Abstract
Densities (ρ), refractive indices (n D) and speeds of sound (u) were measured for the binary mixtures of 2-chloroaniline with butanols (1-butanol, 2-butanol) over the entire range of mole fraction at 303.15, 308.15, 313.15 and 318.15 K under atmospheric pressure. From the experimental data, the values of molar volume (V m), isentropic compressibility (k S ), intermolecular free length (L f), specific acoustic impedance (Z), molar refraction (R m), atomic polarization (P a), polarizability (α), deviation in molar volume (ΔV m), deviation in isentropic compressibility (Δk s ), deviation in intermolecular free length (ΔL f) and deviation in refractive index (Δn D) have been calculated and fitted with Redlich–Kister type polynomial equations by the method of least-squares. The experimental reduced Redlich–Kister deviation properties were also determined, and the results reveal formation of hydrogen bonds between 2-chloroaniline and the butanol mixtures. The formation of hydrogen bonds in the binary mixture systems was further confirmed by FT-IR spectra. The optimized geometry, harmonic vibrational wave numbers and bond characteristics, of pure and equimolar hydrogen bonded complexes, have been calculated theoretically from the ab-intio Hartree–Fock (HF) and density functional theory (DFT-B3LYP) methods with 6-31 + G and 6-311 + G basis sets using Gaussian 09 software.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The hydrogen bond is an important intermolecular interaction in many chemical and biological processes. Hydrogen-bonded complexes in which aromatic amines serve as proton donors have been studied extensively in recent years [1–3]. The formation of a hydrogen bond in solutions and its effect on the physical properties of the mixtures have received much attention. Hydrogen bonding plays an important role in fundamental sciences and in industrial applications. Although many experimental and theoretical studies have been directed towards understanding hydrogen bonding, it remains an area of active research [4]. Knowledge of the physicochemical properties of liquid mixtures formed by two or more components, associated through hydrogen bonds, is important from theoretical and process design aspects. From the theoretical viewpoint, volumetric properties of these mixtures are important sources of information for the characterization of the interactions between the components and they are also useful for understanding liquid state theory. In addition, alcohols and amines are broadly used in a variety of industrial and consumer applications and, hence, information about their physical properties is also of great importance from a practical point of view.
In the present study, the liquids were chosen on the basis of their industrial importance, i.e., 2-chloroaniline is used in petroleum solvents, fungicides, agricultural chemicals, azo dyes, pigments and pharmaceuticals. Butanols are used as hydraulic fluids in pharmaceuticals, medications for animals, manufacturing perfumes and paint removers. 2-Chloroaniline is a polar solvent that is self-associated through hydrogen bonding of their amine group. The amino group in 2-chloroaniline is an electron–donor and the hydrogen atom in the –NH2 group can also play the role of proton–acceptor center, and alcohol molecules are polar and self-associated through hydrogen bonding of their hydroxyl groups.
In the present work, the density (ρ), refractive index (n D) and speed of sound (u) values of 2-chloroaniline + 1-butanol (system 1) and 2-chloroaniline + 2-butanol (system 2), over the entire composition range at 303.15, 308.15, 313.15 and 318.15 K under atmospheric pressure, are reported, and from this experimental data, various acoustical parameters are determined and fitted to Redlich–Kister type polynomial equations by the method of least-squares deviations. Further, computational and spectroscopic studies were carried out to understand the intermolecular interactions in the binary mixture systems under investigation.
2 Experimental and Computational Methods
2.1 Materials
2-Chloroaniline of AR grade was procured from Sigma Aldrich. Butanols (1-butanol, 2-butanol) of AR grade were procured from SD Fine Chemicals, India. All the chemicals were fractionally distilled and dried over 0.4 nm molecular sieves. The mass fraction purities were tested by gas chromatography and are given in Table 1. The purity of the solvents was ascertained by comparing the experimental values of density, refractive index and speed of sound with those reported in the literature [5–10] at temperatures 303.15–318.15 K and the values are given in Table 2. All the binary liquid mixtures were prepared by weighing appropriate amounts of pure liquids on a digital electronic balance (Mettler Toledo AB 135, Switzerland) with an uncertainty of ±0.00001 g, by syringing each component into airtight stoppered bottles to minimize evaporation losses. The estimated uncertainty in mole fraction is ±1 × 10−4.
