Abstract
With respect to different applications of metal–organic framework (MOF) in the medical, industrial and environmental fields, it is very important to choose a new structure that can be synthesized by fast, eco-friendly and affordable methods with distinctive properties so that the properties could be systematically controlled. In this study, new Ta–MOF nanostructures are synthesized by novel methods of microwave (Mw) and ultrasonic assisted microwave (UAMw) in environmental conditions. The final products are characterized by relevant techniques. Although in the both methods, the synthesized products have favourable properties; the use of the UAMw method would produce samples with distinct features such as high thermal stability of 240 °C, average particle size distribution (PSD) of 23 nm and significant specific surface area (SSA) of 2012 m2/g. For a better comprehension of the Ta–MOF formation, computational studies are performed using DFT calculations. In order to investigate the effect of the synthesis parameters on different features of the products, the fractional factorial design is used. The results of analysis of variance confirm that the parameters such as Mw power, Mw duration, ultrasonic temperature, ultrasonic power and ultrasonic duration have a significant effect on PSD and SSA of Ta–MOF samples. Due to the fractional factorial design of the experiments, response surface methodology would optimize the probability of producing samples with the small PSD of 15 nm and high SSA of 2588 m2/g; this desirable amount would provide situations to use these compounds in diverse fields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metal–organic frameworks (MOFs) are a novel class of porous materials, which have recently been used in various fields due to their unique physico-chemical properties [1]. These materials are prepared by various methods under different conditions. It is highly important to choose a method that makes the synthesis of these materials in environmental conditions possible and manufactures high-yielding products with distinctive properties [2]. The ultrasonic and microwave (Mw) methods have advantages including high-speed production, having reactions in environmental conditions, low cost and eco-friendliness [3, 4]. In these methods, compounds with a variety of structures and different physico-chemical properties are synthesized through controlling synthesis conditions [5]. The use of such effective methods, compared to other conventional methods, for the synthesis of samples results in properties of products to be partly improved. However, in some reports, the MOF-samples have low specific surface area (SSA) and bulk size particles [6, 7]. Due to the benefits of ultrasonic and Mw methods, if the both of methods are used together as a novel method, in which the effects of ultrasonic and microwave parameters on properties of products are systematically studied, we expect the production of samples with more favourable properties. To the best of our knowledge, no study has been conducted on the synthesis of MOFs using an ultrasonic assisted microwave (UAMw) method.
As mentioned earlier, the control of the impact of the synthesis parameters on different properties of MOF-samples is highly important. In previous studies, the effect of several synthesis parameters on properties of products has been examined using conventional designs, in which a number of parameters are assumed constant and one factor is variable. This process not only increases the number of experiments, but also ignores the interaction between parameters [8]. Therefore, the systematic control of products’ properties is impossible using conventional designs. In our previous report, the 2K−1 factorial design was used to control products’ properties [9]. Nevertheless, since a large number of experiments should be selected in this method, it is highly important to choose an ideal design that makes the systematic study of products possible and reduces the number of experiments. The fractional factorial design is one of the systematic ways, through which the number of experiments is reduced and synthesis parameters are scientifically investigated [10]. According to the literatures, no reports have been found on the use of this systematic method for controlling the properties of MOFs.
Many samples of MOFs have been synthesized so far [11, 12]; however, it is important to select and introduce a new structure, which can draw the attention of researchers for further studies. In our previous study, due to the numerous uses of tantalum nanostructures in industries, electronics and ceramics [13], we synthesized Ta–MOF nanostructure for the first time. In the present study, we decided to synthesize and introduce C21H14N3O13Ta nanostructure as a new Ta–MOF for manufacturing products with distinctive features using two synthesis methods of Mw and UAMw. To this end, thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), derivative thermogravimetric (DTG) curve, X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared (FT-IR) spectroscopy, CHNS/O elemental analyser, energy dispersive spectrometer (EDS) elemental mapping images, and Brunauer–Emmett–Teller (BET) surface area techniques were used to characterize final products. The Ta–MOF samples were designed using the fractional factorial. For the systematic study of the effect of synthesis parameters on different properties of products, we used analysis of variance and Pareto chart. Finally, response surface methodology (RSM) predicted the possibility of manufacturing products with ideal properties under controlled conditions in the UAMw method.
