Abstract
We report here normal and superconducting properties of Bi1.7Pb0.30Sr2Ca1−xLaxCu2Oy superconductor with various x values (0.00 ≤ x ≤ 0.30). It is found that the replacement of Ca2+ by La3+ does not influence the phase purity of the samples, while the orthorhombic distortion, excess oxygen, effective Cu valance, and hole carriers/Cu ions are affected. Further, the doping distance and crystal geometry factor are decreased by La, but the distance between neighboring Cu atoms and the density of excess doping are increased. Furthermore, the DTA graphs show strong endothermic peaks at a temperature of 844.1, 848.03, 850.10, and 857.41 °C for pure and La-doped samples, respectively. The thermogravimetric analysis diagrams displayed a sharp mass loss started at about 800 °C and extended up to 1000 °C for all samples. Although the critical temperature Tc of the samples is decreased by La from 94 K to 83, 63, and 51 K, the critical concentration for quenching superconductivity can be extended above 0.30. Moreover, an inverse linear relation between Tm and Tc, given by Tc (K) = − 2.544 Tm (°C) + 2272.1, is also estimated. On the other hand, the Vickers hardness Hv is increased by La up to 0.30 (Hv α Tm, Hv α (1/Tc)), but it is decreased by the applied load F. The Hv-F characters are divided according to the applied load into two parts: the first at lower loads (0.00–1.962 N) and the second at higher loads (2.942–4.904 N). It is noted that the surface energy, elastic indentation, and resistance pressure are increased by La, but their values at higher loads are generally more than that of lower loads. Our results are discussed in terms of the balance between the hole carriers lost by La3+ with that introduced by excess oxygen in the Cu-O2 planes, which alters the quenching of superconductivity by La3+ and highlights the present work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The orthorhombic unit cell of Bi:2212 with a~b = 0.544 nm and c = 3.090 nm consists of two units of (Bi,Pb)2Sr2CaCu2O8 and 15 layers. The chemical formula of 8-oxygen atoms (8O2−) needs 16 electrons at least provided by 2Bi3+ + + + 2Sr2+ + 1Ca2+ + 2Cu2+ ions. To be superconductor, Bi (Pb):2212 compound should be over doped with an excess oxygen atom δ in the range of (0.10–0.23) [1]. The extra atom requires two more electrons through transforming 2Cu2+ ions into 2Cu3+ ions, and therefore, the electronic arrangement in the unit cell becomes 2Bi3+ + 2Sr2+ + 1Ca2+ + 2Cu3+ + 9O−2.
The Bi (Pb):2212 superconducting systems with critical temperature Tc of 87 K have been early used in the fabrication of wires and tapes due to their high critical magnetic fields and critical currents [2, 3]. Bi (Pb):2212 phase is thermodynamically stable against cooling over a wide range of temperature up to 300 K [3, 4]. The partial replacement of Bi by Pb in BSCCO system has been used to stabilize the purity and formation of original 2212 phase and also to improve its superconducting critical parameters. However, the highest values of critical temperature Tc, critical field, and critical current have been obtained at Pb = (0.20–0.30) [1,2,3]. Usually, Bi:2201 phase tends to co-exist with Bi:2212 phase during synthesis, but the calcination process helps for increasing the amount of the Bi:2212 as a majority phase and depresses the amount of Bi:2201 as a minority phase [5].
The melt process is considered as the highly essential method for the behavior of thermal optimization of superconductors through solid-liquid phase diagram. Furthermore, the transition points such as melting, sublimation, and solidification can be obtained. Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) are considered the best methods for identifying the above points [6]. TGA is a powerful technique to explore the thermal stability of matter through accurately monitoring the weight of the sample against heating at constant rate. The change in the sample weight during thermal treatments is attributed to the material response as a result of thermal stress. This process can be considered as the perfect way for exploring, in detail, decomposition temperatures and ensuring a material performs adequately in a given temperature range. DTA is used for measuring the temperature difference between the sample and the standard reference for obtaining solid-liquid phase diagram. However, DTA up to 1000 °C with 10 °C min−1 heating rate has been used early to investigate the amount of Bi (Pb):2212 phase relative to the amounts of the other phases such as Bi (Pb):2212 phase. The DTA shows that the structure of Bi (Pb):2212 phase is more stable and can be easily prepared as stable pure phase as well as Bi (Pb):2201 phase [7].