2.2 Density, Refractive Index, and Speed of Sound Measurements
Density and speed of sound of the pure and binary mixtures were measured by using a digital vibrating-tube density meter and speed of sound analyzer (Anton Paar DSA 5000). The calibration of the equipment was carried out with doubly distilled deionized water in the temperature range 303.15–318.15 K, and were compared with values provided by Anton Parr in the instruction manual. Uncertainties in temperature, density and speed of sound are ±0.01 K, ±2 × 10−6 g·cm−3 and ±0.1 m·s−1, respectively.
The refractive index values of pure and binary liquid mixtures were obtained from refractometer measurements using a M/s ASCO make of Abbe’s refractometer, with the sodium D light as source, at different temperatures. The temperature controller system with a water bath, supplied by M/s Sakti Scientific Instruments Company, India, was used to maintain the constant temperature with an uncertainty ±0.01 K. Uncertainty in the refractive index measurement is ±0.0001. The FT-IR-spectra of pure and equimolar binary mixture systems are recorded in the 400–4,000 cm−1 region on an Agilent Cary 630 FTIR spectrometer at room temperature (298.15 K).
2.3 Computational Details
The optimized geometry, harmonic vibrational wave numbers and bond characteristics, of the pure and equimolar hydrogen bonded complexes, have been calculated theoretically from ab-intio Hartree–Fock (HF) and density functional theory (DFT-B3LYP) [11, 12] methods with 6-31 + G and 6-311 + G basis sets. All the calculations have been carried out using the Gaussian 09 computational package [13].
3 Theory
Using the experimentally measured values of density (ρ), refractive index (n D) and speed of sound (u) the following acoustic, optical and thermodynamic parameters were evaluated:
where K j is the Jacobson’s constant, a temperature dependent constant. Its value is 2.0755 × 10−6 at 303.15 K. Also evaluated are the
where N is the Avogadro number. Also evaluated are the
where x 1 and x 2 are the mole fractions of liquid 1 and liquid 2, respectively.
The deviation values were fitted by the method of nonlinear least-squares to a Redlich–Kister [14, 15] polynomial equation of the type:
where x 1 is the mole fraction of 2-chloroaniline, x 2 is the mole fraction of isomeric butanol, and the subscript i in the equation takes values from 0 to 2. The values of A i are the coefficients obtained by the method of least squares.
The standard deviation (σ) was calculated using the relation,
where N represents the number of experimental points and n represents the number of coefficients. The experimental reduced Redlich–Kister [16, 17] deviation properties \( Q_{j,\exp ,T} (x_{1} ) \) are expressed by Eq. 14,
where j = 1, 2, 3 and 4 denotes ΔV m, Δk S , ΔL f or Δn D.
4 Results and Discussions
The experimental values of density, refractive index and speed of sound for system 1 and system 2 are shown in Figs. 1, 2 and 3, respectively. Density, refractive index and speed of sound are important properties needed to understand the nature of molecular interactions between solute and solvent in the binary mixtures. The density, refractive index and speed of sound values increase with increasing concentration of 2-chloroaniline (x 1), at all temperatures in both systems, indicating the presence of solute–solvent interactions in the binary mixtures [18, 19]. The increasing density reveals that the addition of 2-chloroaniline makes the systems more compact, and thereby it discloses the presence of an attractive type of interaction between the components. As the medium becomes more and more compact, the speed of sound also increases. It is also observed that the density decreases with increasing temperature, because the rise in temperature leads to a less ordered structure and more spacing between the molecules. The refractive index values of the pure components and mixtures decrease with increasing temperature in both systems, which may be attributed to the fact that the variation in refractive index with temperature is compensated by the change in density of the liquid mixtures [20].