2 Experimental section
2.1 Materials and instrumentation
Reagent-grade chemicals of tantalum(V) chloride (Mw: 358.21 g/mol, 99.8%), and 2,6-pyridine dicarboxylic acid (Mw: 167.12 g/mol, 99.6%) were purchased from Sigma-Aldrich. All the materials were commercially available and used as received without further purification. TGA (DuPont, TA Q50 analyser), DSC (QMS403C, Netzsch) and DTG (Shimadzu, DTG-60H) were used from room temperature to 800 °C with a heating rate of 5 °C/min under an argon atmosphere. XRD were carried out on Scintag X1 diffractometer with monochromatized CuKα irradiation (λ = 0.1540 nm) in 2θ angles ranging from 10 to 90 °C to collect the crystallographic information. The microscopic features of the Ta–MOF samples were examined using SEM (Nova NanoSEM230). For FT-IR spectroscopy, Thermo Scientific Nicolet-6700 spectrometer was used. CHN/S and EDS elemental mapping images were used to carry out the elemental analysis. BET surface areas of the Ta–MOF samples were determined using a Micromeritics, TriStar II 3020 analyser at 77 K.
2.2 Synthesis of Ta–MOF samples
2.2.1 Microwave (Mw) method
In a typical Mw synthesis, 0.0716 g of TaCl5 (0.2 mmol) and 0.1003 g of pyridine-2,6-dicarboxylic acid (0.6 mmol) were dissolved in 30 mL double-distilled water, and the mixture was stirred approximately 20 min at 85 °C. Then, the compound was put into the Mw reactor and placed under the optimal irradiation with the microwave power of 700 W for 15 min at the ambient temperature.
2.2.2 Ultrasonic assisted microwave (UAMw) method
The synthesis of Ta–MOF nanomaterials using the UAMw method was performed as follows: the mixture, which was obtained in the previous section by microwave irradiation (Sect. 2.2.1), was entered into the ultrasonic bath with the frequency of 20 Hz, undergoing optimal ultrasonic irradiation with the power of 220 W for 10 min at 35 °C. After cooling to room temperature, the products were isolated by washing with distilled water. A simple schematic representation of the synthesis of Ta–MOF is shown in Fig. 1.
3 Results and discussion
3.1 Thermodynamic behaviour
Thermodynamic behaviour of the Ta–MOF samples synthesized using the Mw and UAMw methods is shown in Fig. 2. Based on the results obtained from TGA, the thermal behaviour of the samples in both the methods would follow a certain pattern and the Ta–MOF samples would suffer from weight loss in four steps [14]. The first step can be attributed to the evaporation of adsorbed water. In the second step, the trapped water in the structure is decomposed. After this step, the pure product of Ta–MOF is obtained. In the third step, in both the samples, there is significant weight loss, and the structure of the MOFs is degraded. In the final step, the residual components of the samples are disintegrated. The results show that the use of the UAMw method would produce samples with higher thermal stability (240 °C in the UAMw method compared to 185 °C in the Mw method). Since the pure product obtained contains 87% of the initial sample weight in the UAMw method, while it contains 78% of the initial sample weight in the Mw method, it is concluded that the UAMw method, compared to the Mw method, would produce higher-yielding samples. DSC and DTG analyses approve the results of TGA, representing that the thermodynamic behaviour of the Ta–MOF samples is in four distinct steps and each step requires particular energy [9].
3.2 Crystallinity
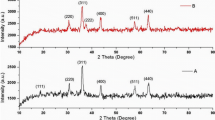
Figure 3 shows the XRD patterns of the Ta–MOF samples with high crystallinity, which are synthesized using the Mw and UAMw methods. Although based on Debye–Scherrer equation, the crystal size of both the samples is in the nanometric range [15], the sample synthesized by the UAMw method has smaller crystal size for having wider peaks (22 nm in the UAMw method compared to 56 nm in the Mw method). Since the samples are synthesized in two different conditions, it is possible that the synthesis parameters would have a great effect on the size of crystals. In both the Mw and UAMw methods, the corresponding XRD patterns were indexed to the monoclinic crystal system of the pure complex of C21H14N3O13Ta with the space group P21/n and unit cell parameters of a = 12.986 Å, b = 8.9104 Å, c = 24.942 Å, and α = 90.049.