The superconductivity of Bi (Pb):2212 system may be directly influenced by the substitution in the CuO2 planes itself or indirectly in place of Ca2+ sites through affecting the charge transfer to or from that planes. However, numerous reports have been carried out on the effect of RE3+ substitutions in place of Ca2+ in Bi (Pb):2212 system (RE3+ rare earth element in lanthanide series) [8,9,10,11,12,13,14,15]. They approved that the replacement of Ca2+ by RE3+ in that systems decreased the hole carrier concentration, and consequently, their normal and superconducting properties are affected. It is also concluded that even though RE3+ improves the structural stability of the system, the superconducting critical parameters show a degrading tendency as the substitution content increases up to critical concentration xc required for quenching superconductivity [16,17,18]. It is also found that xc is decreases as the ionic size of RE3+ increases towards La ion in lanthanum series, while few of the reports show an improvement for these critical parameters by partial substitution of Y into Ca, Ba, and Bi sites, respectively [19,20,21]. Unfortunately, most of these reports have been focused on the RE3+ elements which already show superconductivity in the RE: 123 systems such as Y, Gd, Sm, and Nd elements, and few of them are based on La element due to poor superconductivity of La: 123. Furthermore, the superconductivity is completely quenched at xc = 0.25 for substitution of La in place of Ca in (Bi,Pb):2212 systems, but it is increased above 0.25 for the other RE elements such as Y, Gd, and Nd [22,23,24,25,26,27]. Although the substitution of La3+ at Ca2+ sites in Bi (Pb):2212 shows a depression for superconducting parameters up to 0.25, a question mark should be taken on the Tc variations by the amount of such substitution. Anyhow, the answer is still unclear and needs to pay more attention, especially with systematic La content up to 0.30.
The evaluation of mechanical properties, such as the hardness, elastic modulus, yield strength, fracture toughness, brittleness index, and ductility, is required for superconducting industrial applications such as quasi-permanent magnets and current loads. According to the geometry of indenter and size of applied load, Vickers microhardness has been used to investigate the mechanical properties of solids in the form of bulk samples. Generally, indentation size effect (ISE) occurs when the microhardness decreases as the applied load increases [28, 29], while the reverse indentation size effect occurs when the microhardness increases with the applied load [30,31,32]. Generally, the high Tc superconductors have relatively poor mechanical properties, which limit as possible their practical applications. One of the important strategies to improve these properties is the substitution process. For example, it is found that RE3+ substitution in place of Ca2+ improves the connection between superconducting grains, and consequently, the mechanical resistance has increased [33,34,35].
As a continuation of the above work, and to understand more about the influence of La3+ substitution at Ca2+ site in Bi1.7Pb0.30Sr2Ca1−xLaxCu2Oy samples, a systematic study on the structural, DTA, TGA, mechanical, and superconducting analyses with various x values (0.00 ≤ x ≤ 0.30) is reported. On the light of the present work, it is approved that although the superconducting critical temperatures are decreased by La, the xc for quenching superconductivity can be extended above 0.30. Furthermore, the decomposition temperature, hole carries/Cu ions, and Vickers hardness are increased by La up to 0.30. However, these findings are discussed in terms of the correlation between the hole carriers lost by La with that introduced due to excess oxygen resulting for increasing the xc and solubility of La3+, which highlights the present work.
2 Experimental Details
The Bi2O3, PbO, SrO, La2O3, CaCO3, and CuO oxides and carbonates of 4N purity (Bi,Pb)2Sr2Ca1−xLaxCu2Oy) are thoroughly mixed in required proportions and calcined at 825 °C in air for 24 h. This exercise is repeated three times with intermediate grinding at each stage. The resulting powder was reground, mixed, and pressed into pellets at a force up to 10 tons and sintered in air at 840 °C for 36 h and left in the furnace for slow cooling to room temperature by a rate of 5°/min. For more and more optimization of excess oxygen in the samples, the pellets are annealed in air at 850 °C for 20 h and left in the furnace for slow cooling to room temperature. The phase purity of the samples in powder form is examined by using X-ray diffraction (XRD). DTA and TGA of the samples in powder form are performed in the temperature range (30–1000 °C) with heating rate of 10 °C/min. The melting behaviors of the samples are analyzed by Schimadzu differential thermal analysis-50 (DTA). The electrical resistivity of the samples is obtained using the standard four-probe technique in closed cycle cryogenic refrigerator within the range of 18–300 K (Displex) employing helium gas. A 1.0-mA dc current, I, is supplied by a digital Keithley 6221 constant current source, and the dc voltage drop, V, is measured by using dc Keithley 181 digital nanovoltmeter in the normal and reverse directions across the samples. A calibrated chromel versus gold with 0.07 at % iron (Kp-Au 0.07 at % Fe) thermocouple is used for recording the temperature of the sample. The electrical resistivity of the samples is obtained using the relation,\( \rho =\frac{Vwt}{IL} \), where w, L, and t are the samples width, length, and thickness, respectively. Finally, the Vickers microhardness of the samples at room temperature is determined using manual microhardness tester model IN-412A with an applied loads of (0.49–10 N) for 10 s.
