Abstract
The effect of Ag inclusion on the structure microstructure and the critical current density of the YBa2Cu3O7−δ sample prepared using the planetary ball milling process has been investigated. YBa2Cu3O7−δ ceramics have been synthesized in air by a solid state reaction method from an oxide precursor powder, which was prepared from the starting powders of Y2O3, Ba2CO3, and CuO via a one-step annealing process in air at 950 ∘C. After planetary ball milling for 4 h of the oxide precursor powders, it was mixed with an AgNO3 solution, and then was dried and uniaxially pressed, and subsequently annealed at 950 ∘C in air. Phase analysis by X-ray diffraction (XRD), granular structure examination by scanning electron microscopy (SEM), microstructure investigation by transmission electron microscopy (TEM) coupled with energy dispersive X-ray spectroscopy (EDXS) were carried out. To understand the effects of the ball milling on the pinning behavior, magnetic field and temperature dependences on a critical current density have been studied. Analyses show that Ag-milled YBCO samples exhibit higher values of critical current density in applied magnetic field compared to Ag-unmilled one. The better pinning properties of the Ag-milled samples are believed to be due to the microstructure of more fine and uniform distribution of silver and Y-deficient nanosized generated by ball milling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
For most applications of high temperature superconductors (HTS), high critical current density J c under a high magnetic field is required. Due to the granularity and porous character of HTS, polycrystalline samples exhibit low J c value. Two main requirements must be fulfilled for producing bulk-type superconductors with a high critical current density in YBa2Cu3O7 (Y-123 or YBCO for brevity) superconducting compound. The first is to exclude weak links in this superconductor and the second one is to introduce effective centers for pinning of magnetic flux lines. Among metallic materials, silver inclusion has a large impact on the evolution of microstructure and critical current density [1]. Artificial pinning centers can be created by various methods including chemical doping, addition of second phase particles formation of inhomogeneities, etc. [2–6]. The route of artificially introducing inhomogeneities or second-phase materials as flux pinning sites in the processing of large bulk on YBa2Cu3O7 (Y-123 or YBCO for brevity) is a long standing topic.
Mechanical milling has been proved to be an effective technique for raising the flux pinning properties mainly in the MgB2 compound [7–9]. For the Y-based compound, high energy ball milling has been used to produce nanocrystalline YBa2Cu3O7−d powders [10, 11]. It is reported that after about 1 h of milling, a transition from orthorhombic to tetragonal phase has been observed with total loss of superconductivity. Very recently, we have analyzed the effect of planetary mill process in YBCO compounds [12]. We reported that processing parameters of planetary ball milling technique such as ball-to-powder weight ratio, speed rotation, and number of balls have a substantial influence on the properties of the final product such as, phase homogeneity, grain size, etc. Based on these backgrounds, the ball milling technique and Ag inclusions may be a better approach to improve critical current density and pinning properties. So, it is important to study the influence of silver addition into YBa2Cu3O7−d ceramics prepared using planetary ball milling technique and subsequent sintering at high temperature on the superconducting properties.
2 Experimental Process
Polycrystalline samples of YBa2Cu3O y were synthesized by solid state reaction. Details of the sample preparation have been described previously in [13], and here we give a brief description of our samples preparation. A stoichiometric mixture of Ba2CO3, Y2O3, and CuO was first calcinated in air for 12 h at 950 ∘C in order to produce an oxide precursor without the remainder of any carbonates. The resulting oxide precursor was subjected to two different grinding techniques, one part was milled via the planetary milling technique and another was grounded by hand in an agate mortar with an agate pestle. The precursor oxide characteristics such as size and composition are strongly affected by the impact energy input during the milling that depends on parameters of mechanical milling (e.g., ball to powder weight ratio values, high speeds of rotation, local temperature. In the present work, the ball-milling process was carried out for 4 h with a milling speed of 600 rpm and ball-to-powder weight ratio of about 5:2. The composition of the resulting milled oxide precursor is consisting of a mixture of Y-123, Y-deficient YBCO phases with additional of small quantities of superconducting phases YBa2Cu4O8 (Y-124), Y2Ba4Cu7O14+x (Y-247) and Y2BaCuO5, BaCuO2 such as secondary phases. Silver was added to samples during the final processing stage. In order to mix uniformly the fine Ag particles with constituent of oxide precursor, AgNO3 solution was added to the oxide precursor powder. Appropriate amounts of AgNO3 solution was then mixed with the milled and hand grinding oxide precursor powders. Mixed powders were dried and then pressed uniaxially into pellets of 0.5 mm thick and 7 mm in diameter, under a pressure of 750 MPa. These pellets were sintered in air at 950 ∘C for 8 h and then furnace cooled to room temperature. The samples are labeled as “Ag-milled” (for planetary milled oxide precursor) and “Ag-unmilled” (for hand grinded oxide precursor).