3.3 Morphology and size distribution
Figure 4 demonstrates the SEM images of the Ta–MOF samples synthesized under optimal conditions of the Mw and UAMw methods. Based on the results, the morphology of the sample synthesized by the Mw method is rod-shaped with the average diameter of 76 nm; however, using the UAMw method would produce nanoparticles with flower-like morphology. The sample synthesized by the UAMw method has more uniform morphology than that synthesized by the Mw method; accordingly, no evidence of agglomeration and aggregation of particles can be found while using the UAMw method. However, in the Mw method, the particles are partially aggregated, which can increase the diameter of the Ta–MOF sample.
3.4 Chemical composition
Figure 5 demonstrates the FT-IR spectra of the Ta–MOF samples that have been synthesized by the Mw and UAMw method under optimal condition. In the both spectra, the absorption peak near 3198.44 cm−1 is related to the coordinated water in the structure. The frequency observed at 2847.12 cm−1 confirms the presence of the stretching vibrations of aromatic CH. The absorption peaks near 1605.33 and 1564.47 cm−1 can be assigned to the –COO– groups, and the frequency absorption near 1014.94 cm−1 is ascribed to the C–N bond. The absorption band at 874.06 cm−1 is due to the C–H bending vibrations, and the frequencies around 782.29 and 702.57 cm−1 are assigned to the Ta–O bond.
According to the results of the FT-IR spectra and with regard to various configurations of ligand, the compound I was predicted as the independent unit and the compounds II and III were predicted as the expanded units (Fig. 6). In order to determine the structural stability of the compounds, the density functional theory (DFT/B3LYP) method coupled with 6-31G and 6-311+G functionals were utilized [16]. Since ligands are the base of expansion of structures (Fig. 7), the stability of ligands was examined to determine which of these compounds were stable. The structural information of these ligands, which was obtained using the theoretical methods, is reported in Tables 1 and 2. According to the results, in both the B3LYP/6-31G and B3LYP/6-311G methods, the ligand A had less energy than the ligand B. Since the compounds I, II and III were respectively made of the ligand A, ligands A and B, and ligand B, it can be concluded that the compound III is more stable because it is made of a ligand with less energy. Moreover, since the ligand attacks the metal with its head (OH) and forms a complex, the probability of complex stability is higher in the structures, in which the charge density on oxygen is low. Table 2 shows that the charge density on oxygen atoms is low in the compound III, which implies the stability of this structure. The results of the theoretical methods approve the formation of the compound III as a piece of evidence for the expansion of the primary units. Therefore, this issue strengthens the formation of a MOF with a polymeric structure. The proposed mechanism as well as the final structure of the formation of Ta–MOF is presented in Fig. 8.
The amounts of carbon, hydrogen, nitrogen and oxygen, which were obtained by the experimental methods using CHNS elemental analysis, as well as the results of theoretical computations are shown in Fig. 9. As can be observed, the results of elemental analysis in both the experimental methods of Mw and UAMw are consistent with theoretical calculations. These results confirm the final structure related to the proposed mechanism of the Ta–MOF (C21H14N3O13Ta) shown in Fig. 8. In order to understand the origin of the structure morphology, EDS elemental mapping images were applied on the Ta–MOF sample synthesized by the UAMw method. According to Fig. 10, the flower-like morphology of this nanostructure was due to the presence of Ta, O and C elements; besides, the CHNS elemental analysis confirmed the presence of these elements in the structure.
3.5 Textural properties
The nitrogen adsorption/desorption isotherms of the products synthesized by the methods of Mw and UAMw are shown in Fig. 11a. According to the IUPAC classification of adsorption/desorption isotherms [17, 18], although the Ta–MOF samples produced by both methods have a mesoporous nature, based on the results of BET surface area technique, the sample synthesized by UAMw method has a higher SSA (2012 m2/g in the UAMw method than 1784 m2/g in the Mw method). Figure 11b also shows the pore size distribution of the samples based on the BJH method in both the experimental MW and UAMW methods; as can be seen, the mean pore size distribution of the Ta–MOF samples is 2.6 nm in the UAMw method and 2.1 nm in the Mw method.
