Abstract
La0.5Sr0.5Mn1−x Co x O 3 (0 ≤x ≤ 0.3) is obtained by calcining the precursor carbonates in air. The precursor and its calcined products are characterized by X-ray powder diffraction, scanning electron microscopy, and vibrating sample magnetometer. A highly crystallized La 0.5Sr 0.5Mn 1−x Co x O 3 (0 ≤x ≤ 0.3) with a perovskite structure is obtained when the precursor is calcined at 900 ∘C in air for 3 h. The lattice strains of La 0.5Sr 0.5Mn 1−x Co x O 3 increase with the increase of Co content and/or the decrease of Mn content between x = 0 and x = 0.1 at first, then decreases with the increase of Co content. The magnetic properties of La 0.5Sr 0.5Mn 1−x Co x O 3 depend on the composition and measurement temperature. Cobalt substitution can markedly improve the coercivity of La 0.5Sr 0.5Mn 1−x Co x O 3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Perovskite-type oxides Re 1−x A x MnO 3 (Re = La, Pr, Nd, Sm, etc. and A is an alkaline earth element or a divalent element such as Ca, Sr, Ba, or Pb) have recently attracted much attention due to their interesting properties, such as electrical, mechanical, optical, magnetic, and catalytic properties [1–12]. Thus, perovskite-type oxides have been widely used as electrode materials for solid oxide fuel cells [8, 12–14], chemical sensors [15, 16], oxygen-permeating membranes [17, 18], thermoelectric devices [19], catalysts [7, 20, 21], ferroelectrics, superconductors, and fluorescent materials [22]. Besides, perovskite-type oxide is also a promising magnetic refrigeration material [23–25]. Among the perovskite-type oxides, La 0.5Sr 0.5MnO 3 [26, 27] and doped La 0.5Sr 0.5MnO 3 [28–30] are important perovskite-type oxides. The structures and properties of these materials highly depend on the composition, synthesis method, and calcination temperature. Therefore, the properties of La 0.5Sr 0.5MnO 3 and its related materials can be regulated by the composition and synthesis method. Shi et al. [27] synthesized La 0.5Sr 0.5MnO 3 by conventional solid-state reaction at 1400 ∘C for 24 h using La 2 O 3, SrCO 3, and MnO 2 powders as raw materials. Both the specific saturation magnetizations of the samples annealed in nitrogen and oxygen, respectively, are higher than those of the sample annealed in air. Xia et al. [28] prepared polycrystalline La 0.5Sr 0.5Mn 1−x Ti x O 3 by a standard solid-state reaction method using La 2 O 3, SrCO 3, MnO 2, and TiO 2 as raw materials. The results showed that La 0.5Sr 0.5Mn 1−x Ti x O 3 sintered at 1573 K for 12 h suppresses the antiferromagnetic (AFM) charge ordering and leads to a step-like magnetization behavior below 3 K after doping Ti 4+. Yu et al. [30] synthesized La 0.7Sr 0.3Mn 1−x Co x O 3 by conventional solid-state reaction using La 2 O 3, SrCO 3, MnCO 3, and Co 2 O 3 as precursors, followed by grinding, mixing, and calcination at 1200 ∘C for 24 h. The results showed that the ferromagnetic (FM)–paramagnetic (PM) phase transition temperature (T C) values and magnetization decrease with the increase of Co concentration in the sample, attributed to the presence of Co ions (normally, with oxidation states 3 + and 4 + in manganites) which reduces FM interactions due to a decrease of the Mn 3+/Mn 4+ ratio and to an enhancement of antiferromagnetic interactions between Co and Mn ions. Although many researchers have made great efforts to prepare La 1−x Sr x MnO 3 and/or doped La 1−x Sr x MnO 3, facile and scalable synthesis of La 1−x Sr x MnO 3 and/or doped La 1−x Sr x MnO 3 with high performance is still a significant challenge. Therefore, it is highly desirable and necessary to explore new synthesis methods and composition for the preparation of La 1−x Sr x MnO 3 and/or doped La 1−x Sr x MnO 3. To the best of our knowledge, the synthesis and magnetic properties of La 0.5Sr 0.5Mn 1−x Co x O 3 by calcining precursor carbonates have rarely been reported in previous studies, due to the smaller ionic radii of Co 3+ ion (0.061 nm) [31] and same unpaired electrons in 3d orbit compared to Mn 3+ (0.064 nm) [32]. However, Co 3+ ion (0.061 nm, d 6) has larger ionic radii and more unpaired electrons in 3d orbit than Mn 4+ ion (0.053 nm, d 3) [33]; part of substitution of Mn 3+ and/or Mn 4+ ions by Co 3+ ions in La 0.5Sr 0.5MnO 3 will lead to lattice strain and magnetic property changes.
