Abstract
In the present work, pure and transition metal titanium (Ti)-doped LaFe1−xTixO3(x = 0.0, 0.2, 0.4, and 0.6) nanoparticles have been successfully synthesized by hydrothermal synthesis technique for various molar ratios of 0.2, 0.4, and 0.6. The prepared samples were grinned and annealed in furnace at 600 ∘C/4 h. The X-ray diffraction (XRD) analysis confirms the phase orthorhombic formation of LaFeO3 and Ti-doped LaFeO3. The stoichiometric analysis was performed through energy-dispersive X-ray studies (EDX). The micro-structural features of surface morphology were examined by field emission scanning electron microscopy (FESEM). Further, surface nanocrystalline of the titanium (Ti)-doped LaFe1−xTixO3 was also investigated through high-resolution transmission electron microscopic (TEM) analysis. The enhancement of room temperature magnetization value has been observed from the M–H curve which is measured using vibrating sample magnetometer (VSM).The weak ferromagnetic behavior was observed from hysteresis loop. From hysteresis loop, saturation takes place when the applied magnetic field intensity is 15 kOe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, the perovskite-type transition metal oxides (TMOs) have been proposed as promising ABO3 perovskite-type materials for the coexisting states of multiple coupled such as superconductivity and magnetism. Lanthanum iron oxide (LaFeO3) is an ABO3 perovskite oxide having an orthorhombic structure at room temperature and an antiferromagnetic ordering below 740 K [1,2,3,4]. It has gained a considerable attention because of its wide range of applications in chemical sensors, nonvolatile magnetic memory devices, and ultra-sensitive magnetic read heads of modern hard disk drives, electromechanical devices, solid electrolyte, solid fuel cell, actuators, fixed resistor, transducers, etc. [5,6,7,8,9,10].
It is well known that the undoped LaFeO3 is an antiferromagnetic (AFM) material. The AFM spin order in LaFeO3 is a rare magnetic property due to the finite size effect. However, the decrease in particle size of LaFeO3 has been expected to develop the magnetic property. For example, the ferromagnetism (FM) of LaFeO3 with a particle size of 10–50 nm has been reported [11]. LaFeO3 is a canted G-type AFM structure with a high Neel temperature (NT) of 750 K [12]. Orthoferrites are the weak ferromagnetic materials with interesting magneto-optical properties [13, 14]. It has been credited to the presence of uncompensated surface spin, which is called the ferromagnetic (FM) shell [15, 16]. Besides, the magnetization of LaFeO3 samples increased with decreasing particle size [9, 10] and the change in Curie temperature (CT) for finite size effect has also been studied. It has been examined that both the doping and the preparation method could help in decreasing the particle size which results in the improvement of the magnetization.
Further, the exchange on A and/or B site is also pragmatic to improve the electrical properties. Various types of exchange have been studied, for example, La0.8Sr0.2Fe1−xCuxO3 [17] La1/3Sr2/3FeO3d [18], LaZnFeO3 [19], La0.5Al0.5FeO3 [20], La1−xCxFeO3 [21], and LaZnxFe1−xO3 [22, 23]. These show the interesting properties of electrical materials such as high electrical conductivity, high dielectric constant, low dielectric loss, ferroelectricity or piezoelectricity, thermal stability, etc. Therefore, the substitution of divalent or trivalent ions into the La or Fe sublattices has been specifically investigated for achieving these properties [24,25,26, 34,35,36,37,38,39,40,41,42,43,44] .
The main objective of the present work is to study the effect of substituting Fe ions with Ti ions on the structural and magnetic properties of the compositions of the LaFe1−xTixO3 system with perovskite orthorhombic structure. At present, no studies are available on the magnetic properties of substitution on B site by hydrothermal method of Ti ions. We believe that the structural and magnetic properties that are explored in this paper are useful for various applications as Fe and Ti are present in LaFe1−xTixO3 perovskite systems.
Several methods such as coprecipitation technique [27], combustion synthesis [28], and sol-gel technique [29] were reported to prepare LaFeO3 nanoparticles. In this work, the effect of various doping concentration of titania is investigated in the preparation of LaFeO3 (LaFe1−xTixO3, x = 0, 0.2, 0.4, and 0.6) using hydrothermal synthesis. The prepared sample has been characterized for exploring various structural and magnetic properties.