3 Results and Discussion
3.1 Structural Analysis
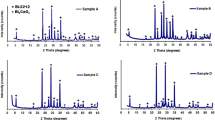
The calculated and measured values of density ρth and ρexp for the samples are listed in Table 1. It is clear that ρth and ρexp are gradually increased by La, but the values of ρexp are higher than that of ρth. The increase of ρ by La may be related to the amount of excess oxygen produced during sample synthesis. The porosity of the samples is calculated using the following relation: PS = [1 − (ρexp/ρth)] and is listed also in Table 1 [6, 36]. The values of PS are gradually decreased by La, which is probably related to decreasing the number of pores due the effective hole carriers as a result for La substitution. It is evident from XRD pattern shown in Fig. 1 that all samples are single phase and free from any impurity phases. The obtained peaks are identified by 2212 (hkl)H and 2201 (hkl)L as majority and minority superconducting phases, respectively [37, 38]. The hkl peaks of high intensities indicated by H belong to the studied Bi (Pb):2212 high phase (Tc = 87 K), while the hkl peaks of lower intensities indicated by L belong to Bi (Pb):2201 low phase (Tc = 23 K). However, the composition of low 2201 superconducting phase is (Bi,Pb)2Sr2CuO6, and it is normally formed as minority phase and cannot be consider as impurity phase as well as the minority 2212 phase formed in 2223 phase. However, there are only four peaks formed and related to 2201 such as (113), (115), (019), and (033).
The volume fraction of the Bi (Pb):2212 superconducting phase (V2212) of the samples is determined by using the following relation [39, 40]:
where I2212, I2201, and Iother are the intensities of peaks for 2212, 2201, and impurity phases, respectively. As listed in Table 1, a little bit decrease of the V2212 by La up to 0.15 followed by an increase with further more increase of La up to 0.30 is obtained. The V2212% of the samples is 92.8%, 92.3%, 92.3%, and 96%, respectively.
The average crystallite diameter Dhkl is evaluated by the following Scherer equation [41,42,43]:
where λ is the X-ray wavelength (λ = 1.5418 Ǻ), ∆θ is the half maximum line width, θ is the Bragg angle, and k is constant (k = 0.93 for most of ceramic materials). By using the Lorentz square method, values of Dhkl listed in Table 1 are 18.76, 22.87, 19.03, and 25.93 nm for pure and La samples, respectively. The dislocation density (β) calculated by, β = 1/D2, and listed in Table 1 is between 0.0029 and 0.0015 for all samples, which indicates that the samples have very few lattice defects and good crystalline quality as obtained by XRD [44].
The variations of lattice parameters a, b, and c listed in Table 1 indicated that b and c/a are slightly decreased by La up to 0.15 followed by an increase at 0.30, while a little bit simultaneous expansion along the c-axes is obtained followed by a decrease at 0.30. The behavior of orthorhombic distortion OD = (b − a)/a) listed in Table 1 is similar to b and c/a variations. This is due to the difference between the ionic radii between La3+ ions (1.18 Ǻ) and Ca2+ (1.12 Ǻ) at the same 8-fold coordination. However, the unusual behavior for the above parameters against La content is often related to the excess of oxygen inserted into BiO layers and also the Cu-O bonding in the Cu-O planes as reported elsewhere [20, 22, 45]. The ratio (c/a) is generally used to characterize the Jahn-Teller distortion of the oxygen octahedron around Cu2+, and therefore, a systematic change of c/a by La content indicates that La release the Jahn-Teller distortion of the CuO6 octahedron markedly [46, 47].
The oxygen content y of superconducting systems can be obtained by titration in terms of a parameter H using the following formula [48]:
where V1, V2, m1, and m2 are the volumes of Na2S2O3 solutions and masses of the sample during the first and second titration, respectively. The values of y listed in Table 2 for the samples are increased from 8.096 for pure sample to 8.168, 8.473, and 8.575 for La samples, respectively. This behavior indicated that La helps for more excess of oxygen to the La free system. On the other hand, the effective Cueff valance can be also obtained with the help of the valence state of the composition elements (Bi+3, Pb+2, Sr+2, Ca+2, La+3, and O−2) as 3 × 1.7 + 2×0.3 + 2 × 2 + 2(1 − x) + 3x + 2Cueff = 2y, using the following relation:
where x is the La content. The values of Cueff listed in Table 2 are also increased by La as well as y. It is increased from 2.246 for pure sample to 2.293, 2.548, and 2.575 for La samples, respectively.
