Abstract
SBA-16, a kind of highly ordered mesoporous silica, was synthesized via a sol–gel method used as the supporter of TiO2 nanoparticles. With variation of Si/Ti molar ratios at 5.6, 1.4 and 0.7, SBA-16/TiO2 nanocomposites were prepared. In this study, pristine TiO2 was also synthesized as a control sample. Prior use, as-synthesized powders were calcined at 500 °C for 4 h. Physicochemical properties of the calcined powders were characterized by using various techniques. Degradation studies of a commercial paraquat (PQ) herbicide, having blue color from addition of the unknown dye, were performed using the SBA-16/TiO2 nanocomposites compared with the SBA-16 support and the pristine TiO2. Experimental results showed that the blue colors of the solutions were faded very quickly within 30 min when tested with SBA-16 and 5.6 SBA-16/TiO2 in dark conditions. The solutions were completely decolorized when the UV light was on for all composites and the pristine TiO2. The degradation of the dye was mainly depended on the adsorption process on SBA-16 surfaces. Photodegradation of PQ in the solutions was started by the nanocomposites and TiO2 tested under UV illumination conditions. The dye was completely degraded and the concentrations of PQ in the prepared solutions were reduced and reached to the maximum reduction at 70% within 24 h by 1.4 SBA-16/TiO2. Kinetic mechanisms for the differences of degradation characteristics by the catalysts under light-on and off conditions were studied, discussed and modelled.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mesoporous silica nanoparticles (MSNs) have been paid much attention to be used in various applications because of their pore characteristics, low cost and good thermal stabilities. Among them, highly ordered MSNs in the family of SBA (Santa Barbara Amorphous), have been recently considered as potential candidates for immobilization and transportation of various active substances due to their high surface areas, large pore volumes and typical characteristics of pore structures [1,2,3,4,5,6,7,8,9,10,11,12,13]. SBA no. 16 (SBA-16) have been extensively researched because of inexpensive pore templates used in the synthesized methods and 3D-channels of large pores being able to allow diffusions of large molecules. From biomedical to environmental applications, SBA-16 has been then used as carriers of drugs [13,14,15], supports of enzymes/bio-catalysts [16], adsorbents of biomolecules [11] and supports of catalysts [5,6,7,8, 12, 17,18,19].
Photocatalytic technology, one of the advanced oxidation processes (AOPs), has been recently applied to treat environmental pollutions [4, 5, 7, 10, 20,21,22,23,24,25,26,27,28,29,30,31,32,33]. The technology, basically, can generate the highly active species under the light illumination activated to decompose the hazardous organic chemicals into the less harmful or non-toxic chemicals as intermediated substances. Titanium dioxide (TiO2) is one of the efficient photocatalyst materials due to its promising properties including non-toxic, low cost, good chemical stability and highly oxidizing activity. Pairs of electrons (e−) and holes (h+) resulted from TiO2 excited by the light illumination are capable to react with organic molecules and mineralize the molecules into the H2O, CO2, etc. [20, 22, 29]. Among the 3-crystalline phases of TiO2 (brookite, anatase and rutile), anatase has been generally reported that it can exhibit highly photocatalytic activities for the pollutant degradation. However, the aggregation of TiO2 nanoparticles and the rapid reactions of e− and h+ recombination at surfaces of the excited TiO2 result in the loss of their photocatalytic activities. Highly porous materials used as supports for TiO2 nanoparticles have been introduced to promote photodegradation activities of organic matters and to reduce the agglomeration of TiO2 nanoparticles. Silica (SiO2), including MSNs, and their based materials have been fascinated as effective supports due to their high surface areas and varieties of pore characteristics [1, 2, 4, 5, 7, 10, 20, 21, 24,25,26,27, 29, 30, 33, 34]. In addition, SiO2 surfaces could inhibit phase transformation TiO2 [1, 2, 35] and retard the electron–hole recombinations [4].
Paraquat dichloride (PQ: 1,1′-dimethyl-4, 4′-bipyridinium dichloride) is classified into the bipyridylium group of herbicides, registered as Restricted Use Pesticides by Environmental Protection Agency (EPA), extensively used to control weeds and grasses in agricultural and non-agricultural areas [28, 33, 36]. PQ could be deactivated when it was adsorbed on soils [34, 36,37,38] due to electrostatic interactions between PQ cations and negative charges on clay surfaces [34]. Photolytic and microbial degradation of PQ were also reported [37]. Due to PQ having high water solubility (625 g l−1 at 25 °C), its residues could be accumulated into crops and moved from soil layers to ground water resulting in the water pollution and human health effect [38]. PQ was reported as a potential dopamine neurotoxin [39].
As reported by EPA [36], many European countries have prohibited the use of PQ. However, due to its rapid action and low cost, PQ is still widely used in many Asian countries [10]. In Thailand, the commercial PQ products have been imported as the second rank of the commercial herbicide products after glyphosate herbicide [40]. Although the command by Thai government for banning the use of 3 pesticides; PQ, glyphosate and chlorpyrifos; are not finally announced and published, the residues of PQ contaminated in water and soils are still needed to manage due to its possibilities contaminated into drinking water. Numerous studies showed that man-made materials could reduce PQ concentrations in wastewater using various kinds of absorbents and photocatalysts. PQ could be adsorbed by porous absorbents [35, 38, 41,42,43,44,45] and degraded by TiO2 [23] and its photocatalysts supported by supports and tested under different conditions of light illuminations [10, 28, 31,32,33, 46].
Dye additives have been practically added in commercial PQ solutions to color the solutions for preventing any accidental event be made by general users [41]. Many studies reported that the spreading of organic dyes into environment was one of the environmental pollutions. Therefore, in the cultivation areas used the commercial PQ products can be contaminated by both of the dye additives and PQ. Only the study of Nur et al. reported on adsorption studies of hydrophobic dyes and hydrophilic PQ by NaY zeolite covered with alkylsilane [41]. They used a well-known chemical structure of a reactive blue dye, Cibracon Blue 3GA [47] and a commercial PQ solution already mixed with an unknown blue dye, for simultaneous adsorption studies.
To our knowledge, studies on degradation of PQ in commercial solutions mixed with the unknown dye additives by photocatalyst nanocomposites have never been implemented before. Thus, in this study, SBA-16/TiO2 nanocomposites were synthesized using a sol–gel method. SBA-16 was used as the support of TiO2 nanoparticles and doped with TiO2 with different molar ratios of Si/Ti. Degradation conditions of PQ and its dye additive by the nanocomposites were compared with both SBA-16 and TiO2 synthesized as control samples. Photocatalytic reactions were tested under a fix intensity of UV illumination to optimize the tested conditions. Kinetic mechanisms of the PQ degradation and its dye additive have been studied, evaluated and discussed by considering experimental results.