The structure and the phase purity were examined by powder X-ray diffraction using a Scintag XDS 2000 diffractometer with CuK α radiation. The scanning electron microscope (SEM) measurements were performed using an FEI Nano Lab 200. Chemical analysis was performed using the energy dispersive X-ray spectroscopy (EDXS) system attached to the SEM. The transport properties of the samples were studied by measuring the electrical resistivity–temperature ρ(T) and current-voltage (I–V) characteristics using the four-probe technique. The pellets were carefully cut into bars shaped samples with almost similar dimensions. Electrical contacts were made using silver paint and the contact resistance value was approximately 0.5 Ω. The temperature dependence of the electrical resistivity ρ(T) was measured by using the standard dc four-probe technique in a DMX-19 SCC cryostat system. The magnitude of the excitation current density, J, used to measure resistivity of the samples is J=40 μA cm−2. The transport critical current density (J cT ) values were determined at various temperatures using a 5 μV/cm criterion and a magnetic field was applied along the short axis of the sample and the excitation current was injected along the length axis of the samples.
3 Results and Discussion
Details of the structural changes associated with the milling process and the effect of different milling parameters on the superconducting properties have already been reported elsewhere [13], and here we give a brief description of our samples characteristics. XRD analysis and SEM observations coupled with EDX analyses indicate that the milled ceramics is consisting of a mixture phases; stoichiometric YBCO and Y-deficient YBCO with a deviation of the yttrium compared to the nominal composition of YBa2Cu3O7−δ and insignificant secondary phases such as Y-124, Y-247, Y2BaCuO5, and BaCuO2. It is known that Y-124 and Y-247 can easily exist as an intergrowth within the Y-123 phase [14]. Y-deficient YBCO phase is presented in milled samples as nanoscale entities surrounded in the superconducting YBa2Cu3O7−d matrix. The result of resistivity versus temperature measurements indicated two resistivity drops, one starting at, \(T_{c1}^{\mathrm{onset}} = 92~\mbox{K}\) and the other starting at \(T_{c2}^{\mathrm{onset}} \approx 90~\mbox{K}\). However, the unmilled sample shows a one step transition at \(T_{c}^{\mathrm{onset}} = 92~\mbox{K}\). So, the observed \(T_{c2}^{\mathrm{onset}} \approx 90~\mbox{K}\) value is consistent with the Y-deficient YBCO phase. The zero resistance temperature, T co , is 86.7 and 90 K for the milled and unmilled samples respectively. The milling process reduces the zero applied field transport critical current density J c at 77 K; J c =115 A cm−2 for the milled sample, and J c =160 A cm−2 for the unmilled one.
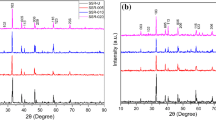
XRD patterns of both milled and unmilled samples sintered with AgNO3 during the final processing stage are shown in Fig. 1. Compared to nonadded samples, it should be mentioned that no noticeable difference appeared in the width and position of the diffraction peaks. Only characteristic peaks corresponding to silver have been appeared. Ag admixture seems thus to not affect the crystallographic structure of grains.
Figure 2 shows the temperature dependence of the electrical resistivity ρ(T) for both milled and unmilled samples sintered with AgNO3 addition. Both curves exhibit a transition to the superconducting state below the onset superconducting temperature \(T_{c}^{\mathrm{onset}}\) and show linear temperature dependence in the normal state. A slight increase in the zero resistance temperature T co with AgNO3 addition has been observed. T co , is 87.4 and 90.7 K for the Ag-milled and Ag-unmilled samples, respectively. Note that the Ag-unmilled sample shows a one step transition at \(T_{c}^{\mathrm{onset}} = 92~\mbox{K}\). The result of resistivity versus temperature measurements indicated two resistivity drops one starting at \(T_{c1}^{\mathrm{onset}} = 92~\mbox{K}\) and the other starting at \(T_{c2}^{\mathrm{onset}} \approx 90~\mathrm{K}\). Double superconducting transitions are also observed in Gd1+2x Ba2+x Cu3+x O7−d [15] in YBCO at different doping levels [16]. AgNO3 addition does not affect the critical temperatures \(T_{c1}^{\mathrm{onset}}\) and \(T_{c2}^{\mathrm{onset}}\) in both samples. This suggests that the grains are not altered, and as a consequence the oxygen content does not change by the presence of silver. This is in excellent agreement with the XRD.