However, the formation of 2212 high Tc requires the existence of Cu3+ ions in the form of excess oxygen in/or near to the CuO2 planes [26, 49, 50]. It is believed that the Coulomb potential caused by the excess oxygen atoms pins the doped holes in the CuO2 planes so as to minimize their total energy [51]. It is found that the excess oxygen atoms introduced in the system have been transformed into a distance assuming a uniform density of excess oxygen positions in the CuO2 planes. This has been done by representing a unit area for one doping element as a square planar configuration. The considered unit area is formed by four doping elements at each corner and having ∑ elements. The density of excess doping Σ−1 in Bi:2212 system is given by Σ−1 = (1 − 8y−1) relation, where ∑ is the doping density [52, 53]. The excess oxygen can be also converted into a doping distance d given by \( d=a\sqrt{\varSigma } \). Furthermore, the distance z between any two neighboring Cu atoms in the designated direction can be calculated in terms of a parameter by \( z=\sqrt{\left[{(2a)}^2+{a}^2\right]}=a\sqrt{5} \). Moreover, the crystal geometry factor (CGF) of the superconducting planes area can be obtained by the following [51,52,53]:
where n is the number of CuO2 planes (n = 2 for 2212) and meff is the effective mass of the hole-doped cuprates (meff = 2me = 18.2 × 10−31 kg). We therefore simply apply the above relations for the samples to check the response of La to the pure system. The values of d, ∑−1, z, and CGF listed in Table 2 indicated that d and CGF are decreased by La, while z and ∑−1 are slightly increased. Decreasing the doping distance d along with increasing the density of excess doping z by La is considered the good evidence for increasing the hole carrier concentration of the system.
3.2 DTA and TGA
Figure 2a shows the DTA patterns of the pure and La samples. It is evident from the figure that with increasing temperature above RT, there are some exothermic and endothermic peaks observed and gradually shifted to higher temperature as La increases. They occurred at temperature ranges of 30–200 °C, 200–450 °C, 450–700 °C, and 650–800 °C for the samples, respectively. This is corresponding to crystallizations of 2201, 2212, and (Sr,Ca)2CuO3 phases, and also due to transformation of 2201 phase to 2212 phase. With increasing temperature at about 800 °C, the tendencies of 2201 crystallization phase decrease and completely disappear. Interestingly, the strong endothermic peaks occurred only at a temperature of 844.1, 848.03, 850.10, and 857.41 °C, and no any other strong peaks could be observed which reinforce the purity of the samples as indicated by XRD. This is due to decomposition reaction of 2212 superconducting phase, and it is usually attributed to the increase of optimum temperature of the mixture due to La, which facilitates the growth of 2212 phase. Such observed increase in Tm emphasizes the role of La addition on the internal structure of the examined samples. Furthermore, the endothermic heats recorded at the endothermic peaks listed in Table 2 are decreased from 2.15 J for pure sample to 1.95, 1.7, and 0.66 J for La samples, respectively. This obtained result claimed that the 2201 phase melts above 800 °C, and after that, it decomposes into 2212 phase, as reported elsewhere [54, 55]. These results indicated that La improves the melting point of 2212 without producing any other additional compound.
The mass loss was determined by TGA shown in Fig. 2b indicating that the samples (x = 0.00, 0.05, and 0.15) displayed a gradual quite of mass loss starting above a temperature of 30 °C to about 450, 600, and 800 °C, followed by a mass gain and then followed by a sharp mass loss at about 800 °C. However, the period of mass gain completely disappears for La = 0.15 sample. But with increasing La up to 0.30, a gradual mass gain occurs with increasing temperature form 35 °C up to 500 °C, followed by a sharp mass loss up to 1000 °C. It is obvious that gradual mass loss is due to de-oxidization, while the mass gain is due oxidation. It is clear that La has an effect on the starting temperature of mass loss and also mass gain. The temperature at which the mass gain begins is shifted to higher values as La increases up to 0.15. Anyhow, a sharp mass gain close to 800 °C may be due to the decomposition temperature as indicated by DTA analysis. Increasing the mass loss/mass gain by La is related to the excess of oxygen as discussed above. Consequently, the sample takes more oxygen atoms to fill these oxygen deficiencies [6, 7, 56]. Interestingly, the oxygen content y of the samples is calculated for Y:123 with the help of TGA analysis using the following empirical relation [57];
where α is a parameter (α = 3.5) for Y:123 systems, x is the doping content, m is the mass of the sample, ℓ is the average mass loss, and M is the atomic weight. Substituting by the values of ℓ, M, and m deduced from TGA, and y from titration method, we found that α = 6.5 for 2212 system.
3.3 Resistivity Measurements
Figure 3 depicts the resistivity versus temperature for pure and La samples. From this figure, it can be observed that the resistivity decreases almost linearly from room temperature and beyond which it turns to zero at a critical temperature Tc. It is also noted that all samples exhibit a clear metallicity and the linear part of curves from RT down to onset temperature has a positive slope dρ/dT and its extrapolation to T = 0 K provides the residual resistivity ρo. Usually, ρo is related with impurity scattering, while dρ/dT is related with carrier-carrier scattering [58]. However, the resistivity drop occurred in a single step for pure sample as indicated in the figure implying a single phase, while La samples have broadened the width of transition temperature, ΔTc [59]. However, the values of ρ300, ρo, and ΔTc presented in Table 3 are increased by La, but dρ/dT is decreased. Furthermore, the Tc is observed at 94 K for pure sample, but it is decreased to 85, 63, and 51 K for La samples.