2 Materials and methods
2.1 Chemicals
Tetraethyl orthosilicate (TEOS, 99%, Merck) and titanium(IV) butoxide (TBUT, 97%, Sigma-Aldrich) were used as Si and Ti precursors for SiO2 and TiO2 synthesis, respectively. Pluronic (F127, Mw = 12,600 Da, Sigma-Aldrich) was used as a pore template. Hydrochloric acid (HCl, 37%, Sigma-Aldrich) and absolute ethanol (ETOH, 99.99%, Merck) were used as reagents for catalyst synthesis. A commercial product of paraquat dichloride (1,1′-dimethyl-4, 4′bipyridinium dichloride, 27.6% (w/v) SL), manufactured by Syngenta company, Thailand under the tradename of Gramoxone, was used in this study. It was produced by the company as green–blue aqueous solution containing 20% (w/v) of 1,1′-dimethyl-4, 4′-bipyridinium ions.
2.2 Synthesis of SBA-16
Figure 1 shows the flowchart for synthesizing all samples used in this study. The synthesized method of SBA-16 was performed based on the previous work [48]. Briefly, a clear solution of pluronic F127 surfactant used as the directing pore agent/template was prepared by dissolution of 2.00 g of pluronic F127 in 15.00 g of distilled water at room temperature. 58 ml of 2 M HCl was then gradually added and followed by the dropwise addition of TEOS, at 0.0078 molar ratio of F127/TEOS into this acidic solution, pH << 1. To reach to the complete hydrolysis and condensation reactions, the solution was continuously stirred with moderated rate at room temperature for 21 h. White precipitated product was further statically incubated at temperature 80 ± 5 °C in an oil bath for 48 h, washed with distilled water several times until pH 4.0 by a centrifugation method operated at a fixed speed of 9000 rpm, and then dried in an oven at 80 °C for 24 h. Dried powder was ground and calcined at 500 °C for 4 h along with a fixed heating and cooling rate at 1 °C min−1 in order to completely remove the pluronic pore-template. In this study, the calcined sample was designated as SBA-16 which was synthesized and calcined by using the same conditions for synthesizing the TEOS-PA 500 sample, referred in the previous study [48].
2.3 Synthesis of SBA-16/TiO2 and TiO2
Titanium(IV) butoxide (TBUT) was used as the Ti precursor. Molar ratios of Si/Ti at 5.6, 1.4, and 0.7 were calculated for the TiO2 additions. Herein, the composite samples were designated as 5.6 SBA-16/TiO2, 1.4 SBA-16/TiO2 and 0.7 SBA-16/TiO2, respectively. For synthesis of SBA-16/TiO2 nanocmposites, equal amounts of TBUT and ethanol solutions were firstly prepared as shown in Fig. 1. The process routes to synthesize SBA-16/TiO2 nanocomposites were performed by following to the synthesized processes of SBA-16. In addition, TBUT-ethanol solutions were quickly dropped into the reactions after the dropwise addition of TEOS for 30 min. For synthesis of the TiO2 nanoparticles, the TBUT-ethanol solution was also quickly dropwise added into the acidic surfactant solution of F127. All of the as-synthesized powders obtained were further calcined using the same heating patterns used for synthesizing SBA-16.
2.4 Characterization of physicochemical properties
2.4.1 XRD analysis
A low angle X-ray diffractometer (LAXRD, TTRAX III, RIGAKU, Japan) was used to analyze highly ordered structures of the synthesized powders. Powder samples were scanned in the range of 2θ = 0.5°–5° at a step size of 0.01° and a step time of 0.50 s. Fraction of rutile phase of TiO2 was calculated as shown in the following equation [49];
where WR is the percentage of rutile fraction, IA is the diffraction peak intensity of anatase at index (101), and IR is the diffraction peak intensity of rutile at index (110).
Crystalline phases of the calcined powder samples were determined using a wide angle X-ray diffractometer (WAXRD, PANalytical X’Pert PRO, The Netherlands) operated at CuKα radiation with 40 kV and 30 mA. The samples were scanned in the range of 2θ = 5°–80°, step size of 0.02° and step time 0.5 s. In addition, the crystal sizes of these catalysts were measured using the Scherrer’s equation [50] as shown in Eq. (2);
where D is the average crystallite size (nm), K is the Scherrer constant (0.9), λ is the x-ray wavelength (CuKα = 0.15418 nm), β is the peak width of half maximum intensity (FWHM, radian), and θ is the Bragg diffraction angle (°).
2.4.2 FTIR and thermal analysis
A Fourier transform infrared spectrometer (FTIR, model 2000, Perkin Elmer, USA) was used to evaluate chemically functional structures of the synthesized powders. Thin pellets of samples/KBr were prepared and then recorded by the FTIR spectroscope in the wavenumber of 4000–400 cm−1 at resolution of 4 cm−1. For thermal analysis, thermogravimetric and differential thermal analysis (TG/DTA) techniques were used. The samples were heated from 30 to 1100 °C with a heating rate of 10 °C min−1 under air atmosphere. TG/DTA thermograms of the samples were analyzed using a simultanious thermal analyzer (TG/DTA, model 320, NETZSCH, Germany) coupled with the Proteus analysis program.
2.4.3 Surface characteristics and band gap energy determination
Surface characteristics of the synthesized powders were determined by physical adsorption of N2 on sample surfaces using a gas sorption analyzer (Autosorb I instrument, Quantachrome, USA). Samples were outgassed at 300 and 200 °C, respectively for 16–36 h prior to adsorption with N2 at − 196 °C. To evaluate band gap energies (Eg) of the calcined samples, a UV–Vis spectroscope (V750, JASCO, Japan) equipped with a solid state mode accessory was used. The samples were pressed as thin discs and then measured by the UV–Vis spectrometer. BaSO4, having white color, was used as a standard sample for the measurement.
2.4.4 Microstructure observation
A field emission scanning electron microscope (FE-SEM, SU5000, Hitachi, Japan), equipped with an energy dispersive X-ray (EDX, EMAX, Horiba, Japan) was used to investigation microstructure of all samples at low magnification. Before the observation, sample surfaces were coated with platinum by a sputtering coater (Q150RS, Quorum, UK) at 15 μA current for 30–45 s. Additionally, typical structures of pore characteristics of the synthesized powders were observed by transmission electron microscopes (TEM, JEOL 2010 and JEOL 2100 plus, Japan) operated at 200 kV. SEM and TEM samples were observed without grinding process to avoid any distortion of the mesopore channels.
2.4.5 Photocatalytic tests
50 mg l−1 of PQ solutions were prepared by dilution of the commercial PQ solution with distilled water and heated at 40 °C for 4–5 h. The prepared solutions were light-blue color. For each batch of photocatalytic testing, a fixed amount of the photocatalyst (100 mg) was added, dispersed into each of the diluted solutions (250 ml) and then sonicated for 5 min. Before UV radiation, the suspension was moderately stirred under a dark condition or light-off condition) at 250 rpm for 30 min using a magnetic stirrer to acquire an equilibrium adsorption of PQ on the sample surfaces. Each solution mixed with the photocatalyst samples was radiated under UV illumination, 8 W × 6 lamps of Philips Blacklight Blue providing total light intensities of UV-A = 53.33 W m−2, UV-B = 0.03 W m−2 and visible light = 0.32 W m−2 measured by light measurement probes (Solar Light company, Inc., USA). 3 ml of the tested solutions was collected at illumination intervals of 0, 30, 60, 90, 120, 240, 360 and 1440 min. The collected solutions were filtrated using 0.2 µm syringe filters, polyethersulfone (PES) and scanned from 190 to 1100 nm by the UV–Vis spectroscope (V750, JASCO, Japan). Intensities of peaks scanned at 257 and 628 nm were used to evaluate the reduction/degradation of PQ and the unknown blue dye, respectively. Calibration equations obtained from linear plots, relationships between intensities of the two scanned peaks and the PQ solution concentrations varied, were used in the calculation for PQ and the dye concentrations reduced.
