Abstract
Easy and convenient synthesis of crystalline cobalt-nanoparticles by a seed mediated growth method with cetyltrimethylammonium bromide used as surfactant, KI as an additive and ascorbic acid as reductant; this synthesized Co-nanoparticle are characterized by XRD and TEM analysis, and is used as a coupling catalyst for alkenes with aryl halides in the presence of a base. The yield of the catalyzed product and the method has many advantages such as affordable and ligand-free condition. Hence overall reaction is augmented to the Heck coupling reaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanoparticles and catalysis are the most thought-provoking combination for the construction of organic molecules. The scope and limitations of organo-metallic catalyst have been well investigated yet. A number of questions become spasmodic, like morphology of the catalyst, controlling of the size and shape of the catalyst and available active surface of the catalyst in solution. Concerning this question, recently nano-catalyst approach was developed [1]. The metal-nanoparticles in the ultra-fine state have been prepared using various methods [2–9] such as chemical reduction, chemical liquid deposition, thermal decomposition, photochemical reduction, radiolysis etc. However in many cases, reproducibility and size of the synthesised particles is not satisfactory; because as many workers suggested there are uncertainty of formation of nucleation centres in the reaction medium. Since seed mediated growth method is a very advantageous way to push back this thermodynamic limit. This seed mediated growth method was initially developed by Murphy et al. [10, 11] and Nikoobakht and El-Sayed [12] In recent years, many researchers reported that in order to obtain a specific size, shape and aspect ratio (AR), by using different modified seed-mediated growth processes have been adopted for the synthesis of many gold nanoparticles, some palladium nanoparticles and bimetallic core shell nanoparticles have been reported by various researchers [13–15]. The detailed literature survey showed many macro size cobalt catalysed Heck reactions are reported but a very limited number of researchers reported nano sized cobalt catalyst and also many of them in-situ generation of nanocobalt which catalyse Heck reaction and need not particularly controlling the size, shape and aseptic ratio.
The Heck reaction is a well-established method for organic synthesis [16–28]. Applications of this reaction used in many area, including the synthesis of natural products having high-performance organic molecules [29–31] and in fine chemicals, syntheses [32–34] aryl halides usually reacted with alkenes in the presence of palladium and a adequate base. Generally, phosphine palladium complexes are the methodical catalysts for the Heck reaction, in which phosphine plays a vital role of stabilizer and prevents the formation of inactive palladium sites [35, 36], subsequently the major drawback with these phosphine–palladium catalysts are that the most of the phosphine derived ligands are highly expensive, toxic and unstable in air and moisture-sensitive. To overcome this problem enormous effort had been made to synthesize a new non-phosphine based catalyst like palladacycles [37, 38] N-heterocyclic carbine palladium catalysts [39–43], macrocyclic recoverable triolefin complex [44]. Along with this, a large number of heterogeneous palladium nanocatalyst supported with silica [45], zeolites [46], carbon nanotubes [47], graphite oxide, graphene [48] and polymers [16], are used as a heterogeneous catalyst wherein effective recovery of the catalyst was reported [24]. In these, the progress of Heck reaction is veering on the basis of halogen atom, i.e., chlorine is less reactive when compared with bromine and iodine, in some cases chlorine gives a good result under a palladium catalyst when bulky phosphine ligands or carbenes are used [49–51] (Fig. 1).
The literature survey showed that a few cobalt (II) catalyzed Heck reaction protocol are also used bulky phosphine ligands [52, 53] or an electrochemical method [54, 55], a five Co hallow nano spheres and bimetallic PdCo hollow nano spheres are used as a catalyst for Heck reaction and Sonogashira reaction [56, 57]. Furthermore, silica supported cobalt catalyzed Heck reaction is reported, in which recovery of catalyst are excellent [58]. In this context in our previous work we reported ligand stabilized heterogeneous palladium nanocomposite for catalysing Heck reaction [59]. In continuation of the previous work herein we report the synthesis of low cost, ligand free cobalt nanoparticles by seed mediated growth method, in which controlling of the size, shape and morphology are very good and availability of active surface in a solution medium is increased because of the smaller the clusters of atoms, higher, the percentage of atoms on the surface. This synthesized cobalt nanoparticles are very cheap when compared to the palladium and also well screening of their catalytic activity with respect to Heck reaction. In this paper detailed account of the work leading to optimization of heck reaction with respect to different organic solvents, base, temperature and Co-NPs loading have also been presented.
