Abstract
FePt magnetic nanoparticles have been synthesized by superhydride reduction of FeCl2 and Pt(acac)2 at high temperature. Adding superhydride (LiBEt3H) to the phenyl ether solution of FeCl2 and Pt(acac)2 in the presence of oleic acid, oleylamine, and 1,2-hexadecanediol at 190 ∘C, followed by refluxing at 245 ∘C, led to monodisperse 3.5 nm FePt nanoparticles. The effect of oleylamine and oleic acid surfactants on the nucleation and growth of FePt nanoparticles were studied. The size of Pt was controlled by oleylamine surfactant in nucleation stage. To prevent sintering of the FePt nanoparticles, oleic acid surfactant was used in growth stage. The energy dispersive spectroscopy results revealed that the particle composition was first Fe11Pt89 in nucleation stage and after adding superhydride the composition changed to Fe63Pt37 in growth stage. The structural and magnetic measurements indicated that the L10 structure of FePt nanoparticles is formed after annealing and the coercivity of superlattice FePt nanoparticles increases to 7.5 kOe after heat treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
FePt nanoparticles have great application potential in advanced magnetic materials such as ultrahigh-density recording media and high-performance permanent magnets [1–3]. The key for applications is the very high uniaxial magnetocrystalline anisotropy of the L10-FePt phase which is based on crystalline ordering of the face-centered tetragonal (fct) structure [4].
Synthesis of magnetic nanoparticles has long been of scientific and technological interest due to their potential applications in tissue imaging [5] drug delivery [6] and information storage [7]. Important progress has been made in chemical synthesis of monodisperse magnetic nanoparticles of metals [9], alloys [8], and oxides [10]. The chemical growth of bulk or nanometer-sized materials inevitably involves the process of precipitation of a solid phase from solution. A good understanding of the process and parameters controlling the precipitation helps to improve the engineering of the growth of nanoparticles to the desired size and shape. For a particular solvent, there is certain solubility for a solute, whereby addition of any excess solute will result in precipitation and formation of nanocrystals. Thus, in the case of nanoparticle formation, for nucleation to occur, the solution must be supersaturated either by directly dissolving the solute at higher temperature and then cooling to low temperatures or by adding the necessary reactants to produce a supersaturated solution during the reaction [11, 12]. The precipitation process then basically consists of a nucleation step followed by particle growth stages [13, 14]. Uniformity of the size distribution is achieved through a short nucleation period that generates all of the particles obtained at the end of the reaction followed by a self-sharpening growth process. If the time of nanocrystal growth during the nucleation period is short compared to the subsequent growth processes, the nanocrystals can become more uniform over time as size focusing takes place [15–17].During nanocrystal growth, the surfactants in solution adsorb reversibly to the surfaces of the nanocrystals, providing a dynamic organic shell that stabilizes the nanocrystals in solution and mediates their growth. Surfactants that bind more tightly to the nanocrystal surface or larger molecules providing greater steric hindrance slow the rate of materials addition to the nanocrystal, resulting in smaller average nanocrystal size.
In the present work, FePt nanoparticles are first synthesized by sol-gel method. The effect of oleic acid and oleylamine are studied on the nucleation and growth of FePt nanoparticles during synthesis by TEM and EDS analysis. In order to phase transition to L10 structure, the nanoparticles are annealed under Ar atmosphere. The structure of FePt nanoparticles is studied after annealing by XRD analysis. The magnetic properties are investigated before and after annealing by VSM analysis.
2 Experimental Details
FePt nanoparticles with size of 3.5 nm were synthesized using the synthesis described by Sun et al. [18]. FePt nanoparticles were prepared by reducing of Pt(acac)2 (197 mg) and FeCl2⋅4H2O (139 mg) in phenyl ether solvent (25 mL) in the presence of 1,2-hexadecanediol (250 mg). Oleic acid (0.16 mL) and oleylamin (0.17 mL) surfactants were added to the solvent at 100 °C as a protective agent, in order to prevent agglomeration and oxidation. By adding LiBEt3H superhydride (2 mL) under a blanket of N2 at 200 °C, followed by refluxing, the FePt nanoparticles were formed. The refluxing temperature was fixed at 245 °C. The black reaction mixture was cooled to room temperature and then combined with ethanol to remove the impurity. The product was first precipitated at the end of process and then separated by centrifugation (9000 rpm, 10 min). In order to purification, any undissolved material was removed by centrifugation. Finally, FePt nanoparticles were dispersed in hexane solution (40 mL) in the presence of surfactants.