The equilibrium adsorption capacities (qe) and the degradation (%) of the dye or PQ were calculated by using Eq. (3) [9, 11, 29, 35, 38, 51, 52] and Eq. (4) [28,29,30], respectively. The dye or PQ remained the solutions was also calculated and presented as the relationship plots between the remained values and time of light-off (the dark condition) and light-on using Eq. (5).
where qe is the equilibrium adsorption capacity of the dye or PQ (mg g−1 of the catalyst used), C0 is the initial concentration of the dye or PQ (mg l−1), Ce is the concentration of the dye or PQ at the equilibrium time (mg l−1), Ct is the concentration of the dye or PQ at time t (mg l−1), m is the mass of the catalyst used (g), and V is the volume of the PQ solutions (l).
3 Results and discussion
3.1 Ordered mesoporous structures
Figure 2 shows LAXRD patterns of the synthesized powders after calcination. The major diffraction peak at 0.74° 2θ together with two minor diffraction peaks at 1.06° 2θ and 1.68° 2θ indicating for (110), (200) and (310) planes, respectively, could be found in the profile of SBA-16 as previously reported [48]. Interplanar spacings or d-spacings of SBA-16 calculated based on Bragg’s Law were 11.91 nm (d110), 8.33 nm (d200), and 5.26 nm (d310) corresponding to the unit cell parameter, a0 = 16.84 nm (a0 = d110 × √2) as summarized in Table 1. As shown in the table, the calculated a0 values of SBA-16/TiO2 nanocomposites decreased when compared to that of SBA-16. Decrease of the cubic unit cell parameters of SBA-16 containing low TiO2 loading as well as disappearance of the LAXRD diffraction peaks [(110), (200) and (310) planes] of SBA-16 containing high TiO2 loading are significantly observed in Fig. 2.
3.2 Phase and crystal size
Figure 3 shows WAXRD patterns of the calcined powders. For SBA-16, only one broaden peak at approximately 23° 2θ could be seen. This peak corresponds to an amorphous phase of SiO2 as reported previously [2]. Based on JCPDS No. 21-1272, the highest intensity of the diffraction peak at 25.28° 2θ corresponding to the anatase phase of TiO2 was found in all SBA-16/TiO2 nanocomposites. It was also found that anatase phase of TiO2 increased with increasing TiO2 loading and the amorphous phase of SiO2 peak disappeared with increasing TiO2 loading (1.4 and 0.7 SBA-16/TiO2). This result suggests that TiO2 can be loaded into mesoporous SiO2 frameworks which is in agreement with the LAXRD result. The WAXRD pattern of the synthesized TiO2 showed 2-highest intensity peaks at 25.28° and 27.45° 2θ corresponding to (101) and (110) planes of anatase and rutile, referred by JCPDS Nos: 21-1272 and 21-1276, respectively.
The phase composition and crystal size of TiO2 and SBA-16/TiO2 nanocomposites calculated by following to Eq. (1) and (2) are summarized in Table 1. After calcination, as-synthesized TiO2 formed a phase mixture ratio of anatase:rutile at 68:32. For SBA-16/TiO2 nanocomposites, retardation of anatase transforming to rutile phase is noticeable when comparing with TiO2. Retarding phase transformation of TiO2 may be due to the presence of SBA-16. Anderson and Bard reported that the Si–O–Ti formed at interfaces was from the insertion of tetrahedral Ti sites into the tetrahedron SiO2 frameworks. This formation could retain the anatase phase and its crystallization [20]. In addition, as shown in Table 1, average crystal sizes of TiO2 (anatase) of 1.4 SBA-16/TiO2 and 0.7 SBA-16/TiO2 were smaller than those of 5.6 SBA-16/TiO2 and TiO2.
3.3 Microstructure investigation and chemical analysis
Figure 4a–e show overall microstructures investigated by SEM for the calcined SBA-16, SBA-16/TiO2 and TiO2, respectively. Roughness of SBA-16 surfaces could be seen clearly as shown in Fig. 4a. Agglomerated TiO2 particles, having nanosized scales, could be found in all SBA-16/TiO2 nanocomposites (Fig. 4b–d). It is obvious that, in particular areas observed, TiO2 particles, having rod shape, agglomerated for 5.6 SBA-16/TiO2 and TiO2 (Fig. 4e).
Figure 5a–d show microstructures investigated by TEM for calcined samples of SBA-16 and typical SBA-16/TiO2 nanocomposites and TiO2, respectively. Mesoporous structures of SBA-16 are shown in Fig. 5a and the inset. Based on the HR-TEM observation and the images of the calcined SBA-16, pore sizes are approximately 6 nm. Figure 5b, c show microstructure of TiO2 nanoparticles attaching on SBA-16 surfaces. It can identify the TiO2 particles agglomerating on the surface of SBA-16 (Fig. 5b). To identify or to separate each particle of TiO2 and SBA-16, it needs to observe the microstructures at higher magnification by considering the different crystal and amorphous structures of TiO2 and SBA-16, respectively (Fig. 5c). However, rod shape particles of TiO2 can be seen locally (Fig. 5d). The rod shape particles may be the rutile TiO2.
3.4 Chemical functionalities
Figure 6 shows a comparison set of FTIR spectra of the calcined samples. The spectra of SBA-16 showed the characteristic absorption peaks at 3458, 1645, 1080, 964, 799 and 461 cm−1 wavenumber. The peak at 3458 cm−1 is assigned to the OH stretching of physical adsorption of water molecules [15, 29]. The peak at 1645 cm−1 corresponds to the OH bending of water molecules [12, 15, 29]. The peak at 1080 cm−1 is assigned to the Si–O–Si asymmetric stretching [29, 30]. The peaks at 964 and 799 cm−1 are defined as the asymmetric bending vibration of the Si–OH [15, 30] and Si–O–Si, respectively [29]. The peak at 461 cm−1 is resulted from the bond of Si–O out of plane deformation, respectively [12, 53]. For SBA-16/TiO2 nanocomposites, the absorption peaks at 461 and 799 cm−1 are gradually decreased with an increase in the TiO2 loading which can be referred to the interruption of the entering of the larger Ti species into the SiO2 frameworks [54].