The values of molar volume (V m), isentropic compressibility (k S ), intermolecular free length (L f), specific acoustic impedance (Z), molar refraction (R m), atomic polarization (P a), and polarizability (α) for system 1 and system 2, at temperatures (303.15, 308.15, 313.15 and 318.15) K under atmospheric pressure, are reported in Table 3.
The molar volume (V m) values, for both systems, increase with increasing mole fraction of 2-chloroaniline in butanols at all temperatures. It is also found that, in both systems at a fixed mole fraction of 2-chloroaniline, the molar volume increases with increasing temperature. The increase in the molar volume of a system on mixing of the components can be attributed to the dissociation of one component or both components and formation of solute–solvent bonds [21].
The isentropic compressibility (k S) and intermolecular free length (L f) are related with the structural effects and packing phenomena. The decrease in isentropic compressibility, as well as intermolecular free length, with the mole fraction of 2-chloroaniline, in both the systems, shows structural compactness. The degree of compressibility is a measure of the ease with which a system can easily be compressed, i.e., the larger the compressibility the easier it can be compressed due to the presence of more free space between the components. In the present study, in both the systems the variations in k S and L f values indicate complex formation through molecular interactions [22, 23]. The increases in k S and L f with rise in temperature imply the weakening of intermolecular attraction due to thermal agitations.
In the present work, the specific acoustic impedance (Z) values are observed to increase with increasing concentration of 2-chloroaniline, in both the systems, at a given temperature. Such an increment in the values of Z supports the possibility of molecular interactions due to H-bonding between 2-chloroaniline and the butanols [24]. Further, the specific acoustic impedance decreases with increase in temperature at a given composition of the mixture, in both the systems, indicating that the variation in temperature has a significant effect on Z and thereby on the molecular interactions.
The polarizability (α) values of 2-chloroaniline, 1-butanol and 2-butanol at 303.15 K are 1.4150, 0.8789 and 0.8745 cm3, respectively. The obvious conclusion is that, in pure 2-chloroaniline, dispersion forces are overwhelming, whereas in the cases of 1-butanol and 2-butanol, intermolecular forces due to hydrogen bonding and dipole–dipole association strengths are more dominating than the dispersion forces. The dispersion forces exist between all molecules and are resolved by the polarizability of the particles. The polarizability depends on the overall number of electrons and the volume over which they are spread. Polarizability values increase with increasing concentration of 2-chloroaniline (x 1) in the mixtures. In both the systems, the polarizability values of all the mixture concentrations increase with increasing temperature, which can be attributed to the increase in the molar volume with temperature.
The molar refraction (R m) reflects the strength of interaction in the binary mixtures and is a sensitive function of wavelength and mixture composition. The magnitude of the molar refraction increases with increase in mole fraction of 2-chloroaniline (x 1) in both the systems. Since the refractive index is measured in the optical region, the polarizability should not include orientational effects. Therefore, the molar refraction should not depend on temperature over a small temperature range and we can observe this from the values of R m (Table 2).
In order to have a comprehensive picture of the nature of molecular interactions between the components of the liquid mixtures, it is of interest to discuss the same parameters in terms of deviation values [25]. In this work, the deviation in molar volume (ΔV m), deviation in isentropic compressibility (Δk S), deviation in intermolecular free length (ΔL f) and deviation in refractive index (Δn D) values were calculated. These values were fitted to the Redlich–Kister polynomial equation and the results are reported in Table 4. The coefficients A i and the corresponding deviations σ obtained from the least-squares fitting method are given in Table 5.
The deviation in molar volume (ΔV m) is an indicator of different effects [26]. The physical contributions that are nonspecific interactions between the real species present in the mixture give rise to a positive value to ΔV m while chemical or specific intermolecular interactions like charge-transfer and other complex-forming interactions result in a volume decrease giving rise to a negative value to ΔV m. The negative ΔV m values, in both the systems at all temperatures, indicate that the mixtures have a compact structure. Further, it is observed that these deviation values become more negative with increase in temperature. Because increasing temperature promotes the breaking of associated species present in the pure liquids thus releasing more and more free dipoles of unlike molecules in the mixture, which in tern form greater number of hydrogen bonds among the interacting molecules, as a result the deviation values become more negative.