To determine the composition of FePt nanoparticles in nucleation and growth stage, energy dispersive spectroscopy (EDS) analysis (15 kV) was carried out. Specification of the size and shape of FePt nanoparticles were examined by transmission electron microscopy (TEM) analysis using a Philips EM 208 TEM (100 kV) with a resolution of 200 kX. The samples were annealed in a 90 % Ar+10 % H2 atmosphere at 800 °C for 4 hours. To determine the nanoparticles structure, X-ray diffraction (XRD) measurements were accomplished by Seifert system with Cu-K α radiation (λ=1.54 Å). The magnetization of the FePt samples in a variable magnetic field was measured using a vibrating sample magnetometer (VSM).
3 Results and Discussion
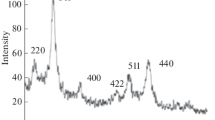
In order to increasing magnetic properties and phase transition from FCC to L10 structure, the FePt nanoparticles were annealed at 800 °C for 4 hours. Figure 1 shows the XRD pattern of superlattice FePt. The peaks appeared provide evidence of a chemical ordering phase transition.
Figure 2 shows the TEM images of the as-synthesized face center cubic (fcc) FePt nanoparticles. In Fig. 2a, the Pt core nucleate with size of 3 nm at 190 °C by 1,2 hexadecadeniol reducing agent. The Fe atoms are released from FeCl2⋅4H2O precursor and penetrate to Pt particles by adding LiBEt3H to the reaction solution at 210 °C. As you can see from Fig. 2b the size of FePt nanoparticles increases to 4 nm.
Figure 3 indicates the TEM images of the L10-FePt nanoparticles after annealing at 800 °C for 4 hours. As you can see, the FePt nanoparticles have been attached to each other. Because annealing process remove the oleic acid and oleylamine surfactants from the surface of nanoparticles. Therefore, FePt nanoparticles agglomerate to each other and the size of FePt nanoparticles increases to 100 nm.
Figure 4 shows a characteristic spectrum collected by EDS analysis. By comparing the area under each peak to a set of standards with known element concentrations, the concentration of the elements could be quantified, and the compositions gave Fe11Pt89 and Fe63Pt37 stoichiometry. It is realized that the Pt atoms are made by 1,2 Hexadecadeniol as the reducing reagent at 190 °C and the particle composition is Fe11Pt89 (Fig. 4a). When the superhydride is added to the solution at 200 °C, the Fe atoms are first released from FeCl2⋅4H2O precursor and then penetrate to Pt particles quickly. Therefore, the composition of nanoparticles changes to Fe63Pt37 (Fig. 4b).
In Table 1, the samples were prepared in different amount of oleic acid and oleylamine surfactants. For sample K, the FePt nanoparticles were made without oleylamine surfactant whereas for sample L, the FePt nanoparticles were prepared without oleic acid surfactant. For sample Q, amount of oleic acid (1.6 mL) and oleylamine (1.7 mL) surfactants were used in the synthesis stage whereas, for sample M half of the amount of Oleic acid (0.8 mL) and oleylamine (0.85 mL) surfactants were used. By comparing samples L and Q at 190 °C, it is realized that for sample L, Pt core are smaller than one for sample K. In fact, 1,2-hexadecanediol first reduce the Pt(acac)2 precursor and then nucleation process are started with Pt atoms. Also, oleylamine molecules slow down the growth of the particles by establishing a layer around the Pt particles. Comparing pair samples K and L at 190 °C with pair samples M and Q at 210 °C, it is found that for pair samples K and L the Fe percent do not change after adding superhydride at 210 °C whereas for pair samples M and Q the composition of the nanoparticles changes from Fe11Pt89 to Fe63Pt37. Because when it is used only oleic acid surfactant, the Fe-complex is formed and the LiBEt3H superhydride cannot release more Fe monomers to attach to the FePt nanoparticles. After adding superhydride, the Fe atoms are released quickly and then a layer of Fe atoms are surrounded around the Pt core. In this step, the oleic acid molecules controls the growth of FePt nanoparticles.