In particular, the development of FTIR characteristic peak at 960 cm−1 may be due to the formation of Si–O–Ti interaction at their interfaces indicating to the bonding interaction of other metal ions (M) into the SiO2 frameworks represented by Si–O–M [5, 30]. On the contrary to previous studies, the slight decrease of the peak at 960 cm−1 of SBA-16/TiO2 nanocomposites was observed with increasing in TiO2 loading. For SBA-16/TiO2 nanocomposites, having high TiO2 loading, the peak at 1080 cm−1 (SiO2) was shifted to 1087 cm−1 (1.4 SBA-16/TiO2) and 1090 cm−1 (0.7 SBA-16/TiO2) as well as the presence of the peak at 570 cm−1 was found. This peak may refer to TiO2 phase composited with SBA-16 [55]. FTIR results suggest that it is successful to incorporate TiO2 into mesostructure of the SiO2 frameworks observed at the low TiO2 loading of 5.6 SBA-16/TiO2. Moreover, the phase separation could be detected at higher TiO2 loading of 1.4 and 0.7 SBA-16/TiO2, in agreement with the XRD results.
3.5 Thermal behaviors
Figure 7a, b show the weight loss and thermal behaviors of SBA and SBA-16/TiO2 nanocomposites depending temperatures heated up to 1100 °C. The major changes of weight as approximately 30% were detected at the temperature higher than 200 °C, while the minor changes of weight (~ 10% loss) were estimated at the temperature in the range of 65–150 °C. The TG/DTA results showed that the former steps were occurred due to the endothermic changes of the degradation of the surfactant template (F127), while the letter steps were generally indicated to an exothermic change of the water evaporation and/or some remaining alcohol.
Evidently, the TG/DTA results showed that the degradation of F127 observed on SBA-16/TiO2 nanocomposites which were gradually extended and slightly shifted to the higher temperature with an increase in TiO2 loading. Increasing of F127 degradation temperatures may be occurred due to the enhancement of interaction between their existing functional groups of F127 and the localized TiO2 at mesoporous SiO2 walls [56]. In contrast to the TG/DTA results of the as-prepared samples in this study, FTIR results showed the disappearance of F127 contained in SBA-16/TiO2 nanocomposites found after calcination at 500 °C. This finding is similar to a previous study of Rankin et al. [57]. Therefore, the calcination temperature and conditions used in this study (500 °C for 4 h with the slow heating rate of 1 °C min−1) are the sufficient conditions for the purification of SBA-16/TiO2 nanocomposites.
3.6 Surface areas, pore sizes and pore distributions
Figure 8a, b show typical characteristics of the calcined powders analyzed by the N2 adsorption isotherm. As shown in Fig. 8a, reversible hysteresis loops at the P/P0 between 0.40–0.75 (H2 type) of the N2 adsorption isotherms could be observed in the SBA-16 and 5.6 SBA-16/TiO2, low TiO2 loading. The presence of the large loops shown in the figure may be related to a highly ordered cubic arrangement of the mesoporous structure of type IV classified by IUPAC [1]. On the other hand, the N2 adsorption isotherms of 1.4 and 0.7 SBA-16/TiO2, high TiO2 loading, show a characteristic of flatten loops at the P/P0 between 0.40 and 0.99 which may be referred to lower ordered arrangement of cubic structure of the mesoporous SiO2 [2, 3]. Obviously, the pore sizes and their distributions of 5.6 SBA-16/TiO2 were similar to those of SBA-16, as seen in Fig. 8b. However, the distribution of pore sizes was observed at high TiO2 loading, 1.4 and 0.7 SBA-16/TiO2.
In addition, the calculated pore wall thickness of 5.6 SBA-16/TiO2 was slightly decreased when compared with that of SBA-16. The results of N2 isotherms suggest that nanocrystalline TiO2 could be inserted into the SiO2 frameworks at low TiO2 loading, 5.6 SBA-16/TiO2, and was localized at extra frameworks at high TiO2 loading (1.4 and 0.7 SBA-16/TiO2). These textural characters insist that nanocrystalline TiO2 can be incorporated into the frameworks of SBA-16, depending on the amount of TiO2 loading. Table 2 lists surface area characteristics of SBA-16 and SBA-16/TiO2 nanocomposites after calcination. N2 adsorption isotherms of SBA-16/TiO2 nanocomposites decreased with an increase in TiO2 loading when comparing to that of SBA-16. Moreover, as shown in the table, BET surface area values of 1.4 and 0.7 SBA-16/TiO2 decreased approximately 50% from that of SBA-16. Pore volumes of SBA-16 also decreased when increasing TiO2 loading into SBA-16.
Shah et al. synthesized SBA-16 containing Ti atoms (Ti-MSM) by the self-assembly method induced the evaporation [5]. In their synthesized method, TEOS and F127 were used as the Si source and pore template, respectively. The incorporation of Ti atoms into the SiO2 frameworks was reported. N2 adsorption–desorption isotherms revealed that BET surface areas and pore volumes of the Ti–MSM composites decreased with increasing Ti contents. Since the pore characteristics of SBA-16/TiO2 nanocomposites synthesized in this study are consistent with the work of Shah et al. it is possible that Ti atoms are possibly inserted into the SiO2 frameworks.
3.7 Band gap energies
Figure 9 shows relationship plots between relative absorption coefficient [F(R)1/2] and hv of the calcined samples. Band gap energy (Eg) value of each sample was determined by the interception of two extrapolated lines (the inset figure) of the plots of the relative absorption coefficient [F(R)1/2] versus hv. The calculated Eg values of 5.6, 1.4 and 0.7 SBA-16/TiO2 and TiO2 were 3.12, 3.02, 3.00 and 2.98 eV, respectively. In this study, the Eg value of SBA-16 could not be determined due to its low UV absorption as similar findings found in a study of Cendrowski et al. [26]. However, some previous studies reported the Eg values of SiO2 as 4.7 [30] and 8.0 eV [58].
3.8 Photocatalytic activities
Photocatalytic activities of the synthesized powders were evaluated on the degradation of the commercial PQ, a recalcitrant pesticide, in terms of % degradation active PQ and the additive dye. For monitoring the UV–vis spectrum of the commercial PQ solution, the first major peak representing for PQ and the second major peak representing for the blue dye could be detected at 257 and 628 nm, respectively. The intensity peak of PQ was higher than that of the dye approximately 270 times. Figure 10a reveals the blue color representing for the presence of the dye additive mixed in the PQ solution still appears after the UV radiation for 24 h. After testing under the UV radiation, the blue color disappeared within 30–60 min depending on the catalyst samples used and light illumination, as shown in Fig. 10b–f.
Figure 11a, b show the evolution of UV–Vis spectra of the dye and PQ in the solutions in the presence of 1.4 SBA-16/TiO2 under UV radiation for 24 h, respectively. Figure 11a demonstrates the absorption spectra of the dye detected at the maximum wavelength of 628 nm by the UV–Vis spectroscope. As shown in the figure, the spectra rapidly decreased. The dye contained in the solutions almost disappeared. Figure 11b shows the spectra of PQ in the solutions. Noticeably, the intensities of the absorption peaks at 257 nm decreased with increasing the radiation time, while the new absorption peaks at 220 nm slightly grow up. The new peaks found suggest the generation of unknown intermediate species formed during photocatalysis.
