Abstract
Structural and electrical properties of the mixed-valence monovalent doped manganites Pr0.6Sr0.4−x K x MnO3 (x=0, 0.05 and 0.1), prepared using the conventional solid-state synthesis method, have been investigated. Rietveld refinement of the X-ray diffraction patterns at room temperature shows a slight increase in unit cell volume with K content and confirms that all powder samples are single phase and crystallize in the orthorhombic structure with Pnma space group. Electrical resistivity measurements under and without magnetic field show a transition from the metallic to insulating behavior. The electrical conductivity is improved with increasing K content. The resistivity data in metallic region were fitted according to the electron–electron scattering process while in insulating region they were fitted using the small polaron hopping SPH model and Mott’s variable range hopping VRH model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Present interest in mixed-valence manganese perovskites with general formula T1−x R x MnO3 (T=rare-earth cation, R=alkaline earth cation) is related to their colossal magnetoresistance (CMR), and their potential technological application [1–3]. These compounds are characterized due to their sensory electronic and magnetic properties, which are dependent on mobile charge carriers, spin ordering, orbital hybridization. The substitution in the A-site influences the electronic and magnetic properties [4, 5]. Zener showed that the metallic and ferromagnetic behavior in manganite can be explained using the so called double exchange mechanism (DE) [6] with mobile electrons e g traveling between the Mn3+ and Mn4+ cations. However, it was suggested that the DE model is not enough to explain the CMR phenomenon. Some authors suggested that other factors such as Jahn Teller [7], phase separation [8–10] are responsible for the behavior observed in manganite. Several studies have been performed on the effect of substitution in the A-site by divalent elements (Ba, Ca) [11, 12]. Recently some authors have reported that the substitution by monovalent element can improve physical properties [13].
In previous studies we have reported that the Pr0.6Sr0.35Na0.05MnO3 exhibits a metal–insulator behavior about 165 K [14, 15]. There have been no previous studies on the effect of monovalent K-doping effect in the Pr0.6Sr0.4−x K x MnO3 system. Each K atom converts Mn3+ ion to Mn4+ and varies the Mn3+/Mn4+ ratio. This results in a larger charge carrier density due to the difference between the valence of K+ and Sr2+ ions. In the present paper we are reporting a systematic investigation of structural and electrical properties of monovalent K-doped series of manganites with chemical compositional Pr0.6Sr0.4−x K x MnO3 (x=0, 0.5 and 0.1).
2 Experimental
Polycrystalline samples of Pr0.6Sr0.4−x K x MnO3 (x=0, 0.05 and 0.1) were prepared using the conventional solid-state reaction method. Stoichiometric ratio of Pr6O11, SrCO3, K2CO3 and MnO2 (99.9 %) was mixed in an agate mortar and then heated in air to 1000 °C for 60 h with intermediate grinding. The obtained mixtures were then pressed into pellets and sintered at 1100 °C in air for 60 h with intermediate regrinding. Phase purity, homogeneity and cell dimensions were checked by X-ray diffraction studies at room temperature with Cu-Kα radiation (1.54 Å) in the 2θ range of 10–80 degrees. Structural analysis was carried out using the standard Rietveld technique [16, 17]. The dc magnetization was measured in a field of 100 Oe. The electrical resistivity versus temperature was measured by the four probe technique under zero field and a magnetic field of 1 T.
3 Structure
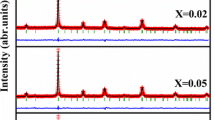
The X-ray diffraction patterns of Pr0.6Sr0.4−x K x MnO3 (x=0, 0.05 and 0.1) shown in Fig. 1A confirms that all manganites studied can be indexed in the orthorhombic structure with Pnma space group without any traces of secondary phases. Figure 1B shows a typical plot of the refined pattern for x=0.1. Structural parameters obtained using Rietveld analysis of the powder XRD data are given in Table 1. It is worth to notice that the unit cell volume is found to increase with increasing K content. This volume enhancement is due to the larger ionic radius of K+ (1.64 Å) as compared to that of Sr2+ (1.44 Å) [18]. A similar tendency is also observed value for other monovalent doped Na-doped manganites with the Na+ ionic radius of 1.39 Å [14]. It is worth to notice that the Mn–O–Mn angles are enhanced with K doping, whereas the Mn–O distances are slightly suppressed.