2 Materials and methods
2.1 General experiments
Cobalt chloride hexahydrate (CoCl2·6H2O), cetyltrimethylammonium bromide (CTAB), ascorbic acid, potassium iodide, doubly distilled water was used throughout the experiments. allylacetates, arylhalides, HPLC grade chloroform, and dichloromethane were procured from Sigma-Aldrich (INDIA), Himedia (INDIA), Lobo Chemicals (INDIA) (Commercially available from local sources) were used as received without further purification. Freshly distilled solvents were employed for all synthetic purposes. Spectroscopic grade solvents were employed for spectral works. All other chemicals were of AR grade. The progress of every coupling reaction was monitored by thin layer chromatography (TLC). Yield refers to isolated yield after column chromatographic purification of compounds that have a purity of ≥95%. The product of Heck reactions were authenticated by matching spectroscopic data of the newly synthesised products with those reported in the literature.
2.1.1 Instrumentation
1H and 13C NMR spectra are recorded on a JNM-ECS-400 NMR spectrometer at 399.78 and 75.03 MHz, respectively with chemical shifts reported in ppm relative to the residual deuterated solvent or the internal standard tetramethylsilane. Transition electron microscopy (TEM) and selected area electron diffraction (SAED) studies were performed on a Jeol/JEM 2100 operated at 200 KV. X-ray diffraction (XRD) samples were prepared by evaporating a drop of nanocrystal solution onto a glass substrate; XRD data were collected using Bruker D8 ADVANCE X-ray diffractometer (CuK radiation) operated at 40 kV and 40 mA over a range of 30–90° by step scanning with a step size of 0.048° at room temperature. Melting point as determined in an electrically heated apparatus by taking the sample in a glass capillary sealed at one end.
2.2 Synthesis of cobalt nanoparticles
A 10 mM H2CoCl4 solution was prepared by dissolving 0.1773 g of CoCl2 in 10 mL of 0.2 M HCl solution and further diluting to 100 mL with doubly distilled water.
2.2.1 Synthesis of small cobalt nano seeds
In a typical synthesis, a 1 mL aliquot of 10 mM H2CoCl4 solution was added to 20 mL of 12.5 mM CTAB solution heated at 95 °C under stirring. After 5 min, 160 μL of freshly prepared 100 mM ascorbic acid solution was added, and the reaction was allowed to proceed for 20 min. The nano seed solution was stored at 30 °C for future use as seeds.
2.2.2 Seed mediate growth of cobalt nano crystals
In a typical synthesis, a 25 μL of KI solutions were added to 5 mL of 100 mM CTAB solution kept at 80 °C temperature. To the above solution a 125 μL portion of 10 mM H2CoCl4 solution and 40 μL of the newly synthesized cobalt nano-seed solution were added. Finally, 50 μL of freshly prepared 100 mM ascorbic acid solution was added, and the solution was mixed thoroughly. The resulting solution was placed on a water bath at 80 °C for about 1 h. The reaction was stopped by centrifugation (6000 rpm, 10 min). The precipitate was re-dispersed in doubly distilled water and subsequently, two more centrifugation (6000 rpm, 10 min) were applied to the samples for transmission electron microscope (TEM) and XRD characterization.