Figure 12a shows the adsorption and degradation of the dye. After 30 min of adsorption, the dye was adsorbed on the surfaces of SBA-16 rather than on the surfaces of SBA-16/TiO2 nanocomposites. All catalyst samples could degrade the dye within 30–60 min. Abilities of adsorbing dyes onto the SiO2 surfaces may depend on pH conditions and ionic characters of the dye. Based on the previous studies, anionic dyes could contact to the SiO2 surfaces via hydrogen bonding and electrostatic interactions between the silanol group of SiO2 and functional groups of organic dye under acidic conditions [51] and cationic dyes could interact with the negatively charged SiO2 surface at neutral conditions [9].
No adsorption of PQ could be observed on all samples tested under dark conditions. However, PQ could be gradually degraded during radiation time as shown in Fig. 12b. The experimental results showed that the degradation of the dye and PQ increased when TiO2 loading content in SBA-16 increased. The dye completely degraded within 30 min of radiation time. The PQ degradation of all samples increased in an order of 1.4 SBA-16/TiO2, 0.7 SBA-16/TiO2, TiO2 and 5.6 SBA-16/TiO2. Percentages of the degradation of PQ tested with SBA-16/TiO2 samples tested under 24 h radiation time were nearly 70 and 60% for 1.4 and 0.7 SBA-16/TiO2, respectively.
A previous study of Lee et al. showed that PQ degradation related to UV-C (254 nm) light intensities which were varied at 4, 8 and 12 W m−2. They reported that without air sparging in the tested system, the PQ degradation rate increased with increasing of UV light intensities. At 60 h of the radiation, PQ, prepared at 100 mg l−1 initial concentrations, could be degraded using 0.1 g l−1 of TiO2 doses at approximately 60, 70 and more than 90% under 4, 8 and 12 W m−2 of the radiation, respectively. They also reported that PQ could be degraded within 40 h when it was radiated under TiO2/air system. However, for 90% PQ degradation, degradation time could be shorter at 18, 12 and 2 h when the intensities were increased to be 12, 24 and 36 W m−2 in the presence of the air sparging, respectively [23].
P25 TiO2 (Degussa-P25), a commercial TiO2 powder, was used as a standard TiO2 for studying on the photodegradation of PQ in the work of Sorolla II et al. [10]. 10 mg l−1 of the prepared PQ solutions (natural pH 6.5) was kept under a dark condition for 1 h and then radiated by 50 W m−2 of the UV light in the presence of 0.5 g l−1 of P25 TiO2. Almost 75% of the PQ degradation was achieved after 4 h. In this study, the highest value of the PQ removal was found to be nearly 70% in the presence of 1.4 SBA-16/TiO2 (0.4 g l−1) after 24 h of the UV radiation. To improve the photodegradation efficiency, increasing the photocatalyst dosage or decreasing the initial concentration should be concerned.
Brigante and Schulz also explained that the high adsorption of PQ on the surface of their synthesized mesoporous SiO2 doped with 28% wt TiO2 under a basic condition (pH 9.0) was due to the interaction of positive PQ ions and negative surface of TiO2. However, no adsorption of PQ observed on the mesoporous SiO2 surfaces having charge balance at neutral conditions [35]. In our study, the low adsorption efficiencies of PQ were observed on the surfaces of SBA-16/TiO2 and SBA-16 and TiO2. Hindrances obtained from dye molecules on the catalyst surfaces and the neutralization of catalyst surfaces under the neutral conditions may be the 2-main reasons of the low adsorption of PQ.
3.9 Kinetic mechanisms
Figure 13a, b show the kinetic linear plots of the dye and PQ containing in the commercial PQ. The kinetic degradation results of the dye and PQ were fitted with the first order Langmuir-Hinshelwood kinetic model. Apparent rate constants (kapp) of each sample were calculated from slopes of the determined linear plots of ln C0/Ct versus reaction time (t), having high values of correlation coefficients (R2), as shown by Eq. (6) [7, 10, 22, 23, 27, 46];
where C0 is the initial concentration of the dye related to PQ or PQ (mg l−1), Ct is the concentration at time of the dye related to PQ or PQ (mg l−1), kapp is the apparent reaction rate constant (min−1) and t is the reaction/radiation time (min).
PQ photodegradation rates represented in terms of kapp values were evaluated and shown in Table 3. The results showed that the calculated kapp values increased with increasing TiO2 loading content. The photocatalytic activity of 1.4 SBA-16/TiO2 showed the highest kapp value on the dye and PQ degradation. The low activities on degradations of the dye and PQ could be observed on 5.6 SBA-16/TiO2 due to the lower amount of TiO2 presented on SBA-16 surfaces. Moreover, aggregation of TiO2 on the mesoporous SiO2 surfaces may be a reason to limit the photocatalytic activity of 0.7 SBA-16/TiO2 having high TiO2 loading.
A possible mechanism of the decolorization and degradation of the blue dye and PQ in aqueous solution by SBA-16, SBA-16/TiO2 nanocomposites and TiO2 proposed is illustrated as shown in Fig. 14. In the adsorption of the dye, SBA-16 mainly takes part in the adsorption process. For the SBA-16/TiO2 nanocomposites, the presence of TiO2 particles is considered as the pore blockage of SBA-16 leading to the decrease in specific surface areas. The adsorption capacities of the composites are subsequently decreased according to the results. Notably, the amount of dye adsorbed during the dark condition was not significantly different in all SBA-16/TiO2 nanocomposites despite the large difference in their specific surface areas. It can be assumed that the adsorption is not related to in the pore characteristics. The adsorption may only occur on the external surface. It is in agreement with the work of Nur et al. [41] studying on the adsorption of a commercial paraquat and the reactive dye Cibacron Blue 3GA (as the model of blue dye containing in PQ) using a commercial NaY zeolite covered with alkylsilane. They suggested that the blue dye was preferentially adsorbed on the external surface of the modified zeolite particles while PQ was adsorbed on the hydrophilic internal pores.
However, it is difficult to distinguish the surface interaction between SBA-16 and the unknown blue dye containing in PQ. The interactions between mesoporous SiO2 gels and dyes corresponding to the dye adsorption were reported in the previous study of Wu et al. [51]. Two types of organic dyes were adsorbed by various kinds of the mesoporous silica gels incorporated with various organic groups. Surface interactions between the dyes and the gels were proposed including electrostatic interaction, hydrophobic interaction and hydrogen bonding. By considering the FTIR spectrum in our work, the silanol groups presenting on SBA-16 and SBA-16/TiO2 surfaces can be protonated, positively charged and electrostatically adsorbed the dye. It indicates that both electrostatic attraction and hydrogen bonding are possible between SBA-16 and the dye.
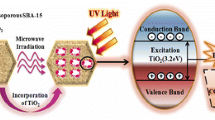
The degradation of the dye is also governed by the photodegradation of TiO2. Following irradiation with UV light, electrons in the valence band of TiO2 particles were excited to the conduction band, leaving holes in the valence band. The holes and electrons migrated to the surface of TiO2 particles and participated in a series of redox reactions. The electrons reacted with the absorbed oxygen onto the TiO2 surface forming O2˙− radicals while the holes reacted with water molecules generating OH˙ radicals. Both radicals possessed the oxidation power to decompose the dye to harmless products. The results from the photocatalytic activities confirm that photodegradation is predominant in the degradation of the dye.
