Abstract
CoCr x Fe2−x O4 (0.0 ≤ x ≤ 1.0) nanoparticles were synthesized by a microwave combustion method and the effect of Cr3+ substitution on structural, morphological, and magnetic properties of CoFe2O4 was studied. The structural, morphological, and magnetic properties of the products were determined and characterized in detail by X-ray diffraction (XRD), high-resolution scanning electron microscopy (HR-SEM), energy-dispersive X-ray spectroscopy (EDX), Fourier transform infrared spectroscopy (FT-IR), and vibrating sample magnetometer (VSM). Cation distribution of calculations confirmed the B site (octahedral site) preference of substituted Cr3+ ions. X-ray analysis showed that all compositions crystallize with a cubic spinel-type structure. The lattice parameter decreased from 8.384 to 8.362 Å with increasing Cr content. The average crystallite size was found in the range of 30.6–45.4 nm. Magnetization measurements showed that saturation magnetization of products decreases with the increase of the Cr substitution (x).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cobalt ferrite is an important compound due to its strong magnetic anisotropy [1], high coercivity at room temperature [2], moderate saturation magnetization [3], magnetostriction [4], and good mechanical and chemical stability [5, 6]. CoFe2O4 is a promising material for high-density recording of its excellent electrical and magnetic properties.
The addition of a small amount of impurity (metal ions with different valence states) leads to effective modification in the electrical and magnetic properties of spinel ferrites [7] because these types of additions lead to various tetrahedral (A) and octahedral (B) site distributions [8, 9]. Hence, the change in cation distribution affects the physical properties of spinel ferrites.
Up to now, various methods have been used for the synthesis of Cr3+-substituted cobalt ferrite nanoparticles. Iqbal and Siddiquah [10] used a micro-emulsion method using polyethylene glycol (PEG) as a surfactant at pH 9.50. Hankarea et al. [11] synthesized the spinel-phase nanocrystalline CoCr x Fe2−x O4 powders (0.0 ≤ x ≤ 2.0) by a citrate-gel precursor method. Singhal et al. [7] and Toksha et al. [12] synthesized the CoCr x Fe2−x O4 powders (0.0 ≤ x ≤ 2.0) by a sol-gel auto-combustion method. Vadivel et al. [9] and Kumari et al. [13] produced the Cr-substituted CoFe2O4 nanoparticles (NPs) by a coprecipitation method. Lastly, Panda et al. [14] investigated the effect of incorporation of Cr3+ into CoFe2O4 NPs on its magnetic and electric properties, prepared by a auto-combustion method.
Literature survey reveals that no systematic studies are available on the synthesis of Cr-substituted CoFe2O4 NPs by a microwave combustion method. Therefore, in the present investigation, pure and Cr-substituted CoFe2O4 NPs were prepared by the combustion method for various concentrations of Cr. The effect of Cr concentration on structural, spectral, morphological, and magnetic properties is reported in the present work.
2 Experimental
2.1 Chemicals and Instrumentation
Analytical grade chemical reagents Co(NO3)2⋅6H2O, Cr(NO3) ⋅9H2O, and Fe(NO3)3⋅9H2O, and citric acid monohydrate, C6H8O7⋅H2O (as fuel), were obtained from Merck and used without further purification process.
The crystalline structure of resultant nanoparticles was determined with X-ray diffraction (XRD) measurements using Rigaku D/Max-IIIC with Cu-K α radiation in the 2 𝜃 range of 20 ∘–70 ∘.
Fourier transform infrared spectroscopy (FT-IR) spectra were recorded in transmission mode with a PerkinElmer BX FT-IR infrared spectrometer. FT-IR spectra in the range of 4000–400 cm−1 were recorded in order to investigate the nature of the chemical bonds formed.
The surface morphology of the composites was analyzed with JEOL JSM-7001F scanning electron microscopy (SEM).