We would like to mention here about the substitution of RE3+ in place of Ca2+ in Bi (Pb):2212 system [8,9,10,11,12,13,14,15,16,17,18,19,20,21]. The reported data of the reduced critical temperature [Tc(x)/Tc(0)] against rare earth elements are drawn and shown in Fig. 4a, and the present data are enclosed for comparison. This behavior indicated that La3+, compared with the other rare earth elements of lower ionic size such as Y and Dy, has nearly a similar behavior. But the present work is less detrimental to the superconductivity as compared to the reported for La as indicated in the figure. On the other hand, it is evident from the Fig. 4a that the critical concentration xc of La required for quenching superconductivity can be extended above 0.30 rather than the reported (0.25). However, different solubilities of rare-earth element R3+ in Bi (Pb):2212 system can be understood by a comparison of their ionic radii of 1.02, 1.03, and 1.18 Ǻ at 8-fold coordination for Y, Dy, and La with respect to 1.12 Ǻ of Ca2+ [60]. It is approved that the solubility has decreased as one move towards the right in lanthanide series such as La, which is not obtained in the present work. An inverse linear relation between Tm and Tc is obtained in Fig. 4c and given by Tc (K) = − 3.29 Tm + 2867.5. This is completely consistent with the reported data for the inverse linear relation between Tc and Tm for Y:123, Bi:2223, and Ti:2223 superconducting systems [61]. Although the Tc values are respectively 92, 110, and 125 K for these systems, the values of Tm are 1010, 867, and 807 °C, respectively. This finding suggests that in order to get higher Tc, a eutectic compound of lower melting point should be looked forward.
The phase diagram of high Tc systems is well described by the parabolic relation [62]:
where Tcm is the maximum critical temperature of the pure sample (94 K) and Tcx is the critical temperature of the La samples. p is the hole-carrier concentration per Cu ion. It is clear from Table 2 that p is increased from 0.16 for pure sample to 0.19, 0.22, and 0.23 for La samples, respectively. This behavior occurs as a result of more positive charges transferred by La to the CuO2 planes. But this disagree with the general role of substitution as a result of replacing Ca2+ by La+3, and therefore, p, should be to decreased. According to the mechanism of the excess of oxygen in BSCCO system, the electrons are transferred from Cu sites to BiO layer leading to the formation of holes on the Cu and electrons on the Bi as follows: Bi3+ + Cu2+ → Bi3-x + Cu2+x [63]. These aforesaid changes in the valence state of Bi are reflected due to the change of hole carriers owing to the excess of oxygen. To clarify this point according the role of substitution, we supposed that La3+ decreased the density of hole carriers, and at the same time, the excess of oxygen introduced some excess of holes in the Cu-O2 planes, and consequently, the effective (net) number of holes will be increased. This can be also supported by decreasing the doping distance and increasing the excess oxygen as discussed in the above paragraph.
3.4 Microhardness Measurements
The Vickers microhardness Hv at different loads for the samples is calculated by the following relation [64]:
where Hv is given by GPa, dp is the diagonal length of the indentation by micrometers, and F is the applied load by N (F = 0.49, 0.981, 1.962, 2.942, and 4.904 N). It is obvious from the curves of Hv versus F shown in Fig. 5 that Hv values are decreased by increasing F up to 4.904 N. This behavior is usually due to increasing the penetration depth where the inner layer effect becomes more prominent as discussed above (the well-known indentation size effect). Interestingly, at constant F, the values of Hv are increased by La, which can be attributed to decreasing the impurities and also increasing the density of the samples as listed in Table 1. This helps for improving the connectivity between the superconducting grains, and consequently, Hv has improved. The elastic Young’s modulus E and yield strength Y are related to the Hv as follows [65, 66]:
However, a similar behavior of both E and Y against F could be obtained and shown in Fig. 6a, b. This is probably due improving the roughness and interlayer bonding of superconducting grains when Ca is replaced by La.
The diagonal length dp is strongly dependent on the applied load F according to the following relation [22, 67,68,69]:
where Ht is the true hardness and γ is the surface energy. As a result of unsystematic linear behavior for the lines, the character is divided into lower and higher loads for simplicity. The plots of (F/dp) against dp for the lower and higher loads are shown in Fig. 7a, b where the slope represents Ht and the intercept represents γ. Interestingly, the slope of the linear plot is positive for all samples at lower loads (0.00–1.962 N). While at higher loads, the slope is negative for La = 0.00 and 0.05 samples and changed to positive for La = 0.15 and 0.30 samples. However, the different values of Ht and γ are listed in Table 4. It is clear that Ht has a similar behavior of Hv, which indicates a decrease of crack faces by La and supports the hardness improvements. But the values of γ are generally decreased from 0.029 N/μm to 0.021 N/μm for pure sample at higher loads, and from 0.008 to 0.005 N/μm at lower loads. From atomistic point of view, the more compacted surface the higher hardness and thus having lower surface energy.
