Abstract
In this study, the effect of temperature on the hydrothermal synthesis of single-phase SrFe12O19 hexaferrite (SrM) was investigated. For this synthesis, annealing or calcination process was applied. The Fe/Ba molar ratio was taken as 8:1. In this study, single-phase SrM NPs were synthesized via hydrothermal method. XRD patterns showed the presence of the hard (SrM) phase in the samples treated at 200 and 220 ∘C. Besides, formation of hexagonal plate-like samples was observed in SEM micrographs. Despite the low magnetization and coercive field values, the presence of the SrM phase was also shown in magnetization measurements. A reduced magnetization was explained by the existence of SrCO3 and Fe2O3 phases, and a high shape anisotropy is probably the reason of low coercivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
SrFe12O19 (SrM) ferrite of hexagonal M-type plays an important role in hard magnetic materials especially for its large magnetic anisotropy, high performance-to-cost ratio, and good chemical stability.
SrFe12O19 had several distinct properties, such as relatively large saturation magnetization, superior coercivity, high uniaxial magnetic crystalline anisotropy, chemical stability, and corrosion resistivity, which stimulated researchers to appreciate its importance in electronic components, magnetic memories, biotechnology, and in magnetic substrate for magnetic catalysts [1, 2].
Because the magnetic properties of SrFe12O19 strongly depend on the size and shape of the particles [3], several routes have been used to prepare strontium ferrite, including the traditional sol–gel process [4], the solid-state method [5], the salt-melting method [6], ball milling [7], self-propagating high-temperature synthesis [8], the chemical coprecipitation method [9], and the hydrothermal method [10]. In addition, the hydrothermal synthesis does not require extremely high processing temperature or sophisticated processing. For example, ferrites can be prepared via the hydrothermal route at a temperature of ∼200 ∘C, whereas the solid-state method requires a temperature of 800 ∘C [11]. Hydrothermal synthesis of hexaferrites was mostly studied by the Drofenik research group [12]. But in all these studies, calcination process was continued at high temperature after hydrothermal synthesis. They accomplished the synthesis of the superparamagnetic BaFe12O19 particles [12]. Hong et al. [13] were successfully prepared by ethylene glycol (EG) assisted hydrothermal synthesis of SrFe12O19 NPs with 25 nm crystallite size without further calcination process. Xia et al. also tried to synthesize SrM NPs with hydrothermal route. But to obtain pure SrM NPs, they calcined the hydrothermal product at 1100 and 1200 ∘C for pure SrM [3]. Jane et al. [14] also tried to synthesize the SrM powders at 180 ∘C via hydrothermal route and noticed that the uncalcinated product was not pure and contained impurities. Ebrahimi et al. [15–20] also synthesized the SrM NPs via many different synthesis methods.
In the present work, we report on the results of the structural analysis of SrFe12O19 powders prepared by hydrothermal processing without calcination process. In particular, we have investigated the effect temperature on the purity and the magnetic properties of the final product.
2 Experimental
2.1 Chemicals
SrCl2⋅2H2O, FeCl3⋅6H2O, ethyl alcohol, and NaOH were obtained from Merck and were used as received, without further purification.
2.2 Instrumentations
X-Ray powder diffraction (XRD) analysis was conducted on a Rigaku Smart Lab Diffractometer operated at 40 kV and 35 mA using Cu K α radiation.
Scanning Electron Microscopy (SEM) analysis was performed, in order to investigate the microstructure of the sample, using FEI XL40 Sirion FEG Digital Scanning Microscope. Samples were coated with gold at 10 mA for 2 min prior to SEM analysis.
Fourier transform infrared (FT-IR) spectra were recorded in transmission mode (Perkin Elmer BX FT-IR) on powder samples that were ground with KBr and compressed into a pellet. FT-IR spectra in the range 4000–400 cm−1 were recorded in order to investigate the nature of the chemical bonds formed.
