Abstract
The effects of 0∽30 mol % excess Mg addition on the superconducting properties of MgB2 bulks were investigated. We found that the excess Mg addition improved the critical current (J c) at low temperatures and low magnetic fields. The critical temperatures (T c) of the excess Mg samples reduced slightly. The excess Mg reduces the MgO content in the system, improves the connectivity, and thus enhances the critical current density at low magnetic fields and low temperature. The highest J c was obtained in the 5-mol% excess Mg sample which has the best grain connectivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since the MgB2superconductor [1] is expected to be useful for various electric power and magnet applications, numerous efforts have been focused on improving basic properties such as the critical current (J c), the upper critical field (H c2), and the irreversibility field (H irr). Because these properties are very sensitive to preparation conditions, a large amount of research about MgB2 preparation conditions was reported, but there are still no optimal results. At present, such work mainly studies precursor powder [2, 3], temperature and rate of heat treatment [4, 5], ion doping [6–8], and the stoichiometric ratio of Mg:B and so on. There have been a number of investigations on the effects of the stoichiometry of MgB2 on superconducting properties [9–15]. These show that the nominal Mg content reduction does not give rise to serious deficiency of Mg and slight Mg deficiency does not affect T c and normal state properties of the samples strongly [12]. Furthermore, Jiang et al. found that the MgB2 tapes prepared with a slight Mg deficiency showed the best J c property in high fields whereas the stoichiometric sample or samples with a slight excess of Mg tended to exhibit higher J c in the low-field region [14]. It has also been reported that excess Mg facilitates oxygen alloying in the MgB2 lattice to form a nanometer-sized coherent Mg(B,O) phase and reduce the MgO formation [16]. The excess Mg improves the connectivity in MgB2 wires and then raises the transport current density of MgB2 wires [17]. The results show that there are considerable variations in the superconducting properties of MgB2 due to the change in stoichiometry. Especially, the effect on low-field J c has remained controversial. Therefore, it is important to clarify and understand the effect of excess Mg on the superconducting properties, especially on the J c.
In this study, the superconducting properties of MgB2 with normal stoichiometry and excess Mg were investigated. Also, we provide an alternative solution for the enhancement of J c by the excess Mg approach without any third element doping.
2 Experiments
MgB2 bulks samples were prepared by an in situ solid-state reaction method. Powders of magnesium (Mg, 99.99 %) and amorphous boron (B, 99.9 %) were mixed for the fabrication of MgB2 bulks. For the excess Mg addition samples, a mixture of (1 + x)Mg:2B was prepared (excess Mg is in the starting composition of Mg and B powder, and the excess content of Mg, x, is from 0 to 30 mol %). The well-mixed powders were pressed into pellets with a diameter of 10 mm and a thickness of about 3 mm under a pressure of ∼20 MPa.
All the pellet samples were sealed in vacuum quartz tubes. These pellets were then sintered in a furnace at 800 °C for 30 min and finally furnace-cooled to room temperature. All samples were characterized by X-ray diffraction (XRD). The magnetization of samples was measured at 10 to 30 K using a Quantum Design physical properties measurement system with a magnetic field sweep rate of 50 Oes −1 and an amplitude of up to 7 T. The magnetic J c was calculated from the height of the magnetization loop M using the extended Bean model: J c = 20 × (ΔM)/[a(1 − a/3b)] with a and b (a ≤ b) being the edge lengths of a thin sheet in the plane. T c was defined as the onset temperature at which diamagnetic properties were observed.