This study aims to prepare La 0.5Sr 0.5Mn 1−x Co x O 3 (0. ≤x ≤ 0.3) by calcining carbonate in air and study the effect of composition and calcination temperature on structure, lattice strains, and magnetic properties of La 0.5Sr 0.5Mn 1−x Co x O 3. Our results clearly show that the specific magnetizations of La 0.5Sr 0.5Mn 1−x Co x O 3 decrease with the increase of Co concentration in the sample. However, the coercivity of La 0.5Sr 0.5Mn 1−x Co x O 3 increases with the increase of Co concentration in the sample.
2 Experimental
2.1 Reagent and Apparatus
All chemicals used are of reagent-grade purity (purity >99.9 %). X-ray powder diffraction (XRD) was performed using an X’pert PRO diffractometer equipped with a graphite monochromator and a Cu target. The radiation applied was Cu K α (λ = 0.15406 nm), operated at 40 kV and 50 mA. The XRD scans were conducted from 5 ∘ to 70 ∘ in 2 𝜃, with a step size of 0.01 ∘ The morphologies of the synthesis products were observed using an S-3400 scanning electron microscope (SEM). The specific magnetizations (M) of the calcined sample powders were carried out at different measurement temperatures using a vibrating sample magnetometer (Lake Shore 7410).
2.2 Preparation of La0.5Sr0.5Mn1−x Co x O3
The La 0.5Sr 0.5Mn 1−x Co x O 3 precursor samples were synthesized by solid-state reaction at low temperatures [34, 35]. In a typical synthesis (La 0.5Sr 0.5MnO 3), La (NO 3) 3⋅6H 2O (15.00 g), SrCl 2 (5.49 g), MnCl 2⋅4H 2O (13.71 g), and Na 2 CO 3 (19.85 g) were placed in a mortar, and the mixture was thoroughly ground by hand with a rubbing mallet for 40 min. The strength applied was moderate. The reactant mixture gradually became damp, and a paste was formed immediately. The reaction mixture was kept at 30 ∘C for 2 h. The mixture was washed with deionized water to remove soluble inorganic salts until SO\(_{\mathrm {4}}^{\mathrm {2-}}\) ion cannot be visually detected with a 0.5 mol L −1 BaCl 2 solution. The mixture was then washed with a small amount of anhydrous ethanol. La 0.5Sr 0.5MnO 3 precursor was obtained after being dried at 80 ∘C for 5 h. A similar synthesis procedure was used to synthesize other La 0.5Sr 0.5Mn 1−x Co x O 3 precursor. Finally, perovskite-type La 0.5Sr 0.5Mn 1−x Co x O 3 was obtained by calcining the precursor at a heating rate of 3 ∘C min −1 from ambient temperature to 900 ∘C in air in a muffle furnace and then kept at 900 ∘C for 3 h.