3.6 Experimental design
Several characterization techniques indicated that although the Ta–MOF samples synthesized by the Mw and UAMw methods had desirable properties, the use of the UAMw method yielded products with higher efficiency and more desirable properties such as higher thermal stability, higher surface area and more uniform morphology. Therefore, this route was selected as the ideal method and employed in the design of the experiment. Since conventional experimental designs result in increased number of experiments and also, do not taken into account the interaction between factors, in case of using these conventional methods, it would not be possible to systematically investigate the effect of synthesis parameters on different properties of products [19]. Accordingly, the scientific experimental design known as fractional factorial design was used in the present study. The fractional factorial range is very critical problem to seek an optimization. At first, we used the experimental condition reported in previous similar works [9, 20]. At last, using XRD, TG/DSC, FTIR and SEM we could find that how theses selected parameters could influence drastically on the final structure and properties of material synthesized by this method. In this scientific design, the synthesis parameters of the UAMw method, including Mw power (A), Mw duration (B), ultrasonic temperature (C), ultrasonic power (D) and ultrasonic duration (E) were selected. Then, effects of these parameters on the properties of the Ta–MOF sample such as their particle size distribution (PSD) and SSA were studied. Coded levels of each parameter were considered equal to + 1, 0 and − 1 (Table 3). Then, several experiments were designed using this method. The design matrix and response data obtained from two replicates of the experiments are shown in Table 4 (18 runs were made in a random order). In contrast to other conventional methods, in which parameters in each experiment are considered constant and only one parameter is considered variable in order to investigate effect of that variable parameter, the parameters in the fractional factorial design were assumed variable [21, 22].
Figure 12 shows the SEM image of the Ta–MOF samples synthesized in different conditions of the synthesis parameters of the UAMw method, which had been designed via fractional factorial. Based on the data obtained from these images, depending on the synthesis conditions, the Ta–MOF samples had various morphologies. Besides, their mean PSD was different so that the sample d was selected as the optimal sample due to having a smaller PSD than the other samples. Figure 13 shows the N2 adsorption/desorption isotherms of the Ta–MOF samples synthesized in different conditions of the UAMw method, including microwave parameters (power and duration) and ultrasonic parameters (temperature, power and duration). As can be observed in this Figure, the samples exhibited various adsorption/desorption behaviours; furthermore, based on the data obtained from the BET technique, these samples (a–h) had different SSAs in different synthesis conditions. A comparison between different synthesis conditions indicated that the condition d, in which the sample had maximum SSA, could be considered as the optimal condition. The obtained results indicated that properties of the Ta–MOF samples obtained in the present study were significantly improved compared to those of other MOF samples that had been previously synthesized through different methods and under different synthesis conditions [23,24,25,26]. Moreover, the thermal stability (240 vs. 218 °C), mean PSD (23 vs. 73 nm) and SSA (2012 vs. 1940 m2/g) of the Ta–MOF sample, which were synthesized by the UAMw method in optimal conditions in the present study, were improved compared to those of the previous sample synthesized using ultrasonic method [9]. Such improvement could be attributed to the type of the synthesized compound, application of the optimal synthesis conditions and use of a novel synthesis method since the UAMw method was employed in the present study.
3.7 Ultrasound assisted microwave (UAMw) effects
To ensure that the design of the experiments is scientific and probability distribution is normal, the residual plots for the results of PSD and SSA of the Ta–MOF samples synthesized in different UAMw conditions are shown in Figs. 14 and 15. It is clear that, in all types of residual plots for every property, the number of positive and negative levels is equal, which is compelling evidence for the scientific design and normal distribution of the experiments [27, 28].
