Abstract
Structural and magnetic properties of La0.67 Ca0.33−x Sr x MnO3 (x = 0.06, 0.07, 0.08) manganites synthesized using the solid-state reaction method were investigated. Rietveld refinement of the X-ray diffraction patterns shows that all our samples crystallize in the orthorhombic structure with Pbnm space group. The temperature dependence of magnetization reveals that all the samples exhibit a ferromagnetic (FM) to paramagnetic (PM) transition at temperatures of 279 K for La0.67 Ca0.27Sr0.06MnO3, 286 K for La0.67Ca0.26Sr0.07MnO3, and 293 K for La0.67Ca0.25 Sr0.08MnO3. Using Arrot plots, the phase transition from FM to PM is found to be of second order. The maximum of themagnetic entropy change (−ΔS M) is found to be 3.37, 2.74, and 2.60 J kg−1 K−1 for x = 0.06, 0.07, and 0.08 under a magnetic field change of 3 T. The relative cooling power (RCP-1) for La0.67Ca0.33−x Sr x MnO3 sample is 152, 162, and 164 J kg−1 for x = 0.06, 0.07, and 0.08. According to these results, these kinds of samples are promising materials for magnetic refrigerants in the room-temperature region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the past few years, the manganites with the formula Ln1−x A x MnO3 (Ln = trivalent rare earth, A = divalent alkaline earth) have attracted much attention due to their extraordinary magnetic and electronic properties and their promise for future technological applications [1]. In particular, the La1−x Sr x MnO3 compounds are given particular attention because of their interesting magnetic properties such as colossal magnetoresistance (CMR) and magnetocaloric effect (MCE) [2]. Guo and colleagues [3] measured the large magnetic entropy change in perovskite-type manganese oxides and found that the compound La1−x Ca x MnO3 (x = 0.2, 0.33) showed larger magnetic entropy change than that of Gd.
Moreover, it has been reported that the magnetocaloric parameters in a series of manganite compounds belonging to the family La0.67Ca0.33−x Sr x Mn3+δ with x ∈ [0; 0.33], and it was found that the Curie temperature can be tailored between 267 and 369 K by the substitution. Also, it was found that the maximum magnetic entropy change of this compound, prepared by the technique of the glycine-nitrate synthesis with composition corresponding to x = 0.055, had the closest value of Curie temperature to that of the gadolinium [4]. In this context, the study of MCE in such lanthanum manganese compounds can be still of great interest. In this work, substitutions of x = 0.06, 0.07, and 0.08 are analyzed.
2 Experimental
Stoichiometric samples of La0.67Ca0.33−x Sr x MnO3 (x = 0.06, 0.07, 0.08) were prepared by the conventional solid-state reaction using purity commercial powders La2O3, MnCO3, SrCO3, and CaCO3 (99 %) as precursors. The precursors were ball-milled in ethanol for 1.5 h. The ball-milled slurry was filtered and dried at 60 °C for 3 h. The as-dried powders were ground and presintered at 1100 °C and sintered at 1150 °C for 8 h with intermediate grinding. The heating temperature and cooling rates for all the samples was 17 °C/min. The X-ray diffraction (XRD) patterns were recorded on a RIGAKU DMAX-2200 diffractometer equipped with a Cu-K α anode (λ = 1.54056 Å) in the 20°−80° 2θ range with a step size of 0.02°. Rietveld refinement analysis of the structure was performed using the MAUD program [5]. Magnetization measurements versus temperature in the range of 234–344 K versus an applied magnetic field up to 3 T were carried out using a vibrating sample magnetometer (VSM).
3 Results and Discussion
3.1 Structural Characterization
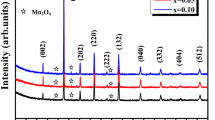
Figure 1 shows the indexed XRD patterns of La0.67 Ca0.33−x Sr x MnO3 (x = 0.06, 0.07, 0.08). The XRD patterns reveal that all solid solutions are a single phase corresponding to the orthorhombic perovskite structure with space group Pbnm. Refined cell parameters and the X-ray density are summarized in Table 1.
3.2 Magnetic Characterization
In order to determine the Curie temperature of all samples, their temperature dependence of magnetization, M(T), was measured under an applied magnetic field of 0.01 T (Fig. 2). All the samples showed a single magnetic transition from ferromagnetic (FM) to paramagnetic (PM) as the temperature increases. The FM-PM transition temperature or Curie temperature (T c) is defined at the inflection point of M(T) curves. The T c was found to be 279, 286, and 293 K for x = 0.06, 0.07, and 0.08, respectively. These temperatures are displaced with respect to the value of 267 K of the parent compound La0.67Ca0.33MnO3 [6].