3 Results and Discussion
3.1 Composition Analysis of the Precursor
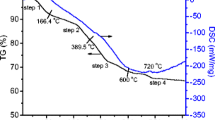
0.0300 g precursor sample was dissolved in 10 mL 50 vol.% HCl solution, then diluted to 100.00 mL with deionized water. Lanthanum (La), strontium (Sr), and manganese (Mn) in the solution were determined by inductively coupled plasma atomic emission spectrometry (ICP-AES, Perkin Elmer Optima 5300 DV). The results showed that the La, Sr, and Mn mass percentages were 21.03, 13.26, and 16.64 %, respectively. In other words, molar ratio of La/Sr/Mn in the precursor is 0.5:0.5:1.00.
3.2 XRD Analyses of the Calcined Products
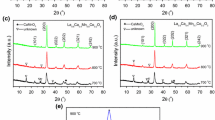
Figure 1 shows the XRD patterns of calcined samples from different calcination temperatures. A highly crystallized La 0.5Sr 0.5Mn 1−x Co x O 3 (0 ≤x ≤ 0.3) with a perovskite structure is obtained when the precursor is calcined at 900 ∘C in air for 3 h (Fig 1a–d). The Co 3+-doped ions do not obviously change the perovskite crystalline structure of ABO 3. As shown in the XRD patterns (Fig. 1e), a slight shift of the peaks of XRD to a higher angle is observed in the Co 3+-doped La 0.5Sr 0.5Mn 1−x Co x O 3 due to the smaller ionic radii of Co 3+ (0.061 nm) [31] than that of Mn 3+ (0.064 nm) [32]. The refined lattice parameters of La 0.5Sr 0.5Mn 1−x Co x O 3 (x = 0, 0.1, 0.2, and 0.3) calcined at 900 ∘C were obtained. The results are shown in Table 1. With the increase of doped Co 3+ amount from x = 0, 0.1, 0.2 to 0.3, lattice parameters a and b values decrease but a slight increase for x = 0.3. This could be due to smaller Co 3+ (0.061 nm) [31] ions substituting Mn 3+ (0.064 nm) [32] initially, and then for higher doping level, some of Co 3+ may substitute the Mn 4+ (with much smaller ionic radius of 0.053 nm [33]). A similar phenomenon was also observed for La 0.5Sr 0.5Mn 1−x Ti x O 3 [33].
Compared to solid-state reaction at high temperatures using a mixture of La 2 O 3, SrCO 3, MnCO 3, and Co 2 O 3 [27, 30], the crystallization temperature of La 0.5Sr 0.5Mn 1−x Co x O 3 in this study is lower, while the crystallinity of La 0.5Sr 0.5Mn 1−x Co x O 3 is higher. This finding is attributed to the fact that direct high-temperature solid-state reaction induces difficult penetration between solid particles, resulting in the crystallization of La 0.5Sr 0.5MnO 3 and La 0.7Sr 0.3Mn 1−x Co x O 3 at higher temperature. However, a mixture of La(NO 3) 3⋅6H 2O, SrCl 2, MnCl 2⋅4H 2O, CoSO 4⋅7H 2O, and Na 2 CO 3 was ground at room temperature in our study. Precursor carbonates can be obtained with molecular-level scale after uniform mixing. Single-phase crystalline La 0.5Sr 0.5Mn 1−x Co x O 3 can then be obtained at a lower temperature when the precursor is calcined in air.