Analysis of variance (ANOVA) was employed to study the effect of UAMw parameters on the PSD and SSA of the Ta–MOF samples. To interpret the results of ANOVA, αvalue and pvalue belonging to each factor were compared together [29, 30]. The αvalue (standard significance level) was set to 0.05 by default. The Pvalue (Pvalue is a number between 0 and 1, and used to weigh the strength of the data validity) varies according to the magnitude of the effect of synthesis parameters on sample properties [31]. The effect of a parameter on the properties in question is revealed if Pvalue is smaller than αvalue for that parameter. Based on the ANOVA results in Table 5, the parameters of Mw power (A), Mw duration (B), ultrasonic temperature (C), ultrasonic power (D) and ultrasonic duration (E) have a small Pvalue (equal or close to 0.0). Thus, all these parameters have significant effects on the PSD and SSA of the Ta–MOF samples. Moreover, small Pvalue in AC and BD inactions confirms that the interaction between ultrasonic and Mw parameters affects the properties of the Ta–MOF products. The magnitude of the effect of synthesis parameters and the interaction among them is illustrated using Pareto charts in Fig. 16.
In the present study, the Ta–MOF samples had various PSDs, morphologies and SSAs depending on the synthesis conditions. The results of ANOVA and Pareto charts showed that the parameters of Mw power, microwave duration, ultrasonic temperature, ultrasonic power and ultrasonic duration had significant effects on these properties. Regarding the systematic studies, the sample d was selected as the optimal sample, and values of the parameters of the UAMw method, which led to the synthesis of this sample with desirable properties, were selected as the optimal value. These synthesis parameters, which are a combination of parameters of the microwave and ultrasonic methods, yielded the Ta–MOF nanostructure (the sample d) with uniform morphology, flower-like structure and average PSD of 23 nm (Fig. 12d). According to the IUPAC classification, the sample d had behaviour similar to that of the fifth type of the N2 adsorption/desorption isotherms series, indicating the meso-size distribution of the pores (Fig. 13d). Furthermore, results of the BET technique showed that the surface area of this sample was higher than that of other samples.
Figure 17 shows the FT-IR spectra of the Ta–MOF samples synthesized under different conditions of the UAMw method (a–h). Accordingly, at the conditions g and h, no bonds related to the formation of Ta–O were observed; thus, it can be concluded that in the samples g and h, the Ta–MOF bonds were not formed properly; however, in the other samples, all the bonds related to the formation of Ta–MOF can be observed. Since at the conditions g and h, some of the synthesis parameters had higher intensity than the optimal parameters, such high intensity could cause destruction of the bonds related to the formation of MOF, which would result in the synthesis of the samples with bulk-range PSD (Fig. 12g, h). Moreover, the interaction between the ultrasonic and microwave parameters, the effect of which was confirmed by ANOVA, would increase the intensity of such destruction. Thus, at the condition g, in which all the microwave and ultrasonic synthesis parameters were at their highest intensity compared to the optimal condition, the sample’s adsorption behaviour (Fig. 13g) was similar to that of the third type of the adsorption isotherms (the behaviour of the nonporous systems) [17, 32]. However, at the condition h, in which the parameters of microwave power, ultrasonic temperature and ultrasonic power were at their highest levels and the other synthesis parameters were in their optimal condition, the N2 adsorption behaviour of the sample h was similar to that of the second type of the isotherms, indicating the irregular interaction between the adsorbent and adsorbate (Fig. 13h) [33]. In the samples e and a, based on the FT-IR, although all the bonds related to the formation of Ta–MOF were formed properly, selection of the synthesis parameters at their lowest level at the condition e compared to the optimal conditions led to the agglomeration of the particles as well as their bulk-range size distribution (Fig. 12e). Furthermore, the N2 adsorption behaviour of the sample e was due to the nonporous system so that the SSA of this sample was considerably reduced (Fig. 13e). However, at the condition a, since the parameters of microwave duration, ultrasonic power and ultrasonic duration were selected at their lowest levels compared to the optimal conditions and only the parameter of microwave power had an optimal value, morphology of the sample a was in a microstructure rod-shaped form (Fig. 12a). Moreover, its adsorption/desorption isotherm was similar to that of the first type of the isotherms, indicating the microporous nature of the pores (Fig. 13a) [20]. In the samples c, f and b, depending on the extent to which the synthesis parameters were close to the optimal parameters’ values, the morphology, N2 adsorption/desorption behaviour and PSD of the samples were different. Table 4 represents the levels of the synthesis parameters as well as the obtained properties of the samples. Figure 18 shows the XRD patterns of the Ta–MOF samples synthesized in different conditions of the UAMw method. The sample d, which was synthesized under optimal conditions, had the peaks related to the formation of Ta–MOF. Presence of the broad peaks indicated the small size of the crystals in this sample. In the samples g and h, due to the high intensity of the synthesis parameters and destruction of the bonds, some of the XRD patterns of these samples were not formed compared to the optimal sample. In the samples e, a, c, b and f, although the XRD patterns of Ta–MOF were formed properly, their crystal sizes differed depending on the synthesis conditions.