Temperature dependences of critical current density J c (T) were measured at self and applied magnetic field for samples sintered with and without AgNO3 addition, and the measurements are shown in Fig. 3a and b. Two distinct behaviors can be observed from these figures. The critical current density increasing monotonically with decreasing temperature from near T c down to T=20 K for each samples. In order to understand the relevant AgNO3 effect, we examined the relative change of the critical current ΔJ c between added and free samples. Plots of ΔJ c versus temperature are illustrated in the inset 1 of Fig. 3a.
Critical current density as function of temperature measured at self (a) and applied (b) magnetic field for milled and unmilled samples sintered with and without AgNO3 addition. Inset ①: critical current density difference for both samples. Inset ②: critical current versus 1−T/T co plotted for samples on a logarithmic scale. The solid red line corresponds to α=1
It is clear that at a temperature less than 77 K, the AgNO3 addition leads to a substantial increase of ΔJ c values in the milled sample. The obtained J c values are very interesting for all samples, especially when compared to the values reported in the literature in the case of polycrystalline samples added with Ag nanoparticles [17]. Generally, it is believed that Ag diffuses into the grain boundary as a metal during thermal processing and it is responsible for the increase of interconnections between the grains [18]. At a low applied magnetic field, J c values of milled samples sintered with and without AgNO3 were larger than those of free and AgNO3-unmilled samples (Fig. 3b). AgNO3 addition does not practically change J c (T) behavior in a low applied magnetic field. For polycrystalline HTS, the type of grain boundaries and its composition are considered as fundamental parameters that control the weak link behavior. We can estimate the electrical character (superconductor–normal–superconductor (S-N-S) or superconductor–insulator–superconductor (S-I-S) junctions) of the weak-link network in our samples using the equation I c =I o (1−T/T co )α for temperatures close to the superconducting transition temperature T co of the intergrain junction where I c is the critical current. The plot of ln(I c ) against ln(1−T/T co ) gives α as the slope value. The results are well approximated with linear fit for both samples (see inset 2 of Fig. 3) with α is about 1 which corresponds to the superconductor–insulate–superconductor (S-I-S) type joints. One can say that grain boundaries behave predominantly as SIS junctions, even in AgNO3 added samples. Our results are in good agreement with ones obtained in the case of Pt doped DyBa2Cu3−x O y [19].
We have also studied the effect of an external magnetic field on the transport critical current density J c (H) at various temperatures. To isolate the flux pinning improvements, the measured J c (H) values in magnetic fields can instead be normalized to the transport critical current density at zero-field values J c (0). The dependence of the normalized critical current density on magnetic field, J cN , (J cN =J c (H)/J c0) in milled and unmilled samples with AgNO3 additives is presented in Fig. 4. All samples show degradation of critical current density with increasing the magnetic field. At T<77 K, the Ag-milled sample shows less sensitivity to magnetic field compared with Ag-unmilled sample over the entire applied magnetic fields. The AgNO3-milled samples seem to have better flux pinning properties at a temperature less than 77 K. SEM micrographs of the transverse cross-section morphology of the sintered samples are shown in Figs. 5a–b. All sintered samples exhibit a granular structure with micrometer grains size. SEM micrographs of the milled sample sintered with AgNO3 (Fig. 5b) exhibited entities bright in contrast with sizes ranging from 60 to 30 nm. These entities take place into the grains along with some segregation in the grain boundaries. Such entities have not been observed in unmilled samples (Fig. 5a). The EDSX resolution in the SEM observations is about 1–2 μm, so it is impractical to determine precisely the composition of entities by conducting a spot analysis on a single particle. To estimate the elemental composition of entities, comparative analyses have been carried out in the regions, where entities are present or absent. For free milled sample (see inset of Fig. 5b), the EDSX analysis performed on the area with high-density of entities clearly reveals that these entities contain Y, Ba, Cu, and O but they are consistently yttrium deficient with a deviation of the yttrium compared to the nominal composition of YBa2Cu3O y . The chemical composition is illustrated in the inset of Fig. 5c. However, for the AgNO3 milled sample, the EDSX spectrum given in the Fig. 5c shows the presence of Ag in addition to Y, Ba, Cu, O elements. It is widely accepted that silver not reacting chemically with the YBaCuO but was shown to reside in the grain boundaries. The Ag particles are more finely and uniformly distributed at the grain boundaries of AgNO3-YBCO for the milled sample than those of AgNO3-YBCO of unmilled powder. For unmilled samples, Ag may be agglomerated with each other to some extent at grain boundaries after sintering and led to the formation of the stack of silver nonuniformly distributed in the sample, which reduces the effectiveness of pinning properties. Since the milled sample with the AgNO3 addition revealed uniformly distributed nanosized inclusions and well dispersed of finer Ag, higher density of flux pinning sites and enhancement of critical current density can be expected at the applied magnetic field.