The average crystallite size evaluated from a width of diffraction peaks using the Scherrer formula is found to be 41, 49 and 52 nm for x=0, 0.05 and 0.1, respectively. We should note that this value is slightly larger than observed value for Na-doped Pr0.6Sr0.35Na0.05MnO3 manganite [14].
4 Magnetic Properties
The magnetization measured in a field of 100 Oe confirms the ferromagnetic ordering of the manganites studied (Fig. 2). The low temperature magnetization becomes reduced with raising K content due to the enhanced Mn4+/Mn3+ ratio. The Curie temperature determined by the dM/dT minimum (not plotted) shifts from 310 K down to 296 K for x varying from 0 to 0.1. It is worth to notice that these Curie temperatures exactly coincide with those reported for the poly- and single crystalline Pr0.6Sr0.4MnO3 manganites [19, 20].
5 Electrical Properties
The absolute values of electrical resistivity varied between 1.2 and 1.4 mΩ m at room temperature for our samples. Temperature dependence of electrical resistivity ρ(T) plotted in Fig. 3 indicates a changeover from the low temperature metallic-like behavior (dρ/dT>0) below T ρ to the semiconductor-like (dρ/dT<0) above T ρ temperature corresponding to the maximum value of resistivity at H=0 T. The T ρ temperature diminishes from 184 down to 170 K, when K content x varies from 0 to 0.1.
The T ρ temperature is more than 100 K lower than the Curie temperature. Such a difference was reported for numerous manganites. The T ρ temperature is found to depend on a microstructure of the manganite as shown for the nanocrystalline manganites studied with the poly- and single crystalline ones [19, 20]. In the Pr0.6Sr0.4MnO3 single crystal the metallic behavior was observed up to 400 K and the temperature derivative of resistivity has a maximum at Curie temperature [20].
In Fig. 4 we have plotted the temperature dependence of the electrical resistivity measured at 0 and 1 T magnetic field. We observe a reduction in resistivity magnitude seen in a whole temperature range for H=1 T. This observation suggests that potassium facilitates electrical conductivity most probably within the grain boundaries, which was also found in various oxide materials. Magnetic field of 1 T shifts T ρ to about 194 K almost independently of x.
Electrical resistivity in ferromagnetic metallic phase (below T ρ ) was numerically fitted by the formula
where ρ 0 is a temperature independent low temperature resistivity due to the scattering by impurities, grain boundaries, domain walls [21]. The A term is ascribed to the electron-electron or single magnon scattering [22, 23]. The obtained values from the best fit of resistivity data are summarized in Table 2. The ρ 0 and parameters are found to decrease with increasing K content. The observed reduction of A parameter by a magnetic field of 1 T can be explained by the weakening of spin fluctuation in a presence of external field.
The temperature dependence of electrical resistivity in semiconducting phase (above T ρ ) was analyzed and compared with the Small Polaron Hopping (SPH) and Mott’s Variable Range Hopping (VRH) models [24, 25]. For the SPH model, the electrical resistivity can be approximated by the following equation:
where E a is the activation energy and B is a constant. According to the VRH model the electrical resistivity is expressed by the formula
where R is the Mott residual resistivity and T 0 the Mott characteristic temperature related to carrier localization length [25]. The collected resistivity data were fitted by the two above models and the resulting parameters are listed in Table 3. The reliability factors are very close for both models tested and they do not point to the better one. The obtained values of E a and T 0 from the fitting (Table 3) are characteristic for manganites and decrease with increasing the magnetic applied field to 1 T. Such behavior can be attributed to the suppression of the transport energy barriers under the influence of magnetic field. The derived activation energies E a are characteristic for manganites. The T 0 values coincide with those reported for La0.85Ag0.15MnO3 [26].
The coexistence of ferromagnetism and metallic conductivity causes the relatively strong effect of negative magnetoresistance (MR) in manganites. The negative magnetoresistance effect is defined as
where ρ(H) and ρ(0) are the resistivities at magnetic field H and at zero field, respectively. Figure 5 shows the temperature evolution of MR for an applied magnetic field of 1 T for all our samples. For x=0 and 0.1 the MR reaches about 17 to 19 % at 20 K and gradually decays around room temperature. The MR plot for x=0.05 is distinguished by a peak at 200 K. In contrast to the single crystal of Pr0.6Sr0.4MnO3 manganite [20] the manganites studied do not exhibit MR peak in vicinity of Curie temperature. The strong MR effect observed at low temperature can be related the extrinsic MR effect involving spin polarized tunneling between grains or spin dependent scattering of polarized electrons at grain boundaries [14].