The deviation in isentropic compressibility (Δk S ) values, which are different from the excess isentropic compressibility (\( k_{S}^{\text{E}} \)), are negative over the entire composition range for both the systems at all temperatures. These negative values of Δk S suggest that the liquid mixtures are less compressible than the pure liquids, indicating that the solution and molecules in the mixture are more tightly bound in the liquid mixtures than in the corresponding pure liquids. According to Fort and Moore [27], negative deviations in isentropic compressibility are an indication of strong heteromolecular interactions in the liquid mixture and are attributed to charge transfer, dipole–dipole, and dipole-induced-dipole interactions and hydrogen bonding between unlike components.
The deviations of intermolecular free length (ΔL f), in both systems, are negative over the entire range of composition at all temperatures. The large negative values in the middle composition range indicate structural readjustments in the liquid mixtures towards a less compressible fluid phase and closer packing of molecules [28]. Thus, the negative values of deviation in intermolecular free length indicate the strengthening of hydrogen bonding between 2-chloroaniline and butanol (1-butanol/2-butanol) molecules. The positive values of refractive index deviations are due to strong specific forces between molecules, such as hydrogen bonding between the constituent molecules [14].
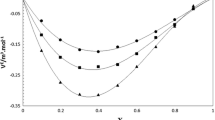
The experimental reduced Redlich–Kister deviation properties \( Q_{j,\exp ,T} (x_{1} ) \) of the deviation in molar volume, deviation in isentropic compressibility, deviation in intermolecular free length, and deviation in refractive index values are shown in Figs. 4, 5, 6 and 7, for systems 1 and 2. For both systems, changes in curvature are found in the reduced Redlich–Kister deviation in molar volume with mole fraction (x 1) are due to hydrogen bonding between unlike molecules of the mixtures, leading to strong intermolecular correlations. The increasingly negative values of the reduced deviation in molar volume with increasing temperature (Fig. 4) can be explained by considering the differences in the molar volumes of the two liquids at different temperatures, and this raises the possibility that the smaller sized molecules of butanol (1-butanol/2-butanol) fit into the voids created by the larger molecules of 2-chloroaniline. Also, significant changes in curvature for the reduced Redlich–Kister deviation in isentropic compressibility and deviation in intermolecular free length with mole fraction (x 1) are observed for both systems (Figs. 5 and 6). From analysis of these results, it is clear that the complexity of cluster formation and mutual solvation are reflected in the results of the reduced functions. The reduced deviation in refractive index values decrease with mole fraction (x 1), pass through a localized minimum at x 1 = 0.8, and then increase continuously in going to the 2-chloroaniline-rich region (Fig. 7). This trend suggests that molecular interactions are present in the mixture containing two liquids of different size and polarity [16].