Magnetic measurements were performed by using a Quantum Design vibrating sample magnetometer (QD-VSM). The sample was measured at ±10 kOe at room temperature (25 ∘C).
2.2 Procedure
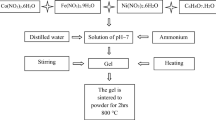
Nanocrystalline powders of CoCr x Fe2−x O4 (0.0 ≤ x ≤ 1.0) were synthesized by a microwave method. For each experiment, a stoichiometric amount of metal salts and 1 g citric acid were taken and ground in an agate mortar for 10 min and then they were placed into a commercial microwave oven. At the beginning of the reaction, there was gas evolution from the crucible and then the color of the reactant mixture turned into red color due to the high temperature inside the oven. Unfortunately, it was possible to measure the temperature inside the oven during the chemical reaction which was completed in 5 min. Then, the solid product was washed with distilled water-ethanol and dried at 150 ∘C to remove remaining water contents.
3 Results and Discussion
3.1 XRD Analysis
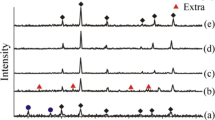
The XRD powder patterns and variation of lattice constants with Cr concentration (x) of CoCr x Fe2−x O4 (0.0 ≤ x ≤ 1.0) NPs are presented in Fig. 1a, b, respectively. The XRD powder patterns indicate that all products contain single-phase spinel cubic structure (Fig. 1a) (except for x = 1.0 which contains a small amount of impurity). The XRD peaks of all products can be indexed to the spinel cubic structure of CoFe2O4 (ICDD card no: 03-0864) [15]. It is observed that lattice parameter goes on decreasing from 8.384 Å (x = 0.0, CoFe2O4) to 8.362 Å (x = 1.0, CoCrFeO4) with increasing Cr content (x). The decrease of a o with an increase in x is due to the fact that the larger ionic radii of Fe3+ (0.67 Å) are replaced by smaller ionic radii of Cr3+ (0.63 Å) (Fig. 1b). Therefore, the lattice parameter decreases because of shrinkage of unit cell dimension. This indicates a difference of Fe3+ and Cr3+ in an oxide solid solution with a spinel-type structure [9, 12]. The variation of lattice parameters with x for all products obeys Vegard’s law [16]. This observation is in good agreement with the reports given in the literature [9, 12, 17, 18]. The variation of crystallite sizes with x is presented in Fig. 2.
3.2 Cation Distribution
Site occupancy of all constituent ions in CoCr x Fe2−x O4 (0.0 ≤ x ≤ 1.0) NPs is presented in Table 1. It is observed that CoFe2O4 (x = 0.0) shows an inverse spinel structure where most of the divalent Co ions occupy octahedral B site and Fe ions are randomly distributed over both the sites. As the Cr3+ substitution in CoFe2O4 increased, it prefers to occupy the octahedral B site only. As the substitution level of Cr3+ ion increased, it pushes some of the Co ions to the tetrahedral A site. However, throughout the substitution level, Co ions show preference toward the octahedral B site along with Cr3+ ions.
3.3 FT-IR Analysis
The FT-IR spectra of CoCr x Fe2−x O4 (0.0 ≤ x ≤ 1.0) NPs are presented in Fig. 3 which showed that all products have two prominent absorption bands, ν 1 (due to the stretching of tetrahedral metal ion and oxygen bonding) and ν 2 (vibrations of oxygen in the direction perpendicular to the axis joining the tetrahedral ion and oxygen), in the range of 600–500 and 430–385 cm−1, respectively [9, 12, 19]. As it can be seen from Fig. 3, the Fe–O bands towards higher frequency with an increase in concentration of Cr3+ ions [9, 11, 20].