3.8 Optimization parameters
Based on the experimental designs performed by fractional factorial and also the ANOVA results [34, 35], the regression equations obtained for each property of the Ta–MOF samples were as PSD = 269 + 11.1 A − 36.7 B + 33.8 C + 96.4 D − 198 E and SSA = 1239 + 206 A − 134 B − 155 C + 65 D + 359 E [34]. According to these equations, which related the synthesis parameters to properties of products, the surface plots are designed (Figs. 19, 20). Based on these plots, various values of PSA and SSA of the Ta–MOF samples are theoretically estimated by selecting different values for Mw power (A), Mw duration (B), ultrasonic temperature (C), ultrasonic power (D) and ultrasonic duration (E). Different synthesis parameters and properties of products (which were experimentally obtained) are reported in Table 4. By generalizing the theoretical equations to the experimental results in Table 4, it is concluded that the experimental results are in good agreement with the theoretical data obtained from the regression models under similar conditions. One of the objectives of this study was to synthesis Ta–MOF samples with ideal properties. Thus, based on the design of the experiments and ANOVA results, RSM was used to achieve the best experimental conditions in order to synthesis products with unique properties (Fig. 21) [36, 37]. Different conditions of UAMw parameters for the production of the Ta–MOF samples with small PSD and high SSA (predicted by RSM) are presented in Table 6.
4 Conclusion
In this study, UAMw was used as a novel method for the facile synthesis of a new Ta–MOF nanomaterial. Since the compounds that were manufactured in this route had ideal properties, UAMw can be used as a new synthesis protocol for the production of a variety of nanostructures. The results showed that synthesis parameters play a major role in the production of Ta–MOF nanostructure with various structures and different physico-chemical properties including PSD of 15 nm and high SSA of 2588 m2/g. For the systematic study of the parameters on various properties of the Ta–MOF samples as well as optimization of the productions with different properties, the fractional factorial design was used.
References
C. McKinstry, R.J. Cathcart, E.J. Cussen, A.J. Fletcher, S.V. Patwardhan, J. Sefcik, Scalable continuous solvothermal synthesis of metal organic framework (MOF-5) crystals. Chem. Eng. J. 285, 718–725 (2016)
G. Sargazi, D. Afzali, A. Ghafainazari, H. Saravani, Rapid synthesis of cobalt metal organic framework. J. Inorg. Organomet. Polym. Mater. 24, 786–790 (2014)
Y. Jiang, X. Zhang, X. Dai, W. Zhang, Q. Sheng, H. Zhuo, Y. Xiao, H. Wang, Microwave-assisted synthesis of ultrafine Au nanoparticles immobilized on MOF-199 in high loading as efficient catalysts for a three-component coupling reaction. Nano Res. 10, 876–889 (2017)
A. Nikseresht, A. Daniyali, M. Ali-Mohammadi, A. Afzalinia, A. Mirzaie, Ultrasound-assisted biodiesel production by a novel composite of Fe (III)-based MOF and phosphotangestic acid as efficient and reusable catalyst. Ultrason. Sonochem. 37, 203–207 (2017)
N.A. Khan, S.H. Jhung, Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 285, 11–23 (2015)
M.R. Armstrong, S. Senthilnathan, C.J. Balzer, B. Shan, L. Chen, B. Mu, Particle size studies to reveal crystallization mechanisms of the metal organic framework HKUST-1 during sonochemical synthesis. Ultrason. Sonochem. 34, 365–370 (2017)
P. George, N.R. Dhabarde, P. Chowdhury, Rapid synthesis of titanium based metal organic framework (MIL-125) via microwave route and its performance evaluation in photocatalysis. Mater. Lett. 186, 151–154 (2017)