The crystallite diameter of La 0.5Sr 0.5Mn 1−x Co x O 3 was estimated using the Scherrer formula [34]:
where D is the crystallite diameter, K = 0.89 (the Scherrer constant), λ = 0.15406 nm (wavelength of the X-ray used), β is the width of line at the half-maximum intensity, and 𝜃 is the corresponding angle. From the position of the (1 0 4) peak (2 𝜃 (1 0 4)) in XRD patterns, the d (1 0 4) interplanar spacing is determined using the Bragg equation [36]:
The crystallite diameter (D) of La 0.5Sr 0.5Mn 1−x Co x O 3 from calcining the precursor at different temperatures and d (1 0 4) interplanar spacing of La 0.5Sr 0.5Mn 1−x Co x O 3 obtained at 900 ∘C are shown in Figs. 2 and 3, respectively. From Fig. 2, it can be seen that after the addition of Co 3+ ions, the average crystallite size of samples decreases slightly and reaches the lowest value (17.5 nm) when x is 0.3. The binding energy of Co 3+–O 2− is larger than that of Mn 3+–O 2−, attributed to the Co 3+ ion (0.061 nm) having smaller radii than Mn 3+ ion (0.064 nm). When Co 3+ ions enter into the lattice to form the Co 3+–O 2− bonds, the crystal nucleation and growth of Co 3+substituted (La, Sr)MnO 3 manganite (LSMO) will consume more energy, which results in a smaller average crystallite diameter for substituted LSMO manganite. The d (1 0 4) values of the samples in Fig. 3 reveal that the interplanar spacing increases slightly with the addition of small amount of Co 3+ ions which is consistent with the XRD result in Fig. 1e. It can be clearly seen from Fig. 2e that the (1 0 4) peak shifts slightly to higher degrees with the increase of Co 3+ content. The increase in interplanar spacing of samples can be explained on the basis of the radii of metal ions. The radius of Co 3+ ion (0.061 nm) is smaller than that of Mn 3+ ion (0.064 nm). Therefore, the Co 3+ ion could locate in the B sublattice with adequate space. The replacement of Mn 3+ ions in La 0.5Sr 0.5MnO 3 by Co 3+ ions could cause the contraction of the unit cell (such as a and b values), resulting in the decrease of interplanar spacing [37, 38].
The crystallinity of La 0.5Sr 0.5Mn 1−x Co x O 3 can be calculated by MDI Jade 5.0 software. The crystallinity of La 0.5Sr 0.5Mn 1−x Co x O 3 (x = 0, 0.1, 0.2, and 0.3) obtained at different temperatures is shown in Fig. 4. The crystallinities of La 0.5Sr 0.5Mn 1−x Co x O 3 (x = 0, 0.1, and 0.2) obtained at 700 ∘C increase with increasing Co 2+ content except for La 0.5Sr 0.5Mn 0.7Co 0.3 O 3. However, the crystallinity of La 0.5Sr 0.5Mn 1−x Co x O 3 obtained above 800 ∘C is approximately 100 %.
Lattice strains of the La 0.5Sr 0.5Mn 1−x Co x O 3 were estimated using the Williamson–Hall formula [39, 40]:
where B is the full width at half of the maximum (in radian) of the peaks, 𝜃 is the peak position, and ε is the lattice strain of the structure. The lattice strains of La 0.5Sr 0.5Mn 1−x Co x O 3 obtained at 900 ∘C are 0.598 % for x = 0, 0.636 % for x = 0.1, 0.552 % for x = 0.2, and 0.541 % for x = 0.3, respectively. That is, the lattice strain of La 0.5Sr 0.5Mn 1−x Co x O 3 increases with the increase of Co 3+ content and/or the decrease of Mn 3+ content between x = 0 and x = 0.1 at first, then decreases with the increase of Co 3+ content, which can be attributed to the Co 3+ ion (0.061 nm) having a smaller radius than the Mn 3+ ion (0.064 nm). The replacement of Mn 3+ ions in La 0.5Sr 0.5Mn 1−x Co x O 3 by Co 3+ ions would cause the decrease of the unit cell (a and b values), resulting in the increase of lattice strain in La 0.5Sr 0.5Mn 1−x Co x O 3. For higher doping level, some of Co 3+ may substitute the Mn 4+ (with much smaller ionic radius of 0.053 nm), which would cause the expansion of the unit cell (c value), resulting in the decrease of lattice strain in La 0.5Sr 0.5Mn 1−x Co x O 3.