3.4 VSM Analysis
The room-temperature M–H graphs of CoCr x Fe2−x O4 (0.0 ≤ x ≤ 1.0) NPs are presented in Fig. 4. The substitution of some trivalent nonmagnetic ions for Fe3+ in spinel ferrites is made for special magnetic and electrical function. From Fig. 4, it can be noticed that the saturation magnetization (∼79 emu/g which is in good agreement with the reported values [20]) is high for CoFe2O4 (x = 0.0) NPs and it decreases gradually with increasing Cr concentration (x) (the saturation magnetization, M s, decreases from 79 to 21.4 emu/g with the increase in Cr3+ concentration) (Fig. 4, inset). This observation can be explained as follows: The introduction of nonmagnetic ions on octahedral B sites in the inverse spinel cobalt ferrite will reduce the saturation magnetization since it has a net uncompensated moment and alters their magnetic and electric properties [18]. As it was stated in various reports [11, 14], there is replacement of Fe3+ ions (3d 5, magnetic moment 5 μ B) by lesser magnetic Cr3+ ions (3d 8, magnetic moment 3 μ B) in the octahedral sites [9]. The substitution of Cr3+ into B site (octahedral site) was also confirmed by the cation distribution calculations above.
3.5 SEM and Energy-Dispersive X-ray Spectroscopy Analysis
The SEM micrographs, the energy-dispersive X-ray spectroscopy (EDX) spectra (along with elemental percentage of each element, O, Co, Cr, and Fe), and the elemental maps (including the mapping of the cation distributions) of CoCr0.4Fe1.6O4 and CoCr0.8Fe1.2O4 NPs are presented in Fig. 5a, b, respectively. The cubic morphology can be clearly seen for both products. It also shows the presence of a large agglomeration phenomenon in the grains, which might be caused by the high temperature during the microwave irradiation for both products. The average particle size of both products is in the range of 100–200 nm. Values of the particle sizes differ significantly from those determined from XRD powder patterns due to the agglomeration. The SEM-EDX results confirmed the formation of exact composition of spinel-type chromium-substituted cobalt ferrite spinel compounds (for all products) with O, Co, Cr, and Fe elements, and no impurity is present in the products [21–24]. Figure 5 shows the representative X-ray elemental maps of all elements detected in EDX, that is, oxygen, chromium, cobalt, and iron, in CoCr0.4Fe1.6O4 and CoCr0.8Fe1.2O4 NPs. Both maps indicate fairly homogeneous elemental distributions in both products.
4 Conclusion
Pure and Cr-doped cobalt ferrite nanoparticles were successfully prepared by the microwave combustion method from nitrate salts of Co, Cr, and Fe using citric as fuel. The crystallite size was found to vary slightly from 30.6 to 45.8 nm. The formation of single and pure spinel phase was confirmed by the Rietveld analysis. The lattice parameter is reduced from 8.384 to 8.362 Å by Cr3+ substitution, leading to the contraction of the unit cell volume. The decrease in lattice constant is due to the smaller ionic radii of the doped cation Cr3+ (0.63 Å) compared to that of Fe3+ (0.67 Å). Fe3+ was replaced at B site (octahedral) by Cr3+ that weakened the magnetic interaction among cations and which caused a decrease in saturation magnetization. Spinel ferrites are widely used in microwave devices due to their high resistivity, and the substitution of Cr3+ increases the resistivity due to the octahedral B site preference of Cr3+. Hence, these materials can be used in microwave devices also. Hence, as a further study, the resistivity studies related to these products are still going on.