3 Results and Discussion
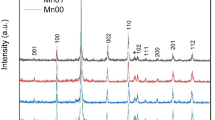
Figure 1a shows the typical θ–2θ X-ray diffraction patterns of MgB2 with the excess Mg content of 0 to 30 mol %. The relative content of MgO in the MgB2 bulk was calculated from the intensity value of MgO(220) and MgB2(101) diffraction peaks by the expression I MgO(220)/(I MgB2(101) + I MgO(220)). Figure 1b shows the relative content of MgO in the MgB2 bulk as a function of the excess Mg content. The X-ray diffraction analysis shows that the relative MgO content decreases for all the excess Mg addition samples compared with the normal sample with stoichiometric ratio. Especially for the 2-mol % excess Mg addition sample, the lowest relative MgO content was obtained. With careful transmission electron microscope (TEM) examination, Liao et al. have shown that oxygen atoms were dissolved into the MgB2 and then segregated to form a nanometer-sized coherent Mg(B,O) phase with the same basic crystal lattice as that of MgB2 [16]. So, the excess Mg facilitates oxygen alloying in the MgB2 lattice and decreases the MgO content. These findings are consistent with this work. Therefore, oxygen incorporation into the MgB2 lattice is the most effective for the sample with 2 mol% excess Mg addition. However, there are no obvious changes of the MgO contents for the samples with the excess Mg content of more than 10 mol%. It seems that there is a saturation of MgO contents for the samples with the excess Mg addition. The possible reason is that the excess Mg was vaporized and absorbed oxygen forming MgO outside the pellet during heat treatment.
Figure 2 shows scanning electron microscope (SEM) images of Mg excess samples. The excess Mg content is 0 to 30 mol%. For excess Mg addition samples, the structures of crystalline grains are irregular but arrangement directions of the grains are basically consistent along one direction, and the grains communicated with each other which show that the samples have good connectivity. For a normal sample with no excess Mg, the MgB2 grain size of it is smaller than that of excess Mg samples; however, the grain size is not uniform, with grain relatively very small inclusions in large grains which along different directions, so the sample connectivity is not good. For the 30-mol% excess Mg samples, the grain structure is the same as with the normal samples. The 2- and 5-mol% excess Mg samples show uniform internal grain and relatively good connectivity. Although the 10- and 20-mol% excess Mg samples have uniform grains and communicate with each other, there are large holes in the interior of the samples.
Figure 3 shows the magnetic moment as a function of temperature for MgB2 with excess Mg content of 0 to 30 mol%. Inserted is the superconductivity critical temperature as a function of excess Mg content for MgB2. It can be seen that the T c decreases with the excess Mg content increasing. These results show that oxygen doping into MgB2 reduced T c [16, 18]. The T c decreasing in this work may be due to the more excess Mg which results in more oxygen doping.
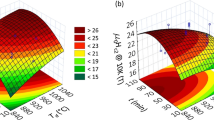
Figure 4 shows the magnetic critical current density (J c) versus the magnetic field for the excess Mg samples at different temperatures. The excess Mg content is 0 to 30 mol% and the temperature is 10 to 30 K. It is noted that the J c increases with excess Mg addition at low-field and low temperatures. The 5-mol% excess Mg sample has the highest J c value. The excess Mg in the precursor facilitates oxygen incorporation into the MgB2 lattice, reduces the MgO content in the system, improves the connectivity, and thus enhances the critical current density at low magnetic fields and low temperature. As observed from Fig. 2, the 2- and 5-mol% excess Mg samples show uniform internal grain, relatively good connectivity, and hence a higher J c value than those of other samples. However, the highest J c value is not for the 2-mol% Mg excess MgB2 sample which has the lowest relative MgO content. It suggested that in the excess Mg <10-mol% samples, the actual content of the MgO is far more than its theoretical content. There is a competition of the Mg consuming in the MgB2 formation, MgO formation, and Mg evaporation during the synthesis process, and for the 5-mol% excess Mg sample, the competition obtains an optimal balance and the sample has the best inter-grain connection. For further investigation, we calculated the density of the MgB2 sample which was prepared with a different excess Mg addition.
The density of MgB2 as a function of the excess Mg content is shown in Fig. 5. The density value of the MgB2 sample increased for the excess Mg content <10 mol%. The highest value was obtained in the 5-mol% excess Mg addition sample. The high density of MgB2 promotes grain connectivity. Therefore, the high J c was obtained for the highest density sample. For the excess Mg content >10-mol% samples, the density value decreased considerably. This is perhaps due to the evaporation of a large amount of Mg which results in large holes in the bulk as observed in Fig. 2. This result is consistent with the variation of J c with the excess Mg content addition increasing.