2.3 General procedure for Heck reaction
To the mixture of K2CO3 (138.25 mg, 1.0 mmol), cobalt nano-catalyst (6.8 mg, 0.1 mmol), TBAB (161.18 mg, 0.5 mmol) and NMP (5 mL) was added arylhalide 3a (78 mg, 0.5 mmol) and allylacetate 4a (100 mg, 1.0 mmol, 2.0 eq.) subsequently. The reaction mixture was vigorously stirred under air or N2 atmosphere at 120 °C for an appropriate time (see Table 1) the progress of the reaction was monitored by TLC, after cooling to room temperature the excess of solvent removed and concentrating in vacuum, the remaining solution was centrifuged filtered and the precipitate was washed three time with dichloromethane (5 mL × 3 times). The three times extracted solutions were combined and washed with water for three times. The crude product was purified by chromatography on a short silica gel (eluent: petroleum ether/ethyl acetate = 20:1) to afford 80 mg (91%) of crude product 3aa. 1H NMR (CDCl3, 399.7 MHz): δ = 7.41–7.24 (m, 5 H), 6.68 (d, J = 15.6 Hz, 1 H), 6.34 (dt, J = 15.6, 6.3 Hz, 1 H), 4.74 (d, J = 6.3 Hz, 2 H), 2.10 (s, 3 H). 13C NMR (CDCl3, 75.4 MHz): δ = 170.9, 136.2, 134.2, 128.6, 128.1, 126.6, 123.1, 65.0, 20.8.
3 Result and discussion
3.1 Characterization of cobalt nanoparticles
In this study the seed-mediated growth method is used, first small cobalt nano seeds were synthesized through a one-step reduction and then employed as seeds for the seed mediated growth procedure. The cobalt nano-seeds were subsequently grown into nanocrystals in growth solutions containing cetyltrimethylammonium bromide (CTAB), dihydrogentetrachlorocobaltate(II) (H2CoCl4), potassium iodide (KI) and ascorbic acid (AA), through manipulation of the concentration of KI and the reaction temperature overall process were controlled. The seed mediated growth mechanism suggested a two-step process, i.e. nucleation and after that successive growth of the particles. In the first step, metal ions in solution is reduced by l-ascorbic acid in seed solution and act as a small metal cluster this metal cluster further act as a tiny nucleation centre in growth solution.
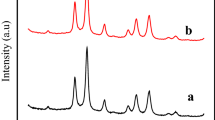
The phase structure and purity of the synthesized sample were analysed by powder XRD. The XRD results confirm a complex composition of nanoparticles is shown in Fig. 2.
Here we observed five diffraction peaks and there 2θ values at 44.2°, 51.5°, 73.8°, 92° and 97.1° can be indexed to the (111), (200), (220), (311), and (222) crystal faces is fcc Co nanoparticles subsequently, it matches with literature JCPDS standard card (#01-1259) the XRD pattern is clearly illustrated that (200) peak was slightly broadened than other diffraction peaks this is most likely due to the formation of stacking faults, leading to energetically growth along the b-axis, indicating that the synthesized nanoparticles might be in the spherical shape rather than other shapes. The XRD diffractogram confirmed the formation of cobalt nanoparticles with spherical shape fcc crystal structure, which is also consistence with the earlier reports. The particle size of Co-nanoparticles as calculated from (111) plane using Scherrer formula is found to be about 25 nm. This shows that Co is present in PVA matrix as nanoparticles.
In order to further characterize the samples, energy-dispersive spectrometry (EDX, Fig. 3), show that cobalt metal atom is associated with CTAB and also revealed that the hollow spheres of Co nanoparticle composed of the minor amount of carbon, oxygen and bromine, which came from the ascorbic acid and CTAB respectively.
The Fig. 4a, a1 show the typical TEM image of Co nanoparticle in a seed solution and Fig. 4b, b1 show the Co nanoparticle after the growth which is conformed that the cobalt nanoparticles are having irregular spherical structure, it clearly indicate that where there is a strong contrast difference in all the circles with an ample dark center surrounded by nadir dark edge, confirming their spherical architecture. The average diameter of the spherical structure is about 15–20 nm, showing relatively narrow size distribution. The enlarged image of an individual sphere is presented in Fig. 4a (inset), which all indicates that the spherical shape of the Co nanoparticle. The unchanged contrast difference between the center and edge in the TEM image of one sphere obtained when the sample grid is rotated by different degrees further proves their spherical structure. The overall result of cobalt nanoparticle showed that in seed