3.3 SEM Analyses of the Calcined Products
The morphologies of the calcined products are shown in Fig. 5. Figure 5a shows that the La 0.5Sr 0.5MnO 3 sample obtained at 900 ∘C is composed of approximately spherical grains and contains particles having a distribution from 80 to 250 nm. Figure 5b–d shows the SEM images of the La 0.5Sr 0.5Mn 1−x Co x O 3 (x = 0.1, 0.2, and 0.3) samples obtained at 900 ∘C, respectively. The La 0.5Sr 0.5Mn 1−x Co x O 3 (x = 0.1, 0.2, and 0.3) sample is also composed of approximately spherical grains. The particle sizes are mainly between 70 and 200 nm. The average crystallite sizes of the calcined samples determined by X-ray diffraction were significantly smaller than the values determined by SEM. This difference can be attributed to the fact that the values observed by SEM have the size of the secondary particles, which are composed of several or many crystallites by soft reunion. In addition, the X-ray line broadening analysis disclosed only the size of a single crystallite.
3.4 Magnetic Properties of La 0.5Sr 0.5Mn 1−x Co x O 3
Hysteresis loops of La 0.5Sr 0.5Mn 1−x Co x O 3 samples calcined at 900 ∘C at different measurement temperatures are shown in Fig. 6. Figure 7 shows the dependence of specific magnetization of La 0.5Sr 0.5Mn 1−x Co x O 3 on doping Co 3+ amount and measurement temperature. The specific magnetization decreases with the increase of Co 3+ concentration in the sample and measurement temperature (see Fig. 7). It can be attributed to the substitution of Mn 3+ (0.064 nm) and/or Mn 4+ (0.053 nm) by Co 3+ (0.061 nm) which promotes a cation arrangement in La 0.5Sr 0.5Mn 1−x Co x O 3, resulting in the decrease of specific magnetization for La 0.5Sr 0.5Mn 1−x Co x O 3. Among La 0.5Sr 0.5Mn 1−x Co x O 3 (x = 0, 0.1, 0.2, and 0.3), La 0.5Sr 0.5MnO 3 at 100 K measurement temperatures has the highest specific magnetization value, 12.69 emu/g. The dependence of remanence (M r) and coercivity (H c) on Co 3+ content is shown in Fig. 8. La 0.5Sr 0.5Mn 0.9Co 0.1 O 3 at 100 K measurement temperatures has the highest remanence (4.26 emu/g) and coercivity (496.24 Oe).
The magnetic moment of La 0.5Sr 0.5Mn 1−x Co x O 3 samples obtained at 900 ∘C was estimated using the following relationship [41, 42]:
where M w is the molecular weight of the composition, M is the specific magnetization (emu/g) at an applied field of 19.5 kOe, and η B is the magnetic moment (Bohr magneton, B.M.). The magnetic moment values of La 0.5Sr 0.5Mn 1−x Co x O 3 are shown in Fig. 9. The magnetic moment value of La 0.5Sr 0.5Mn 1−x Co x O 3 at an applied field of 19.5 kOe decreases with the increase of Co 3+ concentration in the sample and measurement temperature. La 0.5Sr 0.5MnO 3 at 100 K has the highest magnetic moment value (0.491 B.M.).
4 Conclusions
La 0.5Sr 0.5Mn 1−x Co x O 3 (x = 0, 0.1, 0.2, and 0.3) was successfully synthesized by calcining the precursor carbonates in air. The XRD analysis suggests that a rhombohedral La 0.5Sr 0.5Mn 1−x Co x O 3 (x = 0, 0.1, 0.2, and 0.3) with space group Pnma (62) can be obtained by calcining the precursor over 800 ∘C in air for 3 h. Lattice parameters (a and b values) decrease with the increase of Co 3+ additional amount but a slight increase for x = 0.3. Magnetic characterization indicates that the magnetic properties of La 0.5Sr 0.5Mn 1−x Co x O 3 depend on the composition and measurement temperature. Substitution of Mn 3+ and/or Mn 4+ ion by Co 3+ ion in La 0.5Sr 0.5Mn 1−x Co x O 3 decreases specific magnetization. However, the coercivity of La 0.5Sr 0.5Mn 1−x Co x O 3 can be markedly improved after doping Co 3+ ion.