VSM measurements were performed by using a Vibrating sample magnetometer (LDJ Electronics Inc., Model 9600). The magnetization measurements were carried out in an external field up to 15 kOe at room temperature.
2.3 Procedure
Stoichiometric amount of SrCl2⋅2H2O and FeCl3⋅6H2O (Sr/Fe mole ratio: 1/8) were dissolved in 15 ml of distilled water. Then, 3.5.0 g of NaOH was added to the solution with continuous stirring (with the same procedure, six precursor solutions were prepared; then these precursor solutions were put into six different 30 ml of Teflon-lined stainless-steel autoclaves with degree of filling 60 %) and kept it in an oven at 170, 180, 190, 200, 210, and 220 ∘C for 18 h, respectively. The as-prepared ultrafine particles were collected by a magnet and then washed with distilled water, alcohol, and acetic acid for several times and dried at 60 ∘C for 4 h.
3 Results and Discussion
3.1 XRD Analysis
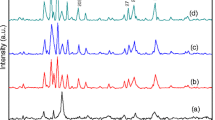
The X-ray diffraction patterns of the as-prepared SrM particles at different temperatures are shown in Fig. 1. Except Fig. 1d, all other XRD powder patterns contain α-Fe2O3 (ICDD card No. 27-2078) and SrCO3 (ICDD card No. 05-0418). For products synthesized at 170, 180, and 190 ∘C, only Fe2O3 and several weak SrCO3 diffraction peaks are found in the XRD pattern, which shows a large amount of Sr2+ ions lost in the solutions, and no hexagonal SrM ferrite was synthesized in the specimen. However, there are typical peaks from hexagonal SrM ferrite, and several sharp Fe2O3 and weak SrCO3 peaks are found in Figs. 1(b)–1(e). Therefore, all the specimens synthesized are not single-phase. For all these products, the presence of strontium carbonate is due to the reaction between sodium hydroxide and the carbon dioxide present in ambient air [21, 22]. As it was seen from Fig. 1f (for 220 ∘C), the formation SrM is more or less complete, although product contains Fe2O3 and SrCO3. But the sample synthesized at 200 ∘C contains only SrM (ICDD card No. 84-1531) as pure phase.
3.2 FT-IR Analysis
FT-IR spectra of all products synthesized at 170, 180, 190, 200, 210, and 220 ∘C are presented in Figs. 2a–2f, respectively. There are typical bands for ferrites observed for all samples utilizing various fuels. The absorption bands observed at around 445, 550, and 590 cm−1 correspond to typical M–O adsorption bands in SrFe12O19 [23–25]. All products except the one synthesized at 200 ∘C contain SrCO3 as impurity (around 1400 cm−1). Also, all products contain different amounts of α-Fe2O3 (the product synthesized at 200 ∘C has very small amount of α-Fe2O3 as indicated in Table 1).
3.3 VSM Analysis
Magnetic characterization of the samples synthesized at different temperatures between 170 and 220 ∘C have been performed by measuring M–H hysteresis curves at room temperature, which was shown in Fig. 3. Magnetization of the samples treated at temperatures below 200 ∘C appeared to be very low (<1 emu/g). This reveals that the hexaferrite phase (SrFe12O19) was not formed at all at this temperature range, which was also supported by XRD patterns of these samples. Samples treated at 200 and 220 ∘C show typical soft ferromagnetic behavior with saturation magnetization (M s ) around 40 emu/g and coercivity of nearly 500 Oe. Both M s and coercivities of the samples are lower than that of the bulk samples which are around 70 emu/g and 5 kOe, respectively [26, 27]. The reason of this reduced magnetization could be the presence of oxygen vacancies, which are used in Fe3+–O2−–Fe2+ exchange interaction. Secondly, the SrCO3 and Fe2O3 (Hematite) phases were clearly detected in XRD patterns of the samples treated at 210 and 220 ∘C. These impurity phases also lead to a decrease in M s values of the samples. At 210 ∘C, the Sr-hexaferrite phase was not detected at all. Accordingly, magnetization of this sample appeared to be very low (<1 emu/g). The reason of the low coercivity values is the high shape anisotropy of the samples, resulting from their high width-to-thickness ratio, see Fig. 4 (SEM micrographs). Namely, the coercivity linearly depends on the magnetocrystalline anisotropy and the shape anisotropy, and in the SrFe12O19 platelets, the two anisotropy fields oppose each other. In addition to this, the crystalline order in the c-direction in thin platelet crystals may be very low and can also have a negative influence on the coercivity. Low coercivities and reduced magnetization values were also reported in Ba-hexaferrite samples synthesized hydrothermally with low-temperature sintering [28, 29].