In the case of PQ, the process of the surface adsorption rarely occurs due to the neutralization of the charges on the sample surfaces. Since PQ is majorly in the neutral form in the studied pH range, which means that there are no opposing charges and no electrostatic interaction. Thus, the degradation mechanism might be mainly controlled by the photocatalytic degradation process.
Based on the previous work studying on intermediate formations during the photodegradation of PQ solutions by TiO2/SiC foams, initially, PQ ions, adsorbing on the surfaces of SBA-16/TiO2 nanocomposites, may transform to bipyridinium ions (e.g. monoquat, reduced paraquat or paraquat monopyridone) as the primary intermediate products by either oxidation or demethylation. The intermediates can be subsequently degraded through demethylation and aromatic ring cleavage, resulting in short chain carboxylic acids (e.g. maleate, succinate, acetate, oxalate or formate) as the final products [32]. Lee et al. reported that acidic compounds such as NO3−, NO2− and HCl were also produced during the PQ photodegradation in the presence of the synthesized TiO2 powder [23]. In the case of the completed photocatalytic reaction, Lee et al. also reported that the compounds could be mineralized as the non-toxic products including N2, O2 and CO2. However, the amount of TiO2 and the crystalline phase of TiO2 on the surfaces of SBA-16/TiO2 nanocomposites were the crucial factors impacting on the photocatalytic activity performance against the dye and PQ in the commercial PQ solution.
4 Conclusions
Highly ordered mesoporous SiO2 (SBA-16) and its nanocomposites (SBA16/TiO2) were successfully synthesized in this study in terms of forming mesoporous structures and being able to degrade a commercial PQ herbicide having blue color. The characterization results revealed the unique structures and properties of SBA-16 and SBA16/TiO2 nanocomposites related to amounts of TiO2 loading into SBA-16. Higher TiO2 loading content could deteriorate the ordered mesoporous structure of SBA-16 because of the attachment of TiO2 nanoparticles on the SBA-16 surfaces. The blue dye in the PQ solution favored to adsorb on the SBA-16 surfaces and could be totally degraded by all SBA-16/TiO2 nanocomposites and the synthesized TiO2 under UV illumination conditions. PQ in the solutions was degraded by the photocatalytic reaction process in the presence of the nanocomposites and TiO2. Within 24 h of the UV illumination, 1.4 SBA-16/TiO2 nanocomposites could maximize the PQ photodegradation in the solution at approximately 70% reduction. The kinetic mechanism of the dye degradation and PQ photodegradation was proposed.
References
D. Zhao, Q. Huo, J. Feng, B.F. Chmelka, G.D. Stucky, Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Ceram. Soc. 120, 6024–6036 (1998)
W. Dong, Y. Sun, C.V. Lee, W. Hua, X. Lu, Y. Shi, S. Zhang, J. Chen, D. Zhao, Controllable and repeatable synthesis of thermally stable anatase nanocrystal-silica composites with highly ordered hexagonal mesostructures. J. Am. Ceram. Soc. 129, 13894–13904 (2007). https://doi.org/10.1021/ja073804oCCC
S. Shen, Y. Deng, G. Zhu, D. Mao, Y. Wang, G. Wu, J. Li, X. Liu, G. Lu, D. Zhao, Synthesis and characterization of Ti-SBA-16 ordered mesoporous silica composite. J. Mater. Sci. 42, 7057–7061 (2007). https://doi.org/10.1007/s10853-007-1608-8
M.V.P. Sharma, V.D. Kumari, M. Subrahmanyam, TiO2 supported over SBA-15: an efficient photocatalyst for the pesticide degradation using solar light. Chemosphere 73, 1562–1569 (2008). https://doi.org/10.1016/j.chemosphere.2008.07.081
A.T. Shah, B. Li, Z.E.A. Abdalla, Direct synthesis of Ti-containing SBA-16-type mesoporous material by the evaporation-induced self-assembly method and its catalytic performance for oxidative desulfurization. J. Colloid Interf. Sci. 336, 707–711 (2009). https://doi.org/10.1016/j.jcis.2009.04.026
M. Boutros, T. Onfroy, P. da Costa, Mesostructured or alumina-mesostructured silica SBA-16 as potential support for NOx reduction and ethanol oxidation. Catal. Lett. 139, 50–55 (2010). https://doi.org/10.1007/s10562-010-0391-3
J. Ma, L. Qiang, X. Tang, A simple and rapid method to directly synthesize TiO2/SBA-16 with different TiO2 loading and its photocatalytic degradation performance on rhodamine B. Catal. Lett. 138, 88–95 (2010). https://doi.org/10.1007/s10562-010-0374-4
J. Ma, L. Qiang, J. Wang, D. Tang, X. Tang, An easy route to synthesize novel mesostructured silicas Al/SBA-16 and its catalytic application. Catal. Lett. 14, 356–363 (2011). https://doi.org/10.1007/s10562-010-0494-x
C.-H. Huang, K.-P. Chang, H.-D. Ou, Y.-C. Chiang, C.-F. Wang, Adsorption of cationic dyes onto mesoporous silica. Microporous Mesoporous Mater. 141, 102–109 (2011). https://doi.org/10.1016/j.micromeso.2010.11.002
M.G. Sorolla II., M.L. Dalida, P. Khemthong, N. Grisdanurak, Photocatalytic degradation of paraquat using nano-sized Cu-TiO2/SBA-15 under UV and visible light. J. Environ. Sci. 24, 1125–1132 (2012). https://doi.org/10.1016/S1001-0742(11)60874-7
S.M.L. dos Santos, K.A.B. Nogueira, M.G. da Souza, J.D.F. Lima, I.J. da Silva Jr, D.C.S. de Azevedo, Synthesis and characterization of ordered mesoporous silica (SBA-15 and SBA-16) for adsorption of biomolecules. Microporous Mesoporous Mater. 180, 284–292 (2013). https://doi.org/10.1016/j.micromeso.2013.06.043
H.-B. Wang, Y.-H. Zhang, H.-L. Yang, Z.-Y. Ma, F.-W. Zhang, J. Sun, J.-T. Ma, Palladium immobilized in the nanocages of SBA-16: an efficient and recyclable catalyst for Suzuki coupling reaction. Microporous Mesoporous Mater. 168, 65–72 (2013). https://doi.org/10.1016/j.micromeso.2012.09.025
G. Gonzalez, A. Sagarzazu, A. Cordova, M.E. Gomes, J. Salas, L. Contreas, K. Noris-Suarez, L. Lascano, Comparative study of two silica mesoporous materials (SBA-16 and SBA-15) modified with a hydroxyapatite layer for clindamycin controlled delivery. Microporous Mesoporous Mater. 256, 251–265 (2018). https://doi.org/10.1016/j.micromeso.2017.07.021