The indentation size effect can be explained by two different methods [70,71,72,73]. The first method assumes that the indentation contains an elastic portion of the deformation which is supposed to relax upon the load beside plastic deformation. This can be accounted by adding an elastic component de to the measured elastic component dp, supposing that they are in the same direction, as follows [28, 74]:
The plot of dp versus F1/2 at lower and higher loads is shown in Fig. 8a, b, in which the slope represents [(1854.4)1/2(Ht)−1/2], and the vertical intercept represents the de. The values de listed in Table 5 are generally decreased by La which emphasizes the hardness suppression as discussed above. Furthermore, the values of de at higher loads are higher than that of lower loads. At lower loads, de is decreased from 13.62 μm for pure sample to 10.07, 8.16, and 5.48 μm for La samples and from 253.72 to 150.34, 88.93, and 42.41 μm at higher loads.
The second method considers the effect of energy dissipative processes, and therefore, the Ht can be defined by subtracting the dissipative part Fo from the applied load F as follows [75]:
The slope of linear plot of F against dp2 is shown in Fig. 9a, b, in which (Ht/1854.4) represents the slope and the intercept represents the resistance pressure Fo. The values of Fo listed in Table 5 are decreased by La, but at higher loads, they are more than that of lower loads (Fo (HL) = 10 Fo (LL). It is decreased at lower load from 0.22 for pure sample to 0.19, 0.16, and 0.12 N and from 2.21 to 1.92, 1.62, and 1.08 N at higher loads.
However, the following points are obtained due substitution of La in place of Ca in Bi (Pb):2212 superconducting phase: (i) A clear 2212 majority superconducting single phase; (ii) increasing the density, c-parameter, and excess of oxygen; (iii) decreasing orthorhombic distortion, doping distance, and crystal geometry factor; (iv) increasing the distance between Cu atoms, density of excess doping, hole carriers per Cu ion, effective Cu valence, critical concentration for quenching superconductivity, and hardness; (v) shifting endothermic peaks to higher temperatures; (vi) a sharp of mass loss starting at about 800 °C; (vii) decreasing the critical temperature, surface energy, resistance pressure, and elastin indentation.
In the above background, the following points are concluded. Firstly, the Tc depression is not related to the phase purity or bad microstructure in the form of secondary phases rich in Sr [76,77,78]. Secondly, although La decreased the hole carriers, the excess oxygen is more than enough to compensate the variation of charge in the system, and consequently, the number of holes in the CuO2 planes increases. The balance between the hole carriers lost by La3+ with that introduced by the excess of oxygen results in increasing the effective (net) of hole carriers, which can be considered as the possible reason for altering the quenching of superconductivity by La3+ in the present work. However, the consistency of these points gave us a fair degree of certainty to the suggestion of La substitution in place of Ca in Bi (Pb):2212 system.
4 Conclusion
Structural and normal properties of Bi1.7Pb0.30Sr2Ca1−xLaxCu2Oy superconductor with various x values (0.00 ≤ x ≤ 0.30) are investigated. We have shown that the replacement of Ca2+ by La3+ increased the excess of oxygen, effective Cu valance, and hole carrier per Cu ion. Further, the doping distance and crystal geometry factor are slightly decreased, while the distance between Cu atoms and the density of excess doping are increased. Furthermore, a strong shift of endothermic peaks to higher temperatures and also a sharp mass loss is obtained. Although the critical temperatures Tc are decreased by La, the critical concentration for quenching superconductivity xc can be extended above 0.30. Moreover, an inverse linear relation between Tm and Tc given by Tc (K) = − 3.29Tm (°C) + 2867.50 could be obtained. On the other hand, Vickers hardness Hv is decreased by the applied loads F, but it is increased by La (Hv α Tm, Hv α (1/Tc)), and the Hv-F character could be divided into lower and higher loads. The surface energy, resistance pressure, and elastic indentation are generally decreased by La, and their values at higher loads are more than that of lower loads. The balance between the hole carriers lost by La3+ with that introduced by excess of oxygen resulting for increasing the effective (net) of hole carriers can be considered as the possible reason for altering the quenching of superconductivity by La3+ which highlights the present work.