By increasing reaction temperature to 245 °C at the beginning of the reflux process, some unattached links for Fe atoms on the surface of FePt nanoparticles are removed from the surface. As you can see from sample Q, the composition of nanoparticles is Fe58Pt42 at the beginning of the reflux process. After 10 min reflux, the EDS results reveals that the percent of Fe shell are increased and the composition changes to Fe62Pt38. This is why more Fe monomers can be attached to FePt nanoparticle and, therefore, the size of FePt particles is increased.
Figure 5 shows the results of magnetic measurements before and after heat treatment. VSM analysis indicated that the as-made FePt are first superparamagnetic at room temperature. The coercivity is increased to 7.5 kOe after annealing at 800 °C for 4 hours.
4 Conclusion
Magnetic FePt nanoparticles were successfully synthesized with mean diameter of 4 nm. The effect of oleic acid and oleylamine surfactants on the size of FePt nanoparticles was studied in the nucleation and growth stage. The TEM and EDS results showed that Pt core are first formed in nucleation stage by 1,2-Hexadecadeniol as reducing reagent and the composition of the nanoparticles changes to Fe11Pt89. After that, the Fe atoms are released by intense LiBEt3H superhydride and surrounded around Pt core in growth stage and the composition changes to Fe63Pt37. At the beginning of the reflux stage, the percent of Fe atoms are decreased and the composition changes to Fe58Pt42. By increasing temperature in reflux stage, the size of FePt nanoparticles are increased to 4.5 nm and the stoichiometery of nanoparticles reaches to Fe62Pt38. The XRD spectra indicated that the L10-FePt nanoparticles are formed after annealing. Finally, the VSM results revealed that the coercivity of annealed FePt nanoparticles is increased to 7.5 kOe.
References
Sun, S.H., Murray, C.B., Weller, D., Folks, L., Moser, A.: Science 287, 2000 (1989)
Zeng, H., Li, J., Liu, J.P., Wang, Z.L., Sun, S.H.: Nature 420, 395 (2002)
Rong, C.B., Zhang, H.W., Du, X.B., Zhang, J., Zhang, S.Y., Shen, B.G.: J. Appl. Phys. 96, 3921 (2004)
Ristau, R.A., Barmak Lewis, K., Coffey, K.R., Howard, J.K.: J. Appl. Phys. 86, 4527 (1999)
Takahashi, Y.K., Ohkubo, T., Ohnuma, M., Hono, K.: J. Appl. Phys. 93, 7166 (2003)
Lee, J.H., Huh, Y.M., Jun, Y.W., Seo, J.W., Cheon, J.W.: Nat. Med. 13, 95 (2007)
Son, S.J., Reichel, J., He, B., Schuchman, M., Lee, S.B.: J. Am. Chem. Soc. 127, 7316 (2005)
Majetich, S.A., Jin, Y.: Science 284, 470 (1999)
Murray, C.B., Sun, S.H., Doyle, H., Betley, T.: Mater. Res. Soc. Bull. 26, 985 (2001)
Sun, S.H., Zeng, H., Robinson, D.B., Raoux, S., Rice, P.M., Wang, S.X., Li, G.X.: J. Am. Chem. Soc. 126, 273 (2004)
Peng, X., Wickham, J., Alivisatos, A.P.: J. Am. Chem. Soc. 120, 5343 (1998)
Murray, C.B., Kagan, C.R., Bawendi, M.G.: Annu. Rev. Mater. Sci. 30, 545 (2000)
Pamplin, B.R.: Crystal Growth. Pergamon Press, New York (1975)
Jiang, Y.: Forced Hydrolysis and Chemical Co-Precipitation. Kluwer Academic, New York (2003)
Murray, C.B., Norris, D.J., Bawendi, M.G.: J. Am. Chem. Soc. 115, 8706 (1993)
Peng, Z.A., Peng, X.: J. Am. Chem. Soc. 123, 183 (2001)
Qu, L., Peng, Z.A., Peng, X.: Nano Lett. 1, 333 (2001)
Sun, S., Anders, S., Thomson, T., Baglin, J.E.E., Toney, M.F., Hamann, H.F., Murray, C.B., Terris, B.D.: J. Phys. Chem. B 107, 5419 (2003)
Acknowledgements
The author is thankful for the financial support of Physics Research Center, and the Science and Research Campus at the Islamic Azad University for analysis and the discussions on the results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farahmandjou, M. Effect of Oleic Acid and Oleylamine Surfactants on the Size of FePt Nanoparticles. J Supercond Nov Magn 25, 2075–2079 (2012). https://doi.org/10.1007/s10948-012-1586-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-012-1586-1