References
Ortega, A.L., Lottini, E., Fernandez, C.J., Sangregorio, C.: Chem. Mater. 27, 4048–4056 (2015)
Chinnasamy, C.N., Jeyadevan, B., Shinoda, K., Tohji, K., Djayaprawira, D.J., Takahashi, M., Joseyphus, R.J., Narayanasamy, A.: Appl. Phys. Lett. 83, 2862–2864 (2003)
Wang, Y.C., Ding, J., Yin, J.H., Liu, B.H., Yi, J.B., Yu, S.: J. Appl. Phys. 98, 124306 (2005)
Wang, J., Gao, X., Yuan, C., Li, J., Bao, X.: J. Magn. Magn. Mater. 401, 662–666 (2016)
Qu, Y., Yang, H., Yang, N., Fan, Y., Zhu, H., Zou, G.: Mater. Lett. 60, 3548 (2006)
Kasapoğlu, N., Baykal, A., Köseoğlu, Y., Toprak, M.S.: Scr. Mater. 57, 441–444 (2007)
Singhal, S., Jauhar, S., Singh, J., Chandra, K., Bansal, S.: J. Mol. Struct. 1012, 182 (2012)
Gul, I.H., Maqsood, A.: J. Alloys Compd. 465, 227 (2008)
Vadivel, M., Ramesh Babu, R., Sethuraman, K., Ramamurthi, K., Arivanandhan, M.: J. Magn. Magn. Mater. 362, 122–129 (2014)
Iqbal, M.J., Siddiquah, M.R.: J. Alloy Compd. 453, 513–518 (2008)
Hankarea, P.P., Sankpal, U.B., Patil, R.P., Mulla, I.S., Lokhandec, P.D., Gajbhiye, N.S.: J. Alloy Compd. 485, 798–801 (2009)
Toksha, B.G., Shirsath, S.E., Mane, M.L., Patange, S.S., Jadhav, S.S., Jadhav, K.M.: J. Phys. Chem. C 115, 20905–20912 (2011)
Kumari, N., Kumar, V., Singh, S.K.: Cer. Int. 40, 12199–12205 (2014)
Panda, R.K., Muduli, R., Jayarao, G., Sanyal, D., Behera, D.: J. Alloy Compd. 669, 19–28 (2016)
Manikandan, A., Sridhar, R., Antony, S.A., Ramakrishna, S.: J. Mol. Struct. 1076, 188–200 (2014)
Vegard, L.: Z. Phys. 15, 17–26 (1921)
Mane, D.R., Patil, S., Birajdar, D.D., Kadam, A.B., Shirsath, S.E., Kadam, R.H.: Mater. Chem. Phys. 126, 755–760 (2011)
Chae, K.P., Lee, Y.B., Lee, J.G., Lee, S.H.: J. Magn. Magn. Mater. 220, 59–64 (2000)
Waldron, R.D.: Phys. Rev. 99, 1727–1735 (1955)
Singhal, S., Barthwal, S.K., Chandra, K.: J. Magn. Magn. Mater. 306, 233–240 (2006)
Khalaf, K.A.M., Al-Rawas, A.D., Widatallah, H.M., Al-Rashdi, K.S., Sellai, A., Gismelseed, A.M., Hashim, M., Jameel, S.K., Al-Ruqeishi, M.S., Al-Riyami, K.O., Shongwe, M., Al-Rajhi, A.H.: J. Alloy Compd. 657, 733–747 (2016)
Padmaraj, O., Venkateswarlu, M., Satyanarayana, N.: Ceram. Int. 41, 3178–3185 (2015)
Wu, K., Hu, G., Du, K., Peng, Z., Cao, Y.: Synthesis and electrochemical characterization of LiCo1/3Fe2/3 PO 4/C composite using nano CoFe2O4 as precursor. J. Alloy Compd. 650, 718–723 (2015)
Baykal, A., Elmal, A.Z., Sertkol, M., Sözeri, H.: J. Supercond. Nov. Magn. 28, 3405–3410 (2015)
Acknowledgments
This study is supported by the Fatih University under BAP grant no. P50031504_B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baykal, A., Eryiğit, Ş., Sertkol, M. et al. The Effect of Cr3+ Substitution on Magnetic Properties of CoFe2O4 Nanoparticles Synthesized by Microwave Combustion Route. J Supercond Nov Magn 29, 2395–2400 (2016). https://doi.org/10.1007/s10948-016-3533-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-016-3533-z