2 Experimental Procedure
Various doping concentrations of LaFe1−xTixO3 (x = 0, 0.2, 0.4, and 0.6) are prepared by hydrothermal method with the aqueous solutions of La(NO3)2 ⋅ 6H2O (99.6% purity, Sigma-Aldrich), Fe(NO3)2 ⋅ 9H2O (99.6% purity, Sigma-Aldrich), and titanium(IV) isopropoxide (99.6% purity, Sigma-Aldrich) mixtures in alkaline medium. The solutions of La(NO3)2 ⋅ 6H2O, Fe(NO3)2 ⋅ 9H2O, and titanium(IV) isopropoxide in their stoichiometry ratio (1 g of La(NO3)2 ⋅ 6H2O in 50 ml), (0.6g of Fe(NO3)2 ⋅ 9H2O in 50 ml), (0.2 g of titanium(IV) isopropoxide in 50 ml) were dissolved in double-distilled water with a constant stirring at 600 rpm. Then, NaOH solution is added and the pH = 10.0 is maintained. The prepared mixed solvent was transferred into a 250-ml Teflon-lined stainless autoclave. The autoclave was sealed and preserved at 120 ∘C for 24 h. After the hydrothermal reaction time, the autoclave was taken out and the autoclave was cooled to room temperature (RT) naturally. The samples obtained were washed four to five times with double-distilled water. A general flow chart of synthesis process is shown in Fig. 1. The sample was dried in a hot-air oven at 100 ∘C overnight (12 h) to remove dampness. The dried powder was mixed homogeneously in an agate mortar and pestle for 2 h. This homogenous mixture was sintered at 600 ∘C for 4 h in a hot-air furnace. After sintering, the sample is again ground for 3 h using the agate mortar, and the resulting powder is analysed using X-ray diffraction (XRD), field emission scanning electron microscope (FESEM), transmission electron microscopy (TEM), and vibrating sample magnetometer (VSM).
2.1 Measurements Techniques
The purity and crystallinity of the samples were characterized by XRD using Bruker, D8 Advance model diffractometer. Measurements were taken in the 2𝜃 range of 15–80∘ , using Cu-Kα1 radiation at a scan rate of 2∘ /min. The average crystallite size was determined based on Scherrer’s formula from the line broadening of the peak. Microstructure and morphology were analyzed using a Carl Zeiss SUPRA-555 FESEM. The scanning electron microscope was fitted with an energy-dispersive X-ray spectrometer (EDX) for the determination of the different elements present in the sample. The morphologies of samples were investigated by TEM using a Philips (model CM200) instrument. Room-temperature magnetic measurement of the samples was carried out with VSM. The parameters like saturation magnetization (Ms), coercive force (Hc), and remanent magnetization (Mr) were evaluated.
3 Results and Discussion
3.1 XRD Structural Analysis
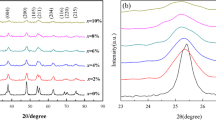
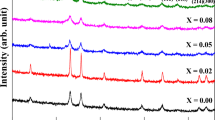
LaFe1−xTixO3 (LFTO) nanoparticles exhibited the orthorhombic symmetry and crystallized with the well-known orthorhombic perovskite structure at 600 ∘C/4 h. In Fig. 2, the XRD data from LFTO are shown. All the reflection planes are in good agreement with the standard JCPDS (No. 82-1958) of orthorhombic phase. Little secondary phases (preceded by an asterisk) corresponding to La2TiO5 are detected for the increased titanium concentrations (x = 0, 0.2, 0.4, and 0.6). The average crystallite size (D) of the sample is evaluated for the intense peak positions using Scherrer’s formula [30].
Here, k is a constant and is approximately equal to 0.9 for a spherical symmetry and λ is X-ray wavelength of Cu-Kα1 = 1.5418 Å, where 𝜃 is diffraction angle and β is full-width half maxima (FWHM).