Summarizing one may notice that the Pr0.6Sr0.4−x K x MnO3 manganites retain the orthorhombic structure up to 10 % potassium doping. The electrical conductivity becomes improved by K doping and the T ρ temperature is shifted more than 100 K than below the Curie temperature. The negative magnetoresistance effect attains 17 to 22 % at 20 K depending on composition.
References
Jin, S., Tiefel, T.H., McCormack, M., Fastnacht, R.A., Ramesh, R., Chen, L.H.: Science 264, 413 (1994)
Rao, C.N., Raveau, B.: Colossal Magnetoresistance, Charge Ordering and Related Properties of Manganese Oxides. World Scientific, Singapore (1998)
Tokura, Y., Tomioka, T.: J. Magn. Magn. Mater. 200, 1 (1999)
Raveau, B., Maignan, A., Caignaert, V.: J. Solid State Chem. 117, 424 (1995)
Tomioka, Y., Asamitsu, A., Moritomo, Y., Kowahara, H., Tokura, Y.: Phys. Rev. Lett. 74, 5108 (1995)
Zener, C.: Phys. Rev. 82, 403 (1951)
Millis, A.J., Shraiman, B.I., Muller, R.: Phys. Rev. Lett. 77, 175 (1996)
Schiffer, P., Ramirez, A.P., Bao, W., Cheong, S.-W.: Phys. Rev. Lett. 75, 3336 (1995)
Kalyana Lakshmi, Y., Venugopal Reddy, P.: J. Alloys Compd. 470, 67 (2009)
Rao, G.N., Saibal Roy, R.C., Yang, Chen, J.W.: J. Magn. Magn. Mater. 260, 375 (2003)
Suresh Kumar, V., Mahendiran, R.: Solid State Commun. 150, 1445 (2010)
Kolat, V.S., Izgi, T., Kaya, A.O., Bayri, N., Gencer, H., Atalay, S.: J. Magn. Magn. Mater. 322, 427 (2010)
Das, S., Dey, T.K.: J. Phys. D, Appl. Phys. 40, 1855 (2007)
Thaljaoui, R., Boujelben, W., Pękała, M., Szydłowska, J., Cheikhrouhou, A.: J. Alloys Compd. 526, 98 (2012)
Thaljaoui, R., Boujelben, .W., Pękała, M., Pociecha, D., Szydłowska, J., Cheikhrouhou, A.: J. Alloys Compd. 530, 138 (2012)
Rietveld, H.M.: J. Appl. Crystallogr. 2, 65 (1969)
Roisnel, T., Rodriguez-Carvajal, J.: Computer program FULLPROF, LLB-LCSIM, May 2003
Shanon, R.D.: Acta Crystallogr., A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 32, 751 (1976)
Chang, C.W., Lin, J.G., Tai, M.F.: Chin. J. Phys. 40, 570 (2002)
Rössler, S., Harikrishnan, S., Naveen Kumar, C.M., Bhat, H.L., Suja, E., Rössler, U.K., Steglich, F., Wirth, S.: J. Supercond. Nov. Magn. 22, 205 (2009)
Snyder, G.J., Hiskes, R., Dicarolis, S., Beasley, M.R., Geballe, T.H.: Phys. Rev. B 53, 14434 (1996)
Ziese, M.: Phys. Rev. B 62, 1044 (2000)
Jaime, M., Lin, P., Salamon, M.B., Han, P.D.: Phys. Rev. B 58, R5901 (1998)
Emin, D., Holstein, T.: Ann. Phys. 53, 439 (1969)
Mott, N.F.: Metal–Insulator Transition, p. 51. Taylor and Francis, London (1990)
Ibrahim, N., Yahya, A.K., Rajput, S.S., Keshri, S., Talari, M.K.: J. Magn. 323, 2179 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thaljaoui, R., Boujelben, W., Pękała, M. et al. Structural and Electrical Properties of Monovalent Doped Manganites Pr0.6Sr0.4−x K x MnO3 (x=0, 0.05 and 0.1). J Supercond Nov Magn 26, 1625–1630 (2013). https://doi.org/10.1007/s10948-012-1913-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-012-1913-6