3.4 SEM Analysis
SEM micrographs of all products are given in Fig. 4 with different magnification for temperatures. The products synthesized at 170, 180, 190, and 210 ∘C do not have plate like hexagonal morphology. One can observe that most of the particles are of hexagonal shape for 200 and 200 ∘C temperatures. The size of these particles varies between 1 and 1.8 μm, and the average thickness was found between 80 and 150 nm. This particle shape was also observed for hydrothermally synthesized BaFe12O19 and Sr Fe12O19 hexaferrites [30–34].
4 Conclusion
SrFe12O19 particles were successfully prepared by a hydrothermal method. The Fe/Ba mole ratio was taken as 8:1, the optimum temperature was determined, and it was observed that the homogeneous platelet-like shape with narrow size distribution was obtained above 200 ∘C. The XRD result showed that the derived sample at 200 ∘C was pure phase with P63/mmc. The FE-SEM results showed that the grains were regular hexagonal platelets with sizes of 1–1.8 μm with 80–150 nm thickness. Magnetization measurements support the existence of hard Sr-hexaferrite phase in samples treated at 200 and 220 ∘C. Samples treated at 210 and 220 ∘C contain SrCO3 and Fe2O3 phases also as an impurity, and thus, the detected magnetization values are lower than reported ones. Besides, due to the high shape anisotropy of the grains, coercivities of the samples appeared to be low.
References
Xie, T.P., Xu, L.J., Liu, C.L.: Powder Technol. 232, 87–92 (2012)
Xie, T., Xu, L., Liu, C., Wang, Y.: Appl. Surf. Sci. 273, 684–691 (2013)
Chen, D.H., Chen, Y.Y.: Mater. Res. Bull. 37, 801 (2002)
Ghasemi, A., Morisako, A.: J. Magn. Magn. Mater. 320, 1167 (2008)
How, H., Zuo, X., Wave, C.V.: IEEE Trans. Magn. 41, 2349 (2005)
Guo, Z.B., Ding, W.P., Zhong, W., Zang, J.R., Do, Y.W.: J. Magn. Magn. Mater. 175, 333 (1997)
Ketov, S.V., Yagodkin, Yu.D., Lebed, A.L., Chernopyatova, Yu.V., Khlopkov, K.: J. Magn. Magn. Mater. 300, e479 (2006)
Qiao, L., You, L.H., Zheng, J.W., Jiang, L.Q., Sheng, J.W.: J. Magn. Magn. Mater. 318, 74 (2007)
Zi, Z.F., Sun, Y.P., Zhu, X.B., Yang, Z.R., Dai, J.M., Song, W.H.: J. Magn. Magn. Mater. 320, 2746–2751 (2008)
Xia, A., Zuo, C., Chen, L., Jin, C., Lv, Y.: J. Magn. Magn. Mater. 332, 186–191 (2013)
D’arrigo, M.C., Leonellic, C., Pellacani, G.C.: J. Am. Ceram. Soc. 81, 3041 (1998)
Drofenik, M., Ban, I., Makovec, D., Žnidaršič, A., Jagličić, Z., Hanžel, D., Lisjak Mater, D.: Chem. Phys. 127, 415–419 (2011)
Tang, X., Hong, R.Y., Feng, W.G., Badami, D.: J. Alloys Compd. 562, 211–218 (2013)