In order to calculate the change of magnetic entropy of the compounds, magnetization curves versus the applied magnetic field, from 0 to 3 T, at different temperatures, were measured in the range from 239 to 334 K with a step of 5 K (Fig. 3). Below T c of every sample, the magnetization increases sharply for values of applied field lower than 0.5 T, and it tends to saturation for higher magnetic fields. Above T c, the magnetization M increases more smoothly, as a typical behavior of paramagnetic materials. This decrease is mainly due to the thermal agitation which tends to disorder the magnetic moments. This variation indicates that there is a large magnetic entropy change associated with the FM-PM transition temperature occurring at T c [7].
The nature of the magnetic transition in the samples was checked using the Banerjee criterion [8]. According to this criterion, the slope of H/M versus M 2 curves denotes whether the observed magnetic transition is of the first order (negative slope) or second order (positive slope). H/M versus M 2 curves obtained for all La0.67Ca0.33−x Sr x MnO3 (x = 0.06, 0.07, 0.08) samples clearly indicate positive slope in their complete M 2 range, and this confirms the transition to be of the second order. A typical set H/M versus M 2 curves for the La0.67Ca0.33−x Sr x MnO3 (x = 0.06) sample is shown in Fig. 4.
Using these data, the magnetic entropy change (ΔS M) versus temperature for a material that undergoes a second-order transition was determined from the discrete form of the following equation [8]:
Experimentally, two different methods are often used to evaluate the magnetic entropy change, ΔS M. The first one is the measurements of the MT curve under different applied magnetic fields. The second one is the measurement of the curve under different temperatures [9]. In this work, we used the second method to calculate the magnetic entropy change.
In the case of isothermal magnetization measurement with small discrete field and temperatures intervals, ΔS M can be approximated as follows [10]:
where M i and M i+1 are the experimental data of the magnetization at T i and T i+1, respectively, and the ΔH is the difference of the applied magnetic field.
The magnetic entropy change (ΔS M) as a function of temperature for the La0.67Ca0.33−x Sr x MnO3 sample investigated at a maximum magnetic field of 3 T is shown in Fig. 5. The magnetic entropy change reaches the maximum value around T c, and it increases with decreasing x. It can be seen that the magnetic entropy change depends on x for this samples. The maximum value of magnetic entropy change decreases from 3.37 J kg−1 K−1 for La0.67Ca0.27 Sr0.06MnO3, 2.74 J kg−1 K−1 for La0.67Ca0.26Sr0.07MnO3, and 2.60 J kg−1 K−1 for La0.67Ca0.25Sr0.08MnO3 samples. Comparing ΔS M values of these compounds with those of more complex manganite system and with higher applied magnetic fields [11], it is found that according to our results, they are more adequate for applications in magnetic refrigeration around room temperature.
Generally, an important index for selecting magnetic material refrigerants is based on the cooling power per unit volume, namely the relative cooling power (RCP) [4], which indicates how much heat can be transferred from the cold end to the hot end of a refrigerator describing a thermodynamic cycle. The first method consist in using not only the value of magnetocaloric effect (ΔS M) but also the width of the ΔS M(T) curve, which is calculated from the product of the maximum ΔS M peak value and the full width at half maximum, δ T FWHM, RCP-1(S) \( = {\Delta } S_{\mathrm {M}}^{\text {max}} \times \delta T_{\text {FWHM}}\) [11]. The higher the δ T FWHM value, the larger the temperature difference between the hot and the cool ends of the cycle that can be used for operation [12]. This method leads to values of 152 J kg−1 for La0.67Ca0.27Sr0.06MnO3, 162 J kg−1 for La0.67Ca0.26Sr0.07MnO3, and 164 J kg−1 for La0.67Ca0.25Sr0.08MnO3.
The second RCP-2 values (Fig. 6) were obtained from the area below the ΔS M(T) curve using T FWHM as the integration limits, according to
where T hot and T cold are the temperatures of the hot and cold sinks of the refrigerant thermodynamic cycle [13]. This method leads to values of 118 J kg−1 for La0.67 Ca0.27Sr0.06MnO3, 127 J kg−1 for La0.67Ca0.26Sr0.07MnO3, and 128 J kg−1 for La0.67Ca0.25Sr0.08MnO3.
4 Conclusions
In summary, good values of magnetocaloric parameters were observed in La0.67Ca0.27Sr0.06MnO3, La0.67 Ca0.26 Sr0.07MnO3, and La0.67Ca0.25Sr0.08MnO3 manganese perovskites. The largest value of the RCP is found in La0.67 Ca0.33−x Sr x MnO3, which exhibits good MCE properties. This kind of samples is suitable for their probable use in magnetic refrigeration at room-temperature region.