4 Conclusion
The effect of AgNO3 additions in the microstructural evolution and related transport properties of YBCO prepared using planetary ball milling process has been investigated in comparison with the YBa2Cu3O7−δ samples subjected to hand grinding. YBa2Cu3O7−δ ceramics have been synthesized in air by a solid state reaction method from an oxide precursor powder, which was prepared from the starting powders of Y2O3, Ba2CO3, and CuO via a one-step annealing process in air at 950 ∘C. After planetary ball milling for 4 h of the oxide precursor powders, it was mixed with AgNO3 solution and then was dried and uniaxially pressed, and subsequently annealed at 950 ∘C in air. XRD analyses indicate that silver does not react with YBCO and the milled ceramics consists of mixture phases; stoichiometric YBCO, Y-deficient YBCO with a small deviation of the yttrium compared to the nominal composition of YBa2Cu3O7−δ and silver phase. SEM observations coupled with EDX analyses reveal that the Y-deficient YBCO phase is presented in milled samples as nanoscale entities surround the superconducting YBa2Cu3O7−d matrix. The critical current density under applied magnetic field (J c ) of the AgNO3-milled sample is higher than that of the Ag-unmilled one. The better pinning properties of the Ag-milled samples are believed to be due to the microstructure of more finely and uniformly distributed of silver and Y-deficient nanosized generated by ball milling.
References
Tepea, M., Avcia, I., Kocoglua, H., Abukayb, D.: Solid State Communications 131, 319 (2004)
Larbalestier, D., Gurevich, A., Feldmann, D.M., Polyanski, A.: Nature 414, 368 (2001)
Goswami, R., Haugan, T.J., Barnes, P.N., Spanos, G., Holtz, R.L.: Physica C 470, 318 (2010)
Ben Azzouz, F., Zouaoui, M., Mellekh, A., Annabi, M., Van Tendeloo, G., Ben Salem, M.: Physica C 455, 25 (2007)
Zhao, Y., Cheng, C.H., Wang, J.S.: Supercond. Sci. Technol. 18, S43 (2005)
Liyanawaduge, N.P., Kumar, A., Kumar, S., Karunarathne, B.S.B., Awana, V.P.S.: J Supercond Nov Magn 25, 31 (2012)
Strickland, N.M., Buckley, R.G., Otto, A.: Appl. Phys. Lett. 83, 326 (2003)
Lee, J.H., Shin, S.Y., Kim, C.J., Park, H.W.: J. Alloys Compd. 476, 919 (2009)
Xu, X., Kim J, H., Duo, S.X.: J. Appl. Phys. 105, 103913 (2009)
Simoneau, M., L’Espérance, G., Trudeau, M.L., Schulz, R.: Mater Res J. 9, 535 (1994)
Xiang, J.Y., Fleck, C., Hampshire, D.P.: Journal of Physics: Conference Series 97, 012237 (2008)
Hamrita, A., Ben Azzouz, F., Madani, A., Ben Salem, M.: Physica C 472, 34 (2012)
Hamrita, A., Ben Azzouz, F., Dachraoui, W., Ben Salem, M.: submitted to Physica C
Van Tendeloo, G., Brodin, D., Zandbergen, H.W., Amelinckx, S.: Physcia C 167, 627 (1990)
Zhang, H., Liu, Y., Li, H.L., Qu, J.F., Li, X.G., Feng, Y.: Supercond. Sci. Technol. 18, 1317 (2005)
Lortz, R., Tomita, T., Wang, Y., Junod, A., Schilling, J.S., Masui, T., Tajima, S.: Physica C 434, 194 (2006)
Farbod, M., Reza Batvandi, M.: Physica C 471, 112 (2011)
Zhao, Y., Cheng, C.H.: Physica C 386, 286 (2003)
Orlova, T.S., Laval, J.Y., Nguyen-van-Huong, C., Dubon, A.: Supercond. Sci. Technol. 1156, 12 (1999)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamrita, A., Ben Azzouz, F., Dachraoui, W. et al. The Effect of Silver Inclusion on Superconducting Properties of YBa2Cu3O y Prepared Using Planetary Ball Milling. J Supercond Nov Magn 26, 879–884 (2013). https://doi.org/10.1007/s10948-012-1976-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-012-1976-4