As the calcination temperature was from 600 ∘C/4 h, the FWHM values of diffraction curve decrease sharply and hence the particle sizes also increased (Table 1). That is, the average crystallite size of Ti content increases and found to vary from 5 ± 1 to 11 ± 1 nm [30]. By increasing the dopant level, Dx exhibits unsystematic trend due to the presence of secondary phases. This behavior is qualified due to fading crystal growth or an increase of elastic strain (å = â/4tan𝜃) by Ti addition.
3.2 Surface Morphology
To well understand the micro-structural features of LaFeO3 and LaFe1−xTixO3 (x = 0–0.2) samples, FESEM was performed. The FESEM showed that the microstructure consisted of submicron-sized particle. However, the rest of the compositions are observed to be almost spherical in shape. Figure 3 shows the FESEM photographs wherein the particle size around 31–39 nm grains were found. FESEM images of the local morphology of LaFeO3 and LaTixFe1−x03 images show good nanocrystalline grain.
EDX in combination with FESEM showed that the synthesized samples have a homogeneous composition. The peaks of La, Fe, and O were perceived for all samples. We also perceived peaks of Ti for the samples with x = 0.2, 0.4, and 0.6. It is found that the improved elements of Ti atomic replies to the reduction of that of Fe atomic as well as the increase of Ti content. It designates that the substitution of Ti ions into Fe site is comprehensive.
3.3 TEM Analysis
The structure and morphology of LaFe1−xTixO3 nanoparticles were investigated by TEM with the selected area electron diffraction (SAED) pattern, as shown in Fig. 4. The TEM images showed the nanoparticles with a size of approximately 21–50 nm. It can be seen from Fig. 4 that the even particle size of the samples decreases with increasing the Ti doping content, which is reliable with the XRD results. The SAED patterns of the sample presented the spotty ring patterns which imply a creation of polycrystalline structure. These rings were indexed as the LaFeO3 perovskite phase with random orientation, which is in agreement with the XRD results [27].
3.4 VSM Analysis
The room temperature hysteresis loops for LaFeO3 samples (x = 0, 0.2, 0.4, and 0.6) are shown in Fig. 5. The substitution of nonmagnetic ion titanium, which has a different B site occupancy results in the decrease of the exchange interaction B sites. Hence, by varying the Ti content, it is possible to vary magnetic properties of the nanoparticles. LaFeO3 had been proven to be an antiferromagnet with a Neel temperature of 750 K. Generally, La3+ is nonmagnetic since all the electrons were paired. The magnetic moments of Fe are the source of magnetic properties. Therefore, the FM behavior in the LaFeO3 nanoparticles is because of the spin-canted Fe magnetic moments due to the disordered surface spins [31]. The VSM measurements for LaFe1−xTixO3 nanoparticles measured at RT for various values of magnetic field (H) are shown in Fig. 5. The Ms, Mr), squareness, and Hc values are listed in Table 2. It can be seen from the table that the above-mentioned physical parameters increase when doping concentration is increased. We emphasize that the coercivity is found to be maximum for pure LaFeO3. Then, it decreases when the doping concentration is increased from 0.2 to 0.4. However, it increases when the doping concentration is 0.6.
All the samples exhibit a weak FM behavior and an increase of magnetization (M) with increased content of Ti. From the magnetization curve, coexistence of antiferromagnetism is observed, i.e., at the high field, the M–H curve does not saturate. Enhanced magnetization is observed in the case of Ti4+ doping which is due to the uncompensated spin of Ti4+ in the Fe3+ site.
The observed change in the magnetic properties, i.e., magnetic moment of undoped LaFeO3, is approximately zero; as a result, it increases with Ti doping along with an increase in magnetization (Fig. 5). This could be understood from the canted-antiferromagnetic (AFM) behavior of these materials. It is known that LaFeO3 exhibits G-type AFM behaviors [30,31,32,33]. The canting of the Fe spins at small angles is due to exchange coupling, which, in turn, results in a small net magnetic moment (Table 2). With increase in Fe4+ content due to Ti doping, net magnetic moment increases. In addition, the presence of oxygen vacancies disturb antiparallel spin ordering in the Fe3+–O–Fe3+ and Fe4+–O2 −Fe4+ by super-exchange interaction. This leads to an increase of magnetization; the maximum value of magnetization shows magnetic memory device applications. The effect of the magnetization curve confirms that the TC of the sample (with x = 0.4) is stable even at higher temperature. We strongly believe that this is foremost and would be useful for the magnetic memory devices in future.