S. Kitagawa, Metal–organic frameworks (MOFs). Chem. Soc. Rev. 43, 5415–5418 (2014)
G. Sargazi, D. Afzali, A. Mostafavi, S.Y. Ebrahimipour, Ultrasound-assisted facile synthesis of a new tantalum (V) metal-organic framework nanostructure: design, characterization, systematic study, and CO 2 adsorption performance. J. Solid State Chem. 250, 32–48 (2017)
N.D. Burrows, S. Harvey, F.A. Idesis, C.J. Murphy, Understanding the seed-mediated growth of gold nanorods through a fractional factorial design of experiments. Langmuir 33, 1891–1907 (2017)
G. Maurin, C. Serre, A. Cooper, G. Férey, The new age of MOFs and of their porous-related solids Chem. Soc. Rev. 46, 3104–3107 (2017)
K.K. Gangu, S. Maddila, S.B. Mukkamala, S.B. Jonnalagadda, A review on contemporary metal–organic framework materials. Inorg. Chim. Acta 446, 61–74 (2016)
L. Xu, H. Gong, L. Deng, F. Long, Y. Gu, J. Guan, Complex-mediated synthesis of tantalum oxyfluoride hierarchical nanostructures for highly efficient photocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 8, 9395–9404 (2016)
G. Sargazi, D. Afzali, N. Daldosso, H. Kazemian, N. Chauhan, Z. Sadeghian, T. Tajerian, A. Ghafarinazari, M. Mozafari, A systematic study on the use of ultrasound energy for the synthesis of nickel–metal organic framework compounds. Ultrason. Sonochem. 27, 395–402 (2015)
H. Fazelirad, M. Ranjbar, M.A. Taher, G. Sargazi, Preparation of magnetic multi-walled carbon nanotubes for an efficient adsorption and spectrophotometric determination of amoxicillin. J. Ind. Eng. Chem. 21, 889–892 (2015)
I. Obot, D. Macdonald, Z. Gasem, Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: an overview. Corros. Sci. 99, 1–30 (2015)
X. Liu, J. Xiong, L. Liang, Investigation of pore structure and fractal characteristics of organic-rich Yanchang formation shale in central China by nitrogen adsorption/desorption analysis. J. Nat. Gas Sci. Eng. 22, 62–72 (2015)
N. Santhosh, G. Radhakrishnamacharya, A.J. Chamkha, Flow of a Jeffrey fluid through a porous medium in narrow tubes. J. Porous Media 18, 71–78 (2015)
L. Esrafili, A.A. Tehrani, A. Morsali, Ultrasonic assisted synthesis of two urea functionalized metal organic frameworks for phenol sensing: a comparative study. Ultrason. Sonochem. 39, 307–312 (2017)
G. Sargazi, D. Afzali, A. Mostafavi, A novel synthesis of a new thorium (IV) metal organic framework nanostructure with well controllable procedure through ultrasound assisted reverse micelle method. Ultrason. Sonochem. 41, 234–251 (2017)
M.-H. Pham, G.-T. Vuong, F.-G. Fontaine, T.-O. Do, Rational Synthesis of metal–organic framework nanocubes and nanosheets using selective modulators and their morphology-dependent gas-sorption properties. Cryst. Growth Des. 12, 3091–3095 (2012)
J.F.S. do Nascimento, B.S. Barros, J. Kulesza, J.B.L. de Oliveira, A.K.P. Leite, R.S. de Oliveira, Influence of synthesis time on the microstructure and photophysical properties of Gd-MOFs doped with Eu+. Mater. Chem. Phys. 190, 166–174 (2017)
D. Chen, C. Chen, W. Shen, H. Quan, S. Chen, S. Xie, X. Luo, L. Guo, MOF-derived magnetic porous carbon-based sorbent: synthesis, characterization, and adsorption behavior of organic micropollutants. Adv. Powder Technol. 28, 1769–1779 (2017)
W.P. Deleu, I. Stassen, D. Jonckheere, R. Ameloot, D.E. De Vos, Waste PET (bottles) as a resource or substrate for MOF synthesis. J. Mater. Chem A 4, 9519–9525 (2016)
J.-C. Yin, T.-Z. Qin, C. Hu, G.-M. He, B.-W. Zhao, C. Zhang, J. Wang, A copper(II)–gadolinium(III) heterometallic MOF: synthesis, structure, and electrochemical property. Mater. Lett. 197, 221–223 (2017)