References
Gamzatov, A.G., Batdalov, A.B., Kaul, A.R., Mel’nikov, O.V.: Correlation of electrical, magnetic, and thermal properties of the La0.85Ag0.15MnO3 manganite near the phase transition temperature. Phys Solid State 53, 182–188 (2001)
Wang, Z.H., Shen, B.G., Tang, N., Cai, J.W., Ji, T.H., Zhao, J.G., Zhan, W.S., Che, G.C., Dai, S.Y., Dickon, H.L.: Colossal magnetoresistance in cluster glass-like insulator La0.67Sr0.33(Mn0.8Ni0.2)O3. J. Appl. Phys. 85, 5399–5401 (1999)
Guo, Z.B., Du, Y.W., Zhu, J.S., Huang, H., Ding, W.P., Feng, D.: Large magnetic entropy change in perovskite-type manganese oxides. Phys. Rev. Lett. 78, 1143–1145 (1996)
Dinesen, A.R., Linderoth, S., Morup, S.: Direct and indirect measurement of the magnetocaloric effect in La0.67Ca0.33−x Sr x MnO3±δ . J. Phys. Condens. Matter 17, 6257–6269 (2005)
MAUD Tutorial Version 2.0, Lutterotti, L. , MAUD: http://www.ing.unitn.it/~maud/tutorial.html., Accessed 4 July 2014
Sun, Y., Xu, X., Zhang, Y.: Large magnetic entropy change in the colossal magnetoresistance material La0.67Ca0.33MnO3. J. Magn. Magn. Mater 219, 183–185 (2000)
Arayedh, B., Kallel, S., Kallel, N., Peña, O.: Influence of non-magnetic and magnetic ions on the magnetocaloric properties of La0.7Sr0.3Mn0.9Mn0.1O3 doped in the Mn sites by M = Cr, Sn, Ti. J. Magn. Magn. Mater 361, 68–73 (2014)
Yang, H., Zhang, P., Wu, Q., Ge, H., Pan, M.J.: Effect of monovalent metal substitution on the magnetocaloric effect of perovskite manganites Pr0.5Sr0.3 M 0.2MnO3 (M = Na, Li,K and Ag). J. Magn. Magn. Mater 324, 3727–3730 (2012)
Fan, J., Ling, L., Hong, B., Pi, L., Zhang, Y.: Magnetocaloric effect in perovskite manganite Nd0.6La0.1Sr0.3MnO3. J. Magn. Magn. Mater 321, 2838–2841 (2009)
Mbarek, H., M’nasri, R., Cheikhrouhou-Koubaa, W., Cheikhrouhou, A.: Magnetocaloric effect near room temperature in (1-y)La0.8Ca0.05 K 0.15MnO3/yLa0.8 K 0.2MnO3 composites. Phys. Status Solidi 211, 975–979 (2014)
Jemaa, F.B., Mahmood, S., Ellouze, M., Hlil, E.K., Halouani, F., Bsoul, I., Awawdeh, M.: Structural, magnetic and magnetocaloric properties of La0.67Ba0.22Sr0.11Mn1−x Fe x O3 nanopowders. J. Magn. Magn. Mater, 121–130 (2014)
Tishin, A. M., Spichkin, Y. I.: The Magnetocaloric Effect and Its Applications (Bristol, Institute of Physics Publishing), 405–407 (2003)
Álvarez-Alonso, P., Santos, J.D., Pérez, M.J., Sánchez-Valdes, C.F., Sánchez Llamazares, J.L., Gorria, P.: The substitution effect of chromium on the magnetic properties of (Fe1−x Cr x )80Si6 B 14 metallic glasses (0.02 ≤ x ≤ 0.014). J. Magn. Magn. Mater, 75–78 (2013)
Acknowledgments
We thank CONACYT (México) for the support given to carry out this work. Thanks also to L.E.S.D. Gerardo Gabriel López Rocha of Physics Institute of the Universidad Autónoma de San Luis Potosí, SLP, México, for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hernández-González, E.L., Palomares-Sánchez, S.A., Elizalde Galindo, J.T. et al. Magnetocaloric Effect Near Room Temperature of La0.67Ca0.33−x Sr x MnO3 (x = 0.06, 0.07, 0.08) Manganites. J Supercond Nov Magn 28, 1635–1638 (2015). https://doi.org/10.1007/s10948-014-2932-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-014-2932-2