4 Conclusion
In summary, nanoparticles of LaFeO3 and LaFe1−xTixO3 were prepared using the hydrothermal method. XRD pattern confirms the phase and the presence of Ti4+ in the lattice. Structural classification showed that the phase of orthorhombic structure (LaFeO3). The average crystallite size calculated from the Scherrer’s formula suggested the formation of nanoparticles, and this was further confirmed by the TEM images. The weak ferromagnetic behavior was observed from hysteresis loop. We found that the coercivity is found to be maximum for pure LaFeO3. Then, it decreases when the doping concentration is increased from 0.2 to 0.4. However, it increases when the doping concentration turns 0.6.
References
Teresita, V.M., Manikandan, A., Josephine, B.A., Sujatha, S., Antony, S.A.: Electro-magnetic properties and humidity sensing studies of magnetically recoverable lamgx fe 1−x o 3− perovskites nano-photocatalysts by sol-gel route. J. Supercond. Nov. Magn. 29, 1691–1701 (2016)
Saad, A.A., Khan, W., Dhiman, P., Naqvi, A.H., Singh, M.: Structural optical and magnetic properties of perovskite (La1xSrx)(Fe1xNix)O 3, (x = 0, 0.1, 0.2) nanoparticles. Electron. Mater. Lett. 9, 77–81 (2013)
Thuy, N.T., Minh, D.L.: Size effect on the structural and magnetic properties of nanosized perovskite lafeo3 prepared by different methods. Adv. Mater. Sci. Eng. 1155, 380306 (2012)
Lee, W.Y., Yun, H.J., Yoon, J.W.: Characterization and magnetic properties of lafeo3 nanofibers synthesized by electrospining. J. Alloys Compd. 583, 320–324 (2014)
Faye, J., Bayleta, A., Trentesauxb, M., Royera, S., Dumeignil, F., Duprez, D., Valange, S.: Influence of lanthanum stoichiometry in la1x feo 3d perovskites on their structure and catalytic performance in CH4 total oxidation. J. Appl. Catal. B 126, 134–143 (2012)
Cristobal, A.A., Botta, P.M., Bercoff, P.G., Porto lópez, J.M.: Mechanosynthesis and magnetic properties of nanocrystalline lafeo3 using different iron oxides. Mater. Res. Bull. 44, 1036–1040 (2009)
Lakshminarayanan, N., Kuhn, J.N., Rykov, S.A., Millet, J.-M.M., Ozkan, U.S.: Doped lafeo3 as SOFC catalysts: control of oxygen mobility and oxidation activity. Catal. Today 157, 446–450 (2010)
Kuiying Li, D., Wang, D., Wu, F., Xie, T., Li, T.: Surface electronic states and photovoltage gas-sensitive characters of nanocrystalline lafeo3. Mater. Chem. Phys. 64, 269–272 (2000)
Parid, K.M., Reddy, K.H., Martha, S., Das, D.P., Biswal, N.: Fabrication of nanocrystallinelafeo3: an efficient sol-gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int. J. Hydrogen Energy 35, 12161–12168 (2010)
Chinnaraj, K., Manikandan, A., Ramu, P., Arul Antony, S., Neeraja, P.: Comparative study of microwave and sol-gel assisted combustion methods of fe3 o 4 nanostructures: structural, morphological, optical, magnetic and catalytic properties. J. Supercond. Nov. Magn. 28, 179–190 (2015)
Koehler, W.C., Wollan, E.O.: Neutron-diffraction study of the magnetic properties of perovskite-like compounds laBO3. J. Phys. Chem. Solids 2, 100–106 (1957)
Treves, D.: Studies on ortho ferrites at the Weizmann Institute of Science. J. Appl. Phys. 36, 1033–1039 (1965)
Didosyan, Y.S., Hauser, H., Wolfmayr, H., Nicolics, J., Fulmek, P.: Sens. Actuators A 106, 168–171 (2003)
Punnoose, A., Magnone, H., Seehra, M.S., Bonevich, J.: Bulk to nanoscale magnetism and exchange bias in CuO nanoparticles. Phys. Rev. B 64, 174420–174427 (2001)