F. Jeremias, S.K. Henninger, C. Janiak, Ambient pressure synthesis of MIL-100 (Fe) MOF from homogeneous solution using a redox pathway. Dalton Trans. 45, 8637–8644 (2016)
G. Sargazi, D. Afzali, A. Mostafavi, S.Y. Ebrahimipour, Synthesis of CS/PVA biodegradable composite nanofibers as a microporous material with well controllable procedure through electrospinning. J. Polym. Environ. (2017). https://doi.org/10.1007/s10924-017-1080-8
V.K. Vankanti, V. Ganta, Optimization of process parameters in drilling of GFRP composite using Taguchi method. J. Mater. Res. Technol. 3, 35–41 (2014)
Y. Hu, H. Yu, K. Dong, S. Yang, X. Ye, S. Chen, Analysis of the tenderisation of jumbo squid (Dosidicus gigas) meat by ultrasonic treatment using response surface methodology. Food Chem. 160, 219–225 (2014)
C. Camposeco-Negrete, Optimization of cutting parameters for minimizing energy consumption in turning of AISI 6061 T6 using Taguchi methodology and ANOVA. J. Clean. Prod. 53, 195–203 (2013)
W. Wang, Q. Li, Y. Liu, B. Chen, Ionic liquid-aqueous solution ultrasonic-assisted extraction of three kinds of alkaloids from Phellodendron amurense Rupr and optimize conditions use response surface. Ultrason. Sonochem. 24, 13–18 (2015)
W. Li, G. Li, Q. Lv, W. Xu, L. Liu, Chromate adsorption on amine-functionalized core/shell magnetic mesoporous material. J. Porous Mater. 22, 965–972 (2015)
Z.-M. Cui, J. Hao, C.-Y. Cao, W. Song, Mesoporous silica (meso-SiO2) was coated on the micro/nanomaterials with hierarchical structure such as flowerlike Fe2O3, flowerlike MgO, SnO2 nanospheres, Co3O4 nanosheets and nanowires from a simple solution method. The structure of the nanomaterials and the silica coating before and after stirring in solution were characterizated by SEM and TEM. The results show that meso-SiO2 coating was an ideal. J. Porous Mater. 24, 103–108 (2017)
M. Ghaedi, M. Rahimi, A. Ghaedi, I. Tyagi, S. Agarwal, V.K. Gupta, Application of least squares support vector regression and linear multiple regression for modeling removal of methyl orange onto tin oxide nanoparticles loaded on activated carbon and activated carbon prepared from Pistacia atlantica wood. J. Colloid Interface Sci. 461, 425–434 (2016)
D. Liu, C. Huang, J. Wang, H. Zhu, P. Yao, Z. Liu, Modeling and optimization of operating parameters for abrasive waterjet turning alumina ceramics using response surface methodology combined with Box–Behnken design. Ceram. Int. 40, 7899–7908 (2014)
V.K. Gupta, S. Agarwal, M. Asif, A. Fakhri, N. Sadeghi, Application of response surface methodology to optimize the adsorption performance of a magnetic graphene oxide nanocomposite adsorbent for removal of methadone from the environment. J. Colloid Interface Sci. 497, 193–200 (2017)
E. Bet-Moushoul, Y. Mansourpanah, K. Farhadi, M. Tabatabaei, TiO2 nanocomposite based polymeric membranes: a review on performance improvement for various applications in chemical engineering processes. Chem. Eng. J. 283, 29–46 (2016)
Acknowledgements
The authors would like to acknowledge financial support for this work from the Shahid Bahonar University of Kerman (Iran).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sargazi, G., Afzali, D. & Mostafavi, A. An efficient and controllable ultrasonic-assisted microwave route for flower-like Ta(V)–MOF nanostructures: preparation, fractional factorial design, DFT calculations, and high-performance N2 adsorption. J Porous Mater 25, 1723–1741 (2018). https://doi.org/10.1007/s10934-018-0586-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-018-0586-3