Y. Hu, J. Wang, Z. Zhi, T. Jiang, S. Wang, Facile synthesis of 3D cubic mesoporous silica microspheres with a controllable pore size and their application for improved delivery of a water-insoluble drug. J. Colloid Interf. Sci. 363, 410–417 (2011). https://doi.org/10.1016/j.jcis.2011.07.022
G.F. Andrde, D.C.F. Soares, R.G. dos Santos, Mesoporous silica SBA-16 nanoparticles: synthesis, physicochemical characterization, release profile, and in vitro cytocompatibility studies. Microporous Mesoporous Mater. 168, 102–110 (2013). https://doi.org/10.1016/j.micromeso.2012.09.034
J. Aburto, M. Ayala, I. Bustos-Jaimes, C. Montiel, E. Terrés, J.M. Domínguez, E. Torres, Stability and catalytic properties of chloroperoxidase immobilized on SBA16 mesoporous materials. Microporous Mesoporous Mater. 83, 193–200 (2005). https://doi.org/10.1016/j.micromeso.2005.04.008
B.R. Jermy, S.-Y. Kim, K.V. Bineesh, M. Selvaraj, D.-W. Park, Easy route for the synthesis of Fe-SBA-16 at weak acidity and its catalytic activity in the oxidation of cyclohexene. Microporous Mesoporous Mater. 121, 103–113 (2009). https://doi.org/10.1016/j.micromeso.2009.01.010
A.T. Shah, B. Li, Z.E.A. Abdalla, Direct synthesis of Cu-SBA-16 by internal pH-modification method and its performance for adsorption of dibenzothiophene. Microporous Mesoporous Mater. 130, 248–254 (2010). https://doi.org/10.1016/j.micromeso.2009.11.017
S. Zhang, S. Muratsugu, N. Ishiguro, M. Tada, Ceria-doped Ni/SBA-16 catalysts for dry reforming of methane. ACS Catal. 3, 1855–1864 (2013). https://doi.org/10.1021/cs400159w
C. Anderson, A.J. Bard, Improved photocatalytic activity and characterization of mixed TiO2/SiO2 and TiO2/Al2O3 materials. J. Phys. Chem. B 101, 2611–2616 (1997)
C. Zhu, L. Wang, L. Kong, X. Yang, L. Wang, S. Zheng, F. Chen, F. MaiZhi, H. Zong, Photocatalytic degradation of AZO dyes by supported TiO2 + UV in aqueous solution. Chemosphere 41, 303–309 (2000)
U.I. Gaya, A.H. Abdullah, Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J. Photochem. Photobiol. C 9, 1–12 (2008). https://doi.org/10.1016/j.jphotochemrev.2007.12.003
J.-C. Lee, M.-S. Kim, C.K. Kim, C.-H. Chung, S.M. Cho, G.Y. Han, K.J. Yoon, B.-W. Kim, Removal of paraquat in aqueous suspension of TiO2 in an immersed UV photoreactor. Korean J. Chem. Eng. 20, 862–868 (2003). https://doi.org/10.1007/BF02697289
S. Artkla, W. Kim, W. Choi, J. Wittayakun, Highly enhanced photocatalytic degradation of tetramethylammonium on the hybrid catalyst of titania and MCM-41 obtained from rice husk silica. Appl. Catal. B 91, 157–164 (2009). https://doi.org/10.1016/j.apcatb.2009.05.019
M. Tasbihi, U.L. Štangar, A.S. Škapin, A. Ristić, V. Kaučič, N.N. Tušar, Titania-containing mesoporous silica powders: Structural properties and photocatalytic activity towards isopropanol degradation. J. Photochem. Photobiol. A 216, 167–178 (2010). https://doi.org/10.1016/j.jphotochem.2010.07.011
K. Cendrowski, X. Chen, B. Zielinska, R.J. Kalenczuk, M.H. Rümmeli, B. Büchner, R. Klingeler, E. Borowiak-Palen, Synthesis, characterization, and photocatalytic properties of core/shell mesoporous silica nanospheres supporting nanocrystalline titania. J. Nanopart. Res. 13, 5899–5908 (2011). https://doi.org/10.1007/s11051-011-0307-1
N.A. Kouamé, D. Robert, V. Keller, N. Keller, C. Pham, P. Nguyen, TiO2/β-SiC foam-structured photoreactor for continuous wastewater treatment. Environ. Sci. Pollut. Res. 19, 3727–3734 (2012). https://doi.org/10.1007/s11356-011-0719-6
E. Kanchanatip, N. Grisdanurak, R. Thongruang, A. Neramittagapong, Degradation of paraquat under visible light over fullerene modified V-TiO2. React. Kinet. Mech. Cat. 103, 227–237 (2011). https://doi.org/10.1007/s11144-011-0293-4
N. Guo, Y. Liang, S. Lan, L. Liu, G. Ji, S. Gan, H. Zou, X. Xu, Uniform TiO2-SiO2 hollow nanospheres: synthesis, characterization and enhanced adsorption-photodegradation of azo dyes and phenol. Appl. Surf. Sci. 305, 562–574 (2014). https://doi.org/10.1016/j.apsusc.2014.03.136
H.B. Yener, ŞŞ Helvacı, Effect of synthesis temperature on the structural properties and photocatalytic activity of TiO2/SiO2 composites synthesized using rice husk ash as a SiO2 source. Sep. Purif. Technol. 140, 84–93 (2015). https://doi.org/10.1016/j.seppur.2014.11.013
S. Wongcharoen, G. Panomsuwan, Easy synthesis of TiO2 hollow fibers using kapok as a biotemplate for photocatalytic degradation of the herbicide paraquat. Mater. Lett. 228, 482–485 (2018). https://doi.org/10.1016/j.matlet.2018.06.089
C.B.D. Marien, M. Le Pivert, A. Azaïs, I.C. M’Bra, P. Drogui, A. Dirany, D. Robert, Kinetics and mechanism of Paraquat’s degradation: UV-C photolysis vs UV-C photocatalysis with TiO2/SiC foams. J. Hazard. Mater. 370, 164–171 (2019). https://doi.org/10.1016/j.hazmat.2018.06.009
A. Pourzad, H.R. Sobhi, M. Behbahani, A. Esrafili, R.R. Kalantary, M. Kermani, Efficient visible light-induced photocatalytic removal of paraquat using N-doped TiO2@SiO2@Fe3O4 nanocomposite. J. Mol. Liq. 299, 112167 (2020). https://doi.org/10.1016/j.molliq.2019.112167
D. Gondar, R. López, J. Antelo, S. Fiol, F. Arce, Adsorption of paraquat on soil organic matter: effect of exchangeable cations and dissolved organic carbon. J. Hazard. Mater. 235–236, 218–223 (2012). https://doi.org/10.1016/j.jhazmat.2012.07.044
M. Brigante, P.C. Schulz, Adsorption of paraquat on mesoporous silica modified with titania: effects of pH, ionic strength and temperature. J. Colloid Interf. Sci. 363, 355–361 (2011). https://doi.org/10.1016/j.jcis.2011.07.061
US EPA archive document, Paraquat dichloride: reregistration eligibility decision (R.E.D. Facts), United States Environmental Protection Agency, US EPA Archives document, EPA-738-F-96-018, 1-12 (1997).