Salabas, E.L., Rumplecker, A., Kleitz, F., Radu, F., Schuth, F.: Exchange anisotropy in nanocasted co3 o 4 nanowires. Nano Lett. 6, 2977 (2006)
Sora, I.N., Caronna, T., Fontana, F., Fernande, C.D.J., Caneschi, A., Green, M.: Crystal structures and magnetic properties of strontium and copper doped lanthanum ferrites. J. Solid State Chem. 191, 33–39 (2012)
Ying, X.N.: Charge order suppression in oxygen nonstoichiometric la1/3sr2/3feo3d. Solid State Commun. 169, 20–23 (2013)
Mukhopadhyay, K., Mahapatra, A.S., Chakrabarti, P.K.: Multiferroic behavior, enhanced magnetization and exchange bias effect of Zn substituted nanocrystalline lafeo3(la(1x)znx feo 3,x = 0.10 and 0.30). J. Magn. Magn. Mater. 329, 133–141 (2013)
Acharya, S., Chakrabarti, P.K.: Some interesting observations on the magnetic and electric peoperties of al3+ doped lanthanum orthoferrite (la0.5 al 0.5 feo 3). Solid State Commun. 150, 1234–1237 (2010)
Andoulsin, R., Horchani-Naifer, K., Ferid, M.: Electrical conductivity of la1x ca x feo 3d solid solutions. Ceram. Int. 39, 6527–6531 (2013)
Hosseini, S.A., Sadeghi, M.T., Alemi, A., Niaei, A., Salar, D., Ahmadi, L.K.: Synthesis characterization, and performance of laznxfe1x o 3 perovskite nanocatlysts for toluene combustion. Chin. J. Catal. 31, 747–750 (2010)
Bhat, I., Husain, S., Khan, W., Patil, S.I.: Effect of Zn doping on structural, magnetic and dielectric properties of lafeo3 synthesized through sol–gel autocombustion process. Mater. Res. Bull. 48, 4506–4512 (2013)
Mahmoud, H., et al.: Synthesis of highly ordered 30 nm nife2 o 4 particles by the microwave-combustion method. J. Magnetism and Magnetic Materials 369, 55–61 (2014)
Bellakki, M.B., Kelly, B., Manivannan, V.: Synthesis and characterization and property studies of (La, Ag) feo3 perovskite materials. J. Alloys Compd. 489, 64–71 (2010)
Karpinsky, D.V., Troyanchuk, I.O., Sikolenko, V., Efimov, V., Kholkin, A.L.: Electromechanical and magnetic properties of bifeo3–lafeo3–catio3 ceramics near the rhombohedral–orthorhombic phase boundary. J. Appl. Phys. 113, 187218 (2013)
Mahmoud, M.H., et al.: Synthesis of highly ordered 30 nm nife2 o 4 particles by the microwave-combustion method. J. Magnetism and Magnetic Materials 369, 55–61 (2014)
Sasikala, C., Durairaj, N., Baskaran, I., Sathyaseelan, B., Henini, M., Manikandan, E.: Transition metal titanium (Ti) doped lafeo3 nanoparticles for enhanced optical structural and magnetic properties. J. Alloys and Compounds 712, 870–877 (2017)
Wang, J.B., Liu, Q.F., Xue, D.S., Li, F.S.: Synthesis and characterization of lafeo3 nanoparticles. J. Matter. Sci. Lett. 21, 1059–1062 (2002)
Rajendran, M., Bhattacharya, A.K.: Nanocrystalline orthoferrite powders: synthesis and magnetic properties. J. Eur. Ceramic Soc. 26, 3675–3679 (2006)
Phokha, S., Hunpratup, S., Pinitsoontorn, S., Putasaeng, B., Rujirawat, S., Maensiri, S.: Structure, magnetic, and dielectric properties of Ti-doped lafeo3ceramics synthesized by polymer pyrolysis method. Mater. Res. Bull. 67, 118–125 (2015)
Phokha, S., Pinitsoontorn, S., Maensiri, S., Rujirawat, S.: structure, optical and magnetic properties of LaFeO3 nanoparticles prepared by polymerized complex method. J. Sol-Gel. Sci. Technol. 71, 333–341 (2014)