4 Conclusions
The effects of 0 ∼ 30 mol% excess Mg addition on the superconductivity of MgB2 bulks were investigated. It was found that the T c decreased slightly for all the samples with excess Mg addition. However, the J c enhanced for the excess Mg samples at low temperatures and low magnetic fields. The highest value of J c was obtained for the 5-mol% Mg excess sample. It suggested that the 5-% mol excess Mg sample has the best inter-grain connection; thus, it contributed to the highest value of J c at low temperatures and low magnetic fields. These results will benefit the preparation method for MgB2 to obtain high superconductivity.
References
Nagamatsu, J., Nakagawa, N., Muranaka, T., Zenitani, Y., Akimitsu, J.: Nature 410, 63–64 (2001)
J iang, J., Senkowicz, B.J., Larbalestier, D.C: Supercond. Sci. Technol. 19, L33–l36 (2006)
Wang, D.L., Ma, Y.W., Yu, Z.G.: Supercond. Sci. Technol. 20, 574–578 (2007)
Chen, S.K., Tan, K.S., Glowacki, B.A.: Appl. Phys. Lett. 87(182), 504–1-3 (2005)
Shcherbakova, O.V., Pan, A.V., Soltanian, S.: Supercond. Sci. Technol. 20, 5–10 (2007)
Dou, S.X., Soltanian, S., Zhao, Y., Getin, E., Chen, Z., Shcherbakova, O., Horvat, J.: Supercond. Sci. Technol. 18, 710–715 (2005)
Ma, Z.Q., Jiang, J.H., Liu, Y.C.: Supercond. Sci. Technol. 23, 025005–1-4 (2010)
DeSilva, K.S.B., Aboutalebi, S.H., Xu, X., Wang, X.L., Li, W.X., Konstantinov, K., Dou, S.X.: ScriptaMaterialia 69, 437–440 (2013)
Sharma, P.A., Hur, N., Horibe, Y., Chen, C.H., Kim, B.G., Guha, S., Cieplak, M.Z., Cheong, S-W.: Phys. Rev. Lett. 89, 167003–1-4 (2002)
Canfied, P.C., Finnemore, D.K., Bud’ko, S.L., Ostenson, J.E., Lapertot, G., Cunningham, C.E., Petrovic, C.: Phys. Rev. Lett. 86, 2423–1-4 (2001)
Zhao, Y.G., Zhang, X.P., Qiao, P.T., Zhang, H.T., Jia, S.L., Sao, C.B., Zhu, M.H., Han, Z.H., Wang, X.L., Gu, B.L.: Phys. C 366, 1–5 (2001)
Xiao, H., Peng, W., Song, W.H., Ma, R.C., Zhang, L., Du, J.J., Sun, Y.P.: Phys. C 386, 648–652 (2003)
Kim, K.H., Betts, J.B., Jaime, M., Lacerda, A.H., Boebinger, G.S.: Phys. Rev. B. 66, 020506–1-4 (2002)
Jiang, C.H., Nakane, T., Kumakura, H.: vol. 87, pp 505–1-3 (2005)
Idrobo, J.C.: vol. 70, pp 172503–1-4 (2004)
Liao, X.Z., Serquis, A.C., Zhu, Y.T., Huang, J.Y., Peterson, D.E., Mueller, F.M., Xu, H.F.: Appl. Phys. Lett. 80, 4398–4400 (2002)
Serquis, A., Civale, L., Hammon, D.L., Liao, X.Z., Coulter, J.Y., Zhu, Y.T., Peterson, D.E., Mueller, F.M.: J. Appl. Phys. 94, 4024–4031 (2003)
Eom, C.B.: Nature 411, 558–560 (2001)
Acknowledgments
In this paper, the authors wish to thank the fund for the project support, including the Fundamental Research Funds for the Central Universities (A0920502051411-5), the National Natural Science Foundation of China (51377138 and 51271155), and the National Science and Technology Projects Foundation (2013DFA51050, 2013GB114003-1, 2014AA032701).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, H., Zhao, Y. & Zhang, Y. The Effects of Excess Mg Addition on the Superconductivity of MgB2 . J Supercond Nov Magn 28, 2711–2714 (2015). https://doi.org/10.1007/s10948-015-3120-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-015-3120-8