Experimental reduced Redlich–Kister deviation properties \( Q_{1,\exp ,T} (x_{1} ) \) for the ratio \( \frac{{\Delta V_{\text{m}} }}{{x_{1} (1 - x_{1} )}} \) of the deviation in molar volumes (Eq. 14) with mole fraction (x 1) in a 2-chloroaniline + 1-butanol and b 2-chloroaniline + 2-butanol binary mixtures at temperatures T = 303.15, 308.15, 313.15, and 318.15 K
Experimental reduced Redlich–Kister deviation properties \( Q_{2,\exp ,T} (x_{1} ) \) for the ratio \( \frac{{\Delta k_{s} }}{{x_{{_{1} }} (1 - x_{{_{1} }} )}} \) of the deviation in isentropic compressibility (Eq. 14) with mole fraction (x 1) in a 2-chloroaniline + 1-butanol and b 2-chloroaniline + 2-butanol binary mixtures at temperatures T = 303.15, 308.15, 313.15, and 318.15 K
Experimental reduced Redlich–Kister deviation properties \( Q_{3,\exp ,T} (x_{1} ) \) for the ratio \( \frac{{\Delta L_{\text{f}} }}{{x_{1} (1 - x_{1} )}} \) of the deviation in intermolecular free length (Eq. 14) with mole fraction (x 1) in a 2-chloroaniline + 1-butanol and b 2-chloroaniline + 2-butanol binary mixtures at temperatures T = 303.15, 308.15, 313.15, and 318.15 K
Experimental reduced Redlich–Kister deviation properties \( Q_{4,\exp ,T} (x_{1} ) \) for the ratio \( \frac{{\Delta n_{\text{D}} }}{{x_{1} (1 - x_{1} )}} \) of the deviation in refractive index (Eq. 14) with mole fraction (x 1) in a 2-chloroaniline + 1-butanol and b 2-chloroaniline + 2-butanol binary mixtures at temperatures T = 303.15, 308.15, 313.15, and 318.15 K
The hydrogen bonding distances and the corresponding angles can be considered as a criterion of the strength of hydrogen bonding [29]. The geometrical parameters, i.e., bond length values of pure compounds and equimolar binary mixtures from DFT (B3LYP) with 6-311 + G basis set, are shown in Figs. 8 and 9. The variations in bond length values of the equimolar binary mixture systems, compared to their monomers, clearly indicate the formation of hydrogen bonds between the complexes.
In order to examine the presence of N–H···O–H bonded complexes and the strength of molecular association at equimolar concentration, for both systems. The infrared spectra were recorded at room temperature (298.15 K). Observing the experimental FT-IR spectra for the equimolar binary mixture of system 1 (2-chloroaniline + 1-butanol), there is a shift of 8 cm−1 in wave number in the position of –NH and 22 cm−1 in the position of –OH for the mixture relative to the pure compound spectra (Fig. 10). Similarly, the FT-IR spectra for the equimolar binary mixture of system 2 (2-chloroaniline + 2-butanol), there is a shift of 11 cm−1 in the position of –NH and 4 cm−1 in the position of –OH, compared with their respective pure compound spectra (Fig. 11). These shifts are caused by strong intermolecular interactions like hydrogen bonding between the oxygen in the hydroxyl group of butanols (1-butanol/2-butanol) and the hydrogen of 2-chloroaniline. Thus, the FT-IR analysis convincingly shows intermolecular hydrogen bonding for the equimolar binary mixture in both systems, with proportionate variations in the stretching wave numbers of –NH and –OH compared to their respective pure components [30]. The comparison of experimental and theoretical (scaled down) FT-IR wave numbers is provided in Table 6 and the obtained theoretical values are in reasonable agreement with the experimental values [31, 32]. The optimized geometrical structures, representing the formation of hydrogen bonding obtained from density functional theory (DFT–B3LYP) method with the 6-311 + G basis set calculation using Gaussian 09 software, are shown for system 1 and system 2 in Fig. 9a, b, respectively.
The Appendix lists the experimental values of density, refractive index and speed of sound.
5 Conclusions
Physicochemical properties like density, viscosity and speed of sound of the binary mixtures containing 2-chloroaniline and butanols (1-butanol/2-butanol), over the entire range of mole fraction at temperatures T = (303.15 to 318.15) K under atmospheric pressure, were measured. From the experimental data, various acoustical parameters were determined and fitted to Redlich–Kister type polynomial equations by the method of least-squares. The sign and magnitude of these quantities indicate the formation of hydrogen bonds. The FT-IR spectra confirm the formation of intermolecular hydrogen bonds between the hydrogen of 2-chloroaniline and hydroxyl group of butanols (N–H···O–H). Further, computational studies were carried out to understand the intermolecular interactions in the binary mixture systems under investigation.