T.R. Roberts, J.S. Dyson, M.C.G. Lane, Deactivation of the biological activity of paraquat in the soil environment: a review of long-term environmental fate. J. Agric. Food Chem. 50, 3623–3631 (2002). https://doi.org/10.1021/jf011323xCCC
M.S.F. Santos, G. Schaule, A. Alves, L.M. Madeira, Adsorption of paraquat herbicide on deposits from drinking water networks. Chem. Eng. J. 229, 324–333 (2013). https://doi.org/10.1016/j.cej.2013.06.008
J.M. Hatcher, K.D. Pennell, G.W. Miller, Parkinson’s disease and pesticides: a toxicological perspective. Trends Pharmacol. Sci. 29, 322–329 (2008). https://doi.org/10.1016/j.tips.2008.03.007
P. Panuwet, W. Siriwong, T. Prapamontol, P.B. Ryan, N. Fiedler, M.G. Robson, D.B. Barr, Agricultural pesticide management in Thailand: situation and population health risk. Environ. Sci. Policy 17, 72–81 (2012). https://doi.org/10.1016/j.envsci.2011.12.005
H. Nur, A.F.N.A. Manan, L.K. Wei, M.N.M. Muhid, H. Hamdan, Simultaneous adsorption of a mixture of paraquat and dye by NaY zeolite covered with alkylsilane. J. Hazard. Mater. B117, 35–40 (2005). https://doi.org/10.1016/j.jcis.2009.04.026
M. Brigante, M. Avena, Synthesis, characterization and application of a hexagonal mesoporous silica for pesticide removal from aqueous solution. Microporous Mesoporous Mater. 191, 1–9 (2014). https://doi.org/10.1016/j.micromeso.2014.02.035
M.M. Desipio, R. Thorpe, D. Saha, Photocatalytic decomposition of paraquat under visible light by carbon nitride and hydrogen peroxide. Optik 172, 1047–1056 (2018). https://doi.org/10.1016/j.ijleo.2018.07.124
C. Keawkumay, W. Rongchapo, N. Sosa, S. Suthirakun, I.Z. Koleva, H.A. Aleksandrov, G.N. Vayssilov, J. Wittayakun, Paraquat adsorption on NaY zeolite at various Si/Al ratios: a combined experimental and computational study. Mater. Chem. Phys. 238, 121824 (2019). https://doi.org/10.1016/j.matchemphys.2019.121824
H. Li, Q. Miao, Y. Chen, M. Yin, H. Qi, M. Yang, Q. Deng, S. Wang, Modified carbon spheres as universal materials for adsorption of cationic harmful substances (paraquat and dyes) in water. Microporous Mesoporous Mater. 297, 110040 (2020). https://doi.org/10.1016/j.micromeso.2020.110040
P. Phuinthiang, P. Kajitvichyanukul, Degradation of paraquat from contaminated water using green TiO2 nanoparticles synthesized from Coffea arbica L. in photocatalytic process. Water Sci. Technol. 79, 905–910 (2019). https://doi.org/10.2166/wst.2018.493
W.O. Smith Jr., S.M. Daniels, Purification of phytochrome by affinity chromatography on agarose-immobilized cibacron blue 3GA. Plant Physiol. 68, 443–446 (1981)
C. Vanichvattanadecha, W. Singhapong, A. Jaroenworaluck, Different sources of silicon precusors influencing on surface characteristics and pore morphologies of mesoporous silica nanoparticles. Appl. Surf. Sci. 513, 145568 (2020). https://doi.org/10.1016/j.apsusc.2020.145568
W. Li, C. Ni, H. Lin, C.P. Huang, S.I. Shah, Size dependence of thermal stability of TiO2 nanoparticles. J. Appl. Phys. 96, 6663–6668 (2004). https://doi.org/10.1063/1.1807520
H.P. Klug, J.E. Alexander, X-Ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd edn. (Wiley, New York, 1997).
Z. Wu, H. Joo, I.-S. Ahn, S. Haam, J.-H. Kim, K. Lee, Organic dye adsorption on mesoporous hybrid gels. Chem. Eng. J. 102, 277–282 (2004). https://doi.org/10.1016/j.cej.2004.05.008
C.-F. Huang, C.-W. Tu, R.-H. Lee, C.-H. Yang, W.-C. Hung, K.-Y.A. Lin, Study of various diameter and functionality of TEMPO-oxidized cellulose nanofibers on paraquat adsorptions. Polym. Degrad. Stabil. 161, 206–212 (2019). https://doi.org/10.1016/j.polymdegradstab.2019.01.023
G. Katumba, B.W. Mwakikunga, T.R. Mothibinyane, FTIR and Raman Spectroscopy of carbon nanoparticles in SiO2, ZnO and NiO matrices. Nanoscale Res. Lett. 3, 421–426 (2008). https://doi.org/10.1007/s11671-008-9172-y
J. Ren, L. Zhong, S. Liu, Y. Xing, K. Xie, Silica-titania mixed oxides: Si-O-Ti connectivity, coordination of titanium, and surface acidic properties. Catal. Lett. 124, 185–194 (2008). https://doi.org/10.1007/s10562-008-9500-y
X. Wang, G. Wu, B. Zhou, J. Shen, Thermal annealing effect on optical properties of binary TiO2-SiO2 sol-gel coatings. Materials 6, 76–84 (2013). https://doi.org/10.3390/ma6010076
J. Jiu, K. Kurumada, F. Wang, L. Pei, Syntheses of highly ordered mesoporous silica by new hybrid template. Mater. Chem. Phys. 86, 435–439 (2004). https://doi.org/10.1016/j.matchemphys.2004.04.013
S.E. Rankin, B. Tan, H.-J. Lehmler, K.P. Hindman, B.L. Knutson, Well-ordered mesoporous silica prepared by cationic fluorinated surfactant templating. Microporous Mesoporous Mater. 73, 197–202 (2004). https://doi.org/10.1016/j.micromeso.2004.05.013
S. Vives, C. Meunier, Optical properties of copper modified sol-gel SiO2 thin films. Mater. Lett. 91, 165–169 (2013). https://doi.org/10.1016/j.matlet.2012.09.081
Acknowledgements
This work was financially supported by National Metal and Materials Technology Center [Grant No. MT-B-55-CER-07-291-I (P-12-00989)]. Some facilities used in this work were granted by European Commission (EC) [Grant No. 309846/FP7 (PCATDES)] and National Metal and Materials Technology Center [Grant No. P-13-00697].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vanichvattanadecha, C., Jaroenworaluck, A., Henpraserttae, P. et al. Ordered mesoporous silica (SBA-16) supporting titania (TiO2) nanoparticles for photodegradation of paraquat (PQ) herbicide. J Porous Mater 28, 1137–1153 (2021). https://doi.org/10.1007/s10934-021-01065-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-021-01065-5