References
Qin, L.Q., Wu, X.H., Wang, K.T., Wu, W.W., Zhou, K.W., Liao, S.: J. Supercond. Nov. Magn. 27, 2751–2756 (2014)
Giri, S.K., Nath, T.K.: J. Supercond. Nov. Magn. 28, 895–900 (2015)
Ho, T.A., Thanh, T.D., Phan, T.L., Oh, S.K., Yu, S.C.: J. Supercond. Nov. Magn. 28, 891–894 (2015)
Sbissi, K., Kahn, M.L., Ellouze, M., Hlil, E.K., Elhalouani, F.: J. Supercond. Nov. Magn. 28, 1433–1438 (2015)
Kolat, V.S., Esturk, U., Izgi, T., Gencer, H., Atalay, S.: J. Alloys Compd. 628, 1–8 (2015)
Zouari, S., Nasri, A., Ellouze, M., Hlil, E.K., Elhalouani, F.: J. Supercond. Nov. Magn. 27, 1473–1481 (2014)
Ran, R., Wu, X.D., Quan, C.Z., Weng, D.: Solid State Ionics 176, 965–971 (2005)
Huang, X.Q., Pei, L., Liu, Z.G., Lu, Z., Sui, Y., Qian, Z.N., Su, W.H.: J. Alloys Compd. 345, 265–270 (2002)
Khlifi, M., Dhahri, E., Hlil, E.K.: J. Supercond. Nov. Magn. 27, 1341–1345 (2014)
Yensano, R., Pinitsoontorn, S., Amornkitbamrung, V., Maensiri, S.: J. Supercond. Nov. Magn. 27, 1553–1560 (2014)
Ma, J., Cai, Y.Q., Wang, W.Z., Cui, Q., Theingi, M., Zhang, H., Chen, Q.M.: Ceram. Int. 40, 4963–4968 (2014)
Sarıboğa, V., Özdemir, H., Faruk Öksüzömer, M.A.: J. Eur. Ceram. Soc. 33, 1435–1446 (2013)
Phillipps, M.B., Sammes, N.M., Yamamoto, O.: J. Mater. Sci. 31, 1689–1692 (1996)
Jiang, S.P., Liu, L., Ong, K.P., Wu, P., Li, J., Pu, J.: J. Power Sources 176, 82–89 (2008)
Morin, F., Trudel, G., Denos, Y.: Solid State Ionics 96, 129–139 (1997)
Liu, X., Cheng, B., Hu, J.F., Qin, H.W., Jiang, M.H.: Sens. Actuators B 129, 53–58 (2008)
Kruidhof, H., Bouwmeester, H.J.M., Doorn, R.H.Ev, Burggraaf, A.J.: Solid State Ionics 63–65, 816–822 (1993)
Wei, Y.Y., Liu, H.F., Xue, J., Li, Z., Wang, H.H.: AIChE. J. 57, 975–984 (2011)
Weidenkaff, A., Robert, R., Aguirre, M., Bocher, L., Lippert, T., Canulescu, S.: Renew. Energy 33, 342–347 (2008)
Fu, S.S., Niu, H.L., Tao, Z.Y., Song, J.M., Mao, C.J., Zhang, S.Y., Chen, C.L., Wang, D.: J. Alloys Compd. 576, 5–12 (2013)
Gaillard, F., Li, X.G., Uray, M., Vernoux, P.: Catal. Lett. 96, 177–183 (2004)
Shimazaki, T., Yamazaki, T., Terayama, K., Yoshimura, M. J. Mater. Sci. Lett 19, 2029–2031 (2000)
Zhang, X.X., Tejada, J., Xin, Y., Sun, G.F., Wong, K.W., Bohigas, X.: Appl. Phys. Lett. 69, 3596–3598 (1996)
Guo, Z.B., Du, Y.W., Zhu, J.S., Huang, H., Ding, W.P., Feng, D.: Phys. Rev. Lett. 78, 1142–1145 (1997)
Szewczyk, A., Szymczak, H., Wiśniewski, A., Piotrowski, K., Kartaszyński, R., Dabrowski, B., Koleśnik, S., Bukowski, Z.: Appl. Phys. Lett. 77, 1026–1028 (2000)