References
Todres, Z.V.: Effects of Hydrogen Bonding. Organic Chemistry in Confining Media, pp. 89–102. Springer International Publishing, Berlin (2013)
Zaitseva, K.V., Varfolomeev, M.A., Novikov, V.B., Solomonov, B.N.: Enthalpy of cooperative hydrogen bonding in complexes of tertiary amines with aliphatic alcohols: calorimetric study. J. Chem. Thermodyn. 43, 1083–1090 (2011)
Vijaya Krishna, T., Madhu Mohan, T.: Study of molecular interactions in the polar binary mixtures of N-methyl aniline and alcohols using excess dielectric and thermodynamic parameters. J. Chem. Thermodyn. 47, 267–275 (2012)
Bricknell, B.C.: Experimental and Theoretical Studies of Hydrogen Bonding. University of Natal, Durban (1995)
Joshi, S.S., Aminabhavi, T.M.: Excess Volumes of binary mixtures of anisole with bromobenzene, o-dichloro-benzene, o-chloroaniline and p-dioxane at 298.15, 303.15, 308.15 and 313.15 K. Fluid Phase Equilib. 60, 319–326 (1990)
Jeevanandham, P., Kumar, S., Periyasamy, P.: Densities, viscosities, refractive indices and excess properties of ortho-and meta-chloroaniline with 2-alkoxyethanols at 303.15 K. J. Mol. Liq. 188, 203–209 (2013)
Pandiyan, V., Oswal, S.L., Malek, N.I., Vasantharani, P.: Thermodynamic and acoustic properties of binary mixtures of ethers. V. Diisopropyl ether or oxolane with 2-or 3-chloroanilines at 303.15, 313.15 and 323.15 K. Thermochim. Acta 524, 140–150 (2011)
Nain, A.K.: Densities and volumetric properties of binary mixtures of formamide with 1-butanol, 2-butanol, 1,3-butanediol and 1,4-butanediol at temperatures between 293.15 and 318.15 K. J. Solution Chem. 36, 497–516 (2007)
Spasojevic, V.D., Djordjevic, B.D., Serbanovic, S.P., Radovic, I.R., Kijevcanin, M.L.: Densities, refractive indices, viscosities, and spectroscopic study of 1-amino-2-propanol + 1-butanol and + 2-butanol solutions at (288.15 to 333.15) K. J. Chem. Eng. Data 59, 1817–1829 (2014)
Nain, A.K., Srivastava, T., Pandey, J.D., Gopal, S.: Densities, ultrasonic speeds and excess properties of binary mixtures of methyl acrylate with 1-butanol, or 2-butanol, or 2-methyl-1-propanol, or 2-methyl-2-propanol at temperatures from 288.15 to 318.15 K. J. Mol. Liq. 149, 9–17 (2009)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Lee, C., Yang, W., Parr, R.G.: Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li., X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, Toyota, M. K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.A., Jr., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E.K. Kudin, N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09, Revision A.02. Gaussian, Inc., Wallingford, CT (2009)
Redlich, O., Kister, A.T.: Algebraic representation of thermodynamic properties and the classification of solutions. J. Ind. Eng. Chem. 40, 345–348 (1948)
Dean, J.A.: Lange’s Handbook of Chemistry. McGraw-Hill, New York (1956)
Desnoyers, J.E., Perron, G.: Treatment of excess thermodynamic quantities for liquid mixtures. J. Solution Chem. 26, 749–755 (1997)
Das, D., Ouerfelli, N.: The relative reduced Redlich-Kister and Herráez equations for correlating excess properties of N,N-dimethylacetamide + formamide binary mixtures at temperatures from 298.15 K to 318.15 K. J. Solution Chem. 41, 1334–1351 (2012)
Ramana, G.V., Raj Gopal, E., Murthy, N.M.: Ultrasonic studies on dilute solutions of water in n-alcohols and 2-alkoxyethanol. Indian J. Pure Appl. Phys. 43, 259–264 (2005)
Glinski, J.: Additivity of sound velocity in binary liquid mixtures. J. Solution Chem. 31, 59–70 (2002)