References
Eisaki, H., Kaneko, N., Feng, D.L., Damascelli, A., Mang, P.K., Shen, K.M., Shen, Z.X., Greven, M.: Phys. Rev. B. 69, 064512 (2004)
Jeremie, A., Yadri, K.A., Grivel, J.C., Fiñkiger, R.: Supercond. Sci. Technol. 6, 730 (1993)
Maeda, H., Tanaka, Y., Fukutomi, M., Asano, T.: Jpn. J. Appl. Phys. 27(2), L209 (1988)
Amirabadizadeh, A., Memarzadeh, S., Tajabor, N., Arabi, H.: World J. Condens. Matter Phys. 2, 148 (2012)
Gul, I.H., Rehman, M.A., Ali, M., Maqsood, A.: Phys. C. 432(1–2), 71 (2005)
Aksan, M.A., Yakinci, M.E.: J. Alloys Compd. 433, 22 (2007)
Hwang, C.H., Kim, G.: Supercond. Sci. Technol. 5, 586 (1992)
dos Santos, C.A.M., Moehlecke, S., Kopelevich, Y., Machado, A.J.S.: Physica C. 390, 21 (2003)
Lu, X.G., Zhao, X., Fan, X.J., Sun, X.F., Wu, W.B., Zhang, H.: Appl. Phys. Lett. 76, 3088 (2000)
Ilyushchkin, A.Y., Yamashita, T., Boskovic, L., Mackinnon, I.D.R.: Supercond. Sci. Technol. 17, 1201 (2004)
Jassim, K.A., Tariq, J., Alwan, J.: Supercond. Nov. Magn. 22, 861 (2009)
Singh, S.: Physica C. 294, 249 (1998)
Nanda Kishore, K., Satyavathi, S., Muralidhar, M., Pena, O., Hari Babu, V.: Physica C. 252, 49 (1995)
Sedky, A.: J. Phys. Chem. Solids. 70, 483 (2009)
Marconi, D., Stiufiuc, G., Pop, A.V.: J. Phys. Conf. Ser. 153, 012022 (2009)
Hudakova, N.: Physica C. 406, 58 (2004)
dos Santos, A.D.M., Pinto, G.S., Ferreira, B., Machado, A.J.S.: Physica C. 354, 388 (2001)
Cao, Q., Ruan, K.Q., Li, S.Y., Chen, X.H., Qian, G.G., Cao, L.Z.: Physica C. 334, 237 (2000)
Khalil, S.M.: J. Phys. Chem. Solids. 64, 855 (2003)
Sun, X., Zhao, X., Wu, W., Fan, X., Li, X.-G., Ku, H.C.: Physica C. 307, 7 (1998)
Awana, V.P.S., Agarwal, S.K., Ray, R., Gupta, S., Narlikar, A.V.: Physica C. 43, 191 (1992)
Yilmazlar, M., Cetinkara, H.A., Nursoy, M., Ozturk, O., Terzioglu, C.: Physica C. 442, 101 (2006)
Sedky, A.: Physica C. 468, 1041 (2008)
Sale, F.R., Mahloojchi, F.: Ceram. Int. 14, 229 (1988)
Yanru, R., Hanpeng, L., Mingzhu, L., Qingyun, T., Lihua, S., Zhenjin, L., Xianren, M.: Physica. 156(5), 799 (1988)
Hwang, J., Timusk, T., Gu, G.D.: Nature. 427, 714 (2004)
Norman, M.: Nature. 427, 692 (2004)
Gong, J., Wu, J., Guan, Z.: J. Eur. Ceram. Soc. 19(15), 2625 (1999)
Khalil, S.M.: J. Phys. Chem. Solids. 62(3), 457 (2001)
Elmustafa, A.A., Stone, D.S.: J. Mech. Phys. Solid. 51, 357 (2003)
Sangwal, K.: Mater. Chem. Phys. 63, 145 (2000)
Musa Abbas, M.: Int. J. Curr. Eng. Tech. 5(3), 1908 (2015)
Khalil, S.M.: Smart Mater. Struct. 14, 804 (2005)
Schuller, I.K., Jorgfnsen, J.D.: MRS Bull. 14, 27 (1989)
Ravinder, D., Alivelu Manga, T.: J. Alloys Compd. 299, 5 (2000)
Khalil, S.M., Sedky, A.: Physica B. 357, 299 (2005)
Aljaafari, A., Sedky, A., Al-Sawalha, A.: J. Phys. Chem. Solids. 69, 2919 (2008)
Simon, A., Mukherjee, P.S., Sarma, M.S., Damodaran, A.D.: J. Mater. Sci. 29, 5059 (1994)
Halim, S.A., Mohamed, S.B., Azhan, H., Khawaldeh, S.A., Sidek, H.A.A.: Physica C. 312, 78 (1999)
Pei, G., Xia, C., Cao, S., Zhang, J., Wu, F., Xu, J.: JMMM. 302(2), 340 (2006)
Shan, F.K., Liu, Z.F., Liu, G.X., Lee, W.J., Lee, G.H., Kim, I.S.: J. Electroceram. 13, 195 (2004)
El-Desoky, M.M., Ali, M.M., Afifi, G., Imam, H.: J. Mater. Sci. Mater. Electron. (11), (2014)
Wang, X.S., Wu, Z.C., Webb, J.F., Liu, Z.G.: Appl. Phys. A Mater. Sci. Process. 77, 561–565 (2003)