Shikha, P., Kang, T.S., Randhawa, B.S.: Effect of different synthetic routes on the structural, morphological and magnetic properties of Ce doped lafeo3 nanoparticles. J. Alloys and Compounds 625, 336–345 (2015)
Bhat, I., Husain, S., Khan, W., Patil, S.I.: Effect of Zn doping on structural, magnetic and dielectric properties of lafeo3 synthesized through sol–gel auto-combustion process. Mater. Res. Bull 48, 4506–4512 (2013)
Abraham, A.G., Manikandan, A., Manikandan, E., Vadivel, S., Jaganathan, S.K.: Enhanced magneto-optical and photo-catalytic properties of TM cobalt (co2+ ions) doped spinel mgfe2 o 4 ferrite nanocomposites. J. Magnetism and Magnetic Materials 452, 380–388 (2018)
Thanigai Arul, K., Manikandan, E., Ladchumananandasivam, R., Maaza, M.: Novel polyvinyl alcohol polymer based nanostructure with ferrites codoped with nickel and cobalt ions for magnetosensor application. Polym. Int. 65, 1482–1485 (2016)
Diallo, A., Doyle, T.B., Mothudi, B.M., Manikandan, E., Maaza, M.: Magnetic behavior of biosynthesized co3 o 4 nanoparticles. J. Magnetism and Magnetic Materials 424, 251–255 (2017)
Arul, K.T., Manikandan, E., Henini, M.: Enhanced magnetic properties of polymer-magnetic nanostructures synthesized by ultrasonication. J. Alloys &, Compounds 720, 395–400 (2017)
Manikandan, A., Manikandan, E., Meenatchi, B., Vadivel, S., Jaganathan, S.K.: Rare earth element (REE) lanthanum doped zinc oxide (La: ZnO) nanomaterials: synthesis structural optical and antibacterial studies. J. Alloys and Compounds 723, 1155–1161 (2017)
Shah, A.H., Manikandan, E., Ahmed, M.B., Irdosh, M.: Nano Ag-doped ZnO particles magnetic, optical and structural studies. AIP Conf. Proc. 1512, 430–431 (2013)
Asiri, S., Sertkol, M., Güngüneş, H., Amir, M., Manikandan, A., Ercan, İ., Baykal, A.: The temperature effect on magnetic properties of NiFe2 O 4 nanoparticles. J. Inorg. Organomet. Polym. Mater. 28, 1587–1597 (2017)
Bomila, R., Srinivasan, S., Gunasekaran, S., Manikandan, A.: Enhanced photocatalytic degradation of methylene blue dye, opto-magnetic and antibacterial behavior of pure and La-doped ZnO nanoparticles. J. Supercond. Novel Magn. 31, 855–864 (2018)
Padmapriya, G., Manikandan, A., Krishnasamy, V., Jaganathan, S.K.: Enhanced catalytic activity and magnetic properties of spinel mnx zn 1−xfe2o4(0.0x1.0) nano-photocatalysts by microwave irradiation route. J. Supercond. Novel Magn. 29, 2141–2149 (2016)
Josephine, B.A., Manikandan, A., Teresita, V.M., Antony, S.A.: Fundamental study of lamgx cr 1−x o− perovskites nano-photocatalysts: sol-gel synthesis, characterization. Kor. J. Chem. Eng. 33, 1590–1598 (2016)
Ravichandran, A.T., Srinivas, J., Karthick, R., Manikandan, A., Baykal, A.: Facile combustion synthesis, structural, morphological, optical and antibacterial studies of bi1−x al x feo 3(0.0x 0.15) nanoparticles. Cer. Inter. 44, 13247–13252 (2018)
Acknowledgments
We also express our thanks to IIT Bombay and IIT Chennai for utilizing TEM facility.
Funding
We gratefully acknowledge support from Arignar Govt. Arts College-Cheyyar and VIT University, Vellore, Tamil Nadu, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sasikala, C., Suresh, G., Durairaj, N. et al. Chemical, Morphological, Structural, Optical, and Magnetic Properties of Transition Metal Titanium (Ti)-Doped LaFeO3 Nanoparticles. J Supercond Nov Magn 32, 1791–1797 (2019). https://doi.org/10.1007/s10948-018-4879-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-018-4879-1