Beatriz, G., Villares, A., Lopez, M.C., Royo, F.M., Lafuente, C.: Refractive indices and molar refractions for isomeric chlorobutanes with isomeric butanols. Phys. Chem. Liquids 43, 13–23 (2005)
Thirumaran, S., George, D.: Ultrasonic study of intermolecular association through hydrogen bonding in ternary liquid mixtures. ARPN J. Eng. Appl. Sci. 4, 1–11 (2009)
Rama Rao, G.V., Sarma, V.A., Rambabu, C.: Excess volumes, deviation in viscosities and compressibilities of binary mixtures consisting of morpholine, piperidine with methanol and pyridine at different temperatures. Indian J. Pure Appl. Phys. 42, 820–826 (2004)
Kannappan, V., Jaya Santhi, R.: Ultrasonic investigation of induced dipole-induced dipole interactions in binary liquid mixtures at 298 K. Indian J. Pure Appl. Phys. 44, 815–819 (2006)
Thirumaran, S., Murugan, R., Prakash, N.: Acoustic study of intermolecular interactions in binary liquid mixtures. J. Chem. Pharm. Res. 2, 53–61 (2010)
Wypych, G.: Handbook of Solvents. Chem Tec Publishing, Ontario (2001)
Nikam, P.S., Kharat, S.J.: Excess molar volumes and deviations in viscosity of binary mixtures of N,N-dimethylformamide with aniline and benzonitrile at (298.15, 303.15, 308.15, and 313.15) K. J. Chem. Eng. Data 48, 972–976 (2003)
Fort, R.J., Moore, W.R.: Adiabatic compressibilities of binary liquid mixtures. Trans. Faraday Soc. 61, 2102–2111 (1965)
Oswal, S.L., Patel, N.B.: Speed of sound, isentropic compressibility, viscosity, and excess volume of binary mixtures. 2. Alkanenitriles + dimethylformamide, + dimethylacetamide, and + dimethyl sulfoxide. J. Chem. Eng. Data 45, 225–230 (2000)
Karthika, M., Senthilkumar, L., Kanakaraju, R.: Theoretical studies on hydrogen bonding in caffeine–theophylline complexes. Comp. Theoretical Chem. 979, 54–63 (2012)
Silverstein, R.M., Bassler, G.C., Morrik, T.C.: Spectroscopic Identification of Organic Compounds, 5th edn. Wiley, Singapore (1991)
Pathak, S., Kumar, A., Tandon, P.: Molecular structure and vibrational spectroscopic investigation of 4-chloro-4′dimethylamino-benzylidene aniline using density functional theory. J. Mol. Struc. 981, 1–9 (2010)
Mohan, T.M., Sastry, S.S., Murthy, V.R.K.: Conformational and dielectric relaxation studies on hydrogen bonded binary mixture of isopropyl alcohol in methyl benzoate and ethyl benzoate. J. Mol. Struc. 973, 157–162 (2010)
Acknowledgments
The authors are thankful to the Managements of Vignan Institute of Technology & Science, Hyderabad, and Vasireddy Venkatadri Institute of Technology, Guntur, for their encouragement towards the present research work. One of the authors (M. Chandra Sekhar) is thankful to Dr. M. Venkataramana, Principal, Vignan Institute of Technology & Science, Deshmukhi, for his moral support in all aspects of the research work. They are also thankful to Dr. R. L. Gardas, Department of Chemistry, Indian Institute of Technology, Chennai, for his valuable suggestions and moral support in all aspects of the research work.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Experimental values of the densities, speeds of sound and refractive indices (Table 7).
Rights and permissions
About this article
Cite this article
Chandra Sekhar, M., Madhu Mohan, T., Vijaya Krishna, T. et al. Density, Refractive Index, Speed of Sound and Computational Studies of Intermolecular Interactions in Binary Mixtures of 2-Chloroaniline with Butanols (1-Butanol, 2-Butanol) at T = (303.15–318.15) K. J Solution Chem 44, 237–263 (2015). https://doi.org/10.1007/s10953-015-0306-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0306-4