Jean, M., Nachbaur, V., Bran, J., Breton, J.M.L.: J. Alloys Compd. 496, 306–312 (2010)
Hasab, M.G., Ebrahimi, S.A.S., Badiei, A.: J. Eur. Ceram. Soc. 27, 3637–3640 (2007)
Koohdar, H.R., Ebrahimi, S.A.S., Yourdkhani, A., Dehghan, R., Zajkaniha, F.: J. Alloys Compd. 479, 638–641 (2009)
Yourdkhani, A., Ebrahimi, S.A.S., Koohdar, H.R.: J. Alloys Compd. 470, 561–564 (2009)
Masoudpanah, S.M., Ebrahimi, S.A.S., Ong, C.K.: J. Magn. Magn. Mater. 324, 2894–2898 (2012)
Masoudpanah, S.M., Ebrahimi, S.A.S.: J. Magn. Magn. Mater. 324, 2239–2244 (2012)
Masoudpanah, S.M., SeyyedEbrahimi, S.A.: J. Magn. Magn. Mater. 323, 2643–2647 (2011)
Jean, M., Nachbaur, V., Bran, J., Breton, J.M.L.: JALCOM 496, 306–312 (2010)
Lin, C.H., Shih, Z.W., Chin, T.S., Wang, M.L., Yu, Y.C.: IEEE Trans. Magn. 26, 15–17 (1990)
Iqbal, M.J., Ashiq, M.N.: Chem. Eng. J. 136, 383–389 (2008)
Baykal, A., Toprak, M.S., Durmus, Z., Sozeri, H.: J. Supercond. Nov. Magn. 25, 2081–2085 (2012)
Durmus, Z., Sozeri, H., Toprak, M.S., Baykal, A.: J. Supercond. Nov. Magn. 25, 1957–1963 (2012)
Shirk, B.T.: Mater. Res. Bull. 5, 771 (1970)
Kojima, H.: In: Wohlfarth, E.P. (ed.) Ferromagnetic Materials, vol. 3, pp. 305–391. North-Holland, Amsterdam (1982)
Primc, D., Makovec, D., Lisjak, D., Drofenik, M.: Nanotechnology 20, 315605–315614 (2009)
Tomohisa, Y., Yasunori, T., Takao, S., Hirotaro, M., Tsukasa, C., Shunsaku, K., et al.: J. Magn. Magn. Mater. 321, 8–11 (2009)
Ataie, A., Harris, I.R., Ponton, C.B.: In: Lee, W.E., Bell, A. (eds.) Proc. of a Symposium Held as Part of Condensed Matter and Materials Physics Conference, University of Leeds, 20–22 December 1993, pp. 273–281 (1994).
Lin, C.H., Shih, Z.W., Chin, T.S., Wang, M.L., Yu, Y.C.: IEEE Trans. Magn. 26, 15–17 (1990)
Liu, X., Wang, J., Gan, L.M., Ng, S.C.: J. Magn. Magn. Mater. 195, 452–459 (1999)
Zi, Z.F., Sun, Y.P., Zhu, X.B., Yang, Z.R., Dai, J.M., Song, W.H.: J. Magn. Magn. Mater. 320, 2746–2751 (2008)
Baykal, A.: Solvothermal synthesis of pure SrFe12O19 hexaferrite nanoplatelets. J. Supercond. Nov. Magn. doi:10.1007/s10948-013-2369-z
Acknowledgements
This work is supported by Fatih University under BAP grant No. P50021203-Y (2282).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shafiu, S., Sözeri, H. & Baykal, A. Solvothermal Synthesis of SrFe12O19 Hexaferrites: Without Calcinations. J Supercond Nov Magn 27, 1593–1598 (2014). https://doi.org/10.1007/s10948-014-2490-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-014-2490-7