Teng, F., Han, W., Liang, S.H., Gaugeu, B., Zong, R.L., Zhu, Y.F.: J. Catal. 250, 1–11 (2007)
Shi, L., Yang, H.P., Zhou, S.M., Zhao, J.Y., He, L.F., Zhao, S.Y., Guo, Y.Q., Chen, L.: Solid State Commun. 150, 371–374 (2010)
Shang, C., Xia, Z.C., Wei, M., Chen, B.R., Jin, Z., Huang, J.W., Shi, L.R., Ouyang, Z.W., Huang, S. Ceram. Int. 41, 9708–9714 (2015)
Taran, S., Sun, C.P., Huang, C.L., Yang, H.D., Nigam, A.K., Chaudhuri, B.K., Chatterjee, S.: J. Alloys Compd. 644, 363–370 (2015)
Phan, T.L., Thanh, T.D., Yu, S.C.: J. Alloys Compd. 615, S247—S251 (2014)
Amer, M.A., Meaz, T.M., Mostafa, A.G., El-Kastawi, M., Ghoneim, A.I.: Ceram Int. 40, 241–248 (2014)
Güner, S., Amir, M., Geleri, M., Sertkol, M., Baykal, A.: Ceram Int. doi: 10.1016/j.ceramint.2015.05.034
Shang, C., Xia, Z.C., Jin, Z., Shi, L.R., Huang, J.W., Chen, B.R., Wei, M., Xiao, L.X., Liu, L., Huang, Y.: J Alloys Compd. 588, 53–58 (2014)
Wu, W.W., Cai, J.C., Wu, X.H., Liao, S., Wang, K.T., Tao, L.: Adv Powder Technol. 24, 154–159 (2013)
Zhou, K.W., Wu, X.H., Wu, W.W., Xie, J., Tang, S.Q., Liao, S.: Adv Powder Technol. 24, 359–363 (2013)
Li, D.Y., Sun, Y.K., Xu, Y., Ge, H.L., Wu, Q., Yan, C.: Ceram. Int. 41, 4581–4589 (2015)
Ateia, E.E., Ahmed, M.A., Salah, L.M., El-Gamal, A.A.: Physica B: Condens. Matter 445, 60–67 (2014)
Peng, Z.J., Fu, X.L., Ge, H.L., Fu, Z.Q., Wang, C.B., Qi, L.H., Miao, H.Z.: J. Magn. Magn. Mater. 323, 2513–2518 (2011)
Williamson, G.K., Hall, W.H.: Acta Metall. 1, 22–31 (1953)
Chen, W., Wu, W.W., Liu, S.Q., Xu, J.W., Liu, D.S., Wu, X.H., Zhou, Y., Wu, J.: Mater. Sci. Semicond. Process 39, 544–550 (2015)
Venkata Reddy, C., Byon, C., Narendra, B., Baskar, D., Srinivas, G., Shim, J., Prabhakar Vattikuti, S.V.: Superlattices Microstruct. 82, 165–173 (2015)
Mane, D.R., Birajdar, D.D., Shirsath, S.E., Telugu, R.A., Kadam, R.H.: Phys. Status Solidi A 207, 2355–2363 (2010)
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Grant no. 21161002) and the Guangxi University Student Innovation Foundation of China (Grant no. 30).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Chen, Y., Wu, W. et al. Lattice Strain and Magnetic Property Evolution of La0.5Sr0.5Mn1−x Co x O3 Synthesized by Solid-State Reaction at Low Temperatures. J Supercond Nov Magn 29, 115–122 (2016). https://doi.org/10.1007/s10948-015-3218-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-015-3218-z