared. The reactivity ratio values have been discussed. The reactivity ratio values derived from the F–R and K–T methods were in good agreement with each other. The value of the product r 1 × r 2 indicates a strong tendency to alternation. The antimicrobial characterization and thermal stability of the polymers were investigated. The decomposition activation energies of the polymers were calculated with the Ozawa method. GPC data imply that the polydispersity index of the copolymers is nearly equal to 2, and this implies a strong tendency for chain termination by disproportionation. Tg of the copolymers increased with increasing TMA content in the copolymers.
References
Klein J, Heitzmann R (1978) Makromol Chem 179(8):1895
Smith EA, Oehme FWJ (1993) Chromatrog Sci 31(5):192
Teixeira SCS (1995) In: Proceedings of the 3rd Congresso Brasileiro de Polimeros, p 925
Jose L, Pillai VNR (1996) Eur Polym J 32(12):1431
Parada LG, Cesteros LC, Meaurio E, Katime I (1997) Polymer 39(5):1019
Kim SR, Yuk SH, Jhon M (1997) Eur Polym J 33(7):1009
Onda N, Furusawa K, Yamaguchi N, Komuro S (1979) J Appl Polym Sci 23:3631
Halverson F, Panzer HP (1980) In: Grayson M (ed) Encyclopedia of chemical technology. vol. 10. Wiley, New York
Bune YV, Barabanova AI, Bogachev YS, Gromov VF (1997) Eur Polym J 33(8):1313
Gendy TS, Barakot Y, Mohamed AI, Youssef M (1991) Polym Int 24:235
Kalal J (1978) J Polym Sci Polym Symp Ed 62:251
Feit ED, Wurtz ME, Kammlott GW (1978) J Vac Sci Technol 1:944
Kalal J, Svec F, Marousek V (1974) J Polym Sci Polym Symp 47:155
Lee H, Newille K (1967) Handbook of epoxy resins. McGraw-Hill, New York
Worley SD, Sun, G (1996) Trends Polym Sci 32:375
Bayer H (1963) Organic chemistry. Verlag Harry Deutsch, Frankfurt/Main, Zurich, pp 609–610
Ibatullen UG, Petrushina TF, Leitis LY, Minibaev IZ, Logvin BO (1993) Khim Geterotsikl Soedin, p 15
Erol I, Yavuz F, Durgun M (2004) Polymer J 36(4):303
Erol I, Soykan C, Ahmedzade M (2002) J Polym Sci Part A: Polym Chem 40:1756
Erol I (2004) J Polym Sci Part A: Polym Chem 42:3157
Tirrell DA (1985) In: Mark H, Bikales NM, Overberger CG, Menges G (eds) Encyclopedia of polymers sciences and engineering. vol. 4. 2nd edn. Wiley, New York, p 192
Zutty NL, Faucher JA (1962) J Polym Sci 60:536
Rees RW, Vaughan D (1965) J Am Chem Soc Div Polym Chem Polym Prepr 61(1):287
Arshady R, Kenner GW, Ledwith A (1974) J Polym Sci Polym Chem Ed 12:2017
Ham G (1964) Copolymerization, high polymers, vol. 18. Interscience, New York
Azab MM (2005) J Polym Res 12:9
Soykan C, Erol I, Kırbağ S (2003) J App Polym Sci 90:3244
Bilmeyer FW (1984) Textbook of polymer science, 3rd edn. Wiley, New York, pp 119–120
Melville HW, Noble B, Watson WF (1949) J Polym Sci 4:629
Kennedy JP, Kelen T, Tüdös F (1975) J Polym Sci A1:2277
Finemann M, Ross S (1950) J Polym Sci 5:259
Kelen T, Tüdös F (1975) J Macromol Sci Chem A9:1
Tüdös F, Kelen T, Földes-Berezsnich T, Turcsanyi B (1976) J Macromol Sci Chem A10:1513
Grassie N, McNeill IC, Samson JNR (1978) Eur Polym J 14(11):931
Minsk LM, Kotlarchik C, Meyer GN, Kenyon WO (1974) J Polym Sci 12(1):133
e Silva MESR, Dutra ER, Mano V, Machado JC (2000) Polym Deg Stab 67(3):491
Wendlandt WW (1986) Thermal analysis. Wiley, New York, p 57
Ozawa T (1965) Bull Chem Soc Jpn 38:1881
Regnier N, Guibe C (1997) Polym Degrad Stab 55:165
Connerton IF (1994) Analysis of membrane proteins. In: Gould GW (ed) Membrane protein expression systems: a user’s guide. Portland, London, p 177
Chan ECZ, Pelczar MJ, Krieg NR (1993) Agar diffusion method. In: Chan MJ (ed) Laboratory exercises in microbiology. McGraw-Hill, New York, p 225
Desai JA (1996) J Macromol Sci Pure Appl Chem 33:1113
Patel JN, Dolia MB, Patel KH et al (2006) J Polym Res 13(3):219–228
Acknowledgements
This work was supported with 042-FENED09 by Afyon Kocatepe University research found.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erol, I., Poyraz, B., Arif Koroğlu, M. et al. Copolymerization of 2-methyl-N-1,3-thiazole-2-ylacrylamide with glycidyl methacrylate: synthesis, characterization, reactivity ratios and biological activity. J Polym Res 16, 19–28 (2009). https://doi.org/10.1007/s10965-008-9198-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-008-9198-y