Sedky, A., Abu-Abdeen, M., Almulhem, A.-A.A.: Physica B. 388, 266 (2007)
Khalil, S.M., Low, J.: Temp. Phys. 143(112), 31 (2006)
Mandel, P., Poddar, A., Ghosh, B., Choudhury, P.: Phys. Rev. B. 43(16), 13102 (1991)
Biju, A., Sarun, P.M., Aloysius, R.P.: Physica C. 433, 68 (2007)
Chen, W.M., Wu, X.S., Geng, J.F., Chen, J., Chen, D.B., Jin, X., Jiang, S.S.: J. Supercond. 10(1), 41 (1997)
Jean, F., Collin, G., Andrieux, M., Blanchard, N., Marucco, J.F.: Phys. C. 339, 269 (2000)
Norman, M.R., Ding, H., Randeria, M., Campuzano, J.C., Yokoya, T., Takeuchi, T., Takahashi, T., Mochiku, T., Kadowaki, K., Guptasarma, P., Hinks, D.H.: Nature. 392, 157 (1998)
Alldredge, J.W., Lee, J., McElroy, K., Wang, M., Fujita, K., Kohsaka, Y., Taylor, C., Eisaki, H., Uchida, S., Hirschfeld, P.J., Davis, J.C.: Nat. Phys. 4, 319 (2008)
Roesera, H.P., Hetfleischa, F., Huberb, F.M., von Schoenermarka, M.F., Steppera, M., Moritza, A., Nikoghosyanc, A.S.: Acta Astronaut. 63, 1372 (2008)
Roesera, H.P., Huberb, F.M., von Schoenermarka, M.F., Nikoghosyanc, A.S.: Acta Astronaut. 65, 489–494 (2009)
Bernik, S., Hrovat, M., Kolar, D.: Supercond. Sci. Technol. 7, 920 (1994)
Honda, T., Wada, T., Sakai, M., Miyanaga, M., Nishikawa, N., Uchida, S., Uchinokawa, K., Tanaka, S.: Jpn. J. Appl. Phys. 27, L545 (1988)
Broido, A.: J Polymar Sci. A-2. 7, 1761–1773 (1969)
Venkataramani, R., Garg, S.P., Mazumder, S.: Bull. Mater. Sci. 14(3), 665 (1991)
Ravi, S., Seshu Bai, V.: Phys. Rev. B. 49, 13082 (1994)
Nkum, R.K., Datars, W.R.: Supercond. Sci. Technol. 8, 822 (1995)
Sedky, A., Gupta, A., Awana, V.P.S., Narlikar, A.V.: Phys. Rev. B. 58(18), 12495 (1998)
A.A. Bahgat, High Tc Updata (USA) Techinical Report 6, 19 (1992)
Presland, M.R., Tallon, J.L., Buckley, R.G., Liu, R.S., Floer, N.E.: Physica C. 176, 95 (1991)
Konsin, P., Sorkin, B.: Supercond. Sci. Technol. 13, 301 (2000)
Leenders, A., Ullrich, M., Freyhardt, H.C.: Physica C. 279, 173 (1997)
Veerender, C., Dumke, V.R., Nagabhooshanam, M.: Phys. Status Solid A. 144, 199 (1994)
Tabor, D.: The Hardness of Metals. Clarendon, Oxford (1951)
Lawn, B.R., Wilshaw, T.R.: J. Mater. Sci. 10, 1049 (1975)
Quinn, J.B., Quinn, G.D.: J. Mater. Sci. 32, 4331 (1997)
Dutta, A.K., Narasaiah, N., Chattopadhyaya, A.B., Ray, K.K.: Ceram. Int. 27, 407 (2001)
Farber, B.Y., et al.: Superconductivity. 4, 2296 (1991)
Fröhlinch, F., Grau, P., Grellmann, W.: Phys. Status Solidi. 42, 79 (1997)
Li, H., Bradt, R.C.: J. Mater. Sci. 22, 917 (1993)
Hays, C., Kendall, E.G.: Metallography. 6(4), 275 (1973)
Aydin, H., Cakiroglu, O., Nursoy, M., Terzioglu, C.: Chin. J. Phys. 47(2), 192 (2009)
Li, Z., Ghosh, A., Kobayashi, A.S.: J. Am. Soc. 72, 904 (1989)
Prabitha, V.G., Biju, A., Abhilash Kumar, R.G., Sarun, P.M., Aloysius, R.P., Syamaprasad, U.: Physica C. 433, 28 (2005)
Biju, A., Aloysius, R.P., Syamaprasad, U.: Physica C. 440, 52 (2006)
Munakata, F., Kawano, T., Yamauchi, H., Inoue, Y.: Physica C. 190, 471 (1992)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sedky, A., Salah, A. & Abou-Aly, A. Normal and Superconducting Properties of Bi1.7Pb0.30Sr2Ca1−xLaxCu2Oy Superconductor with 0.00 ≤ x ≤ 0.30. J Supercond Nov Magn 33, 3349–3359 (2020). https://doi.org/10.1007/s10948-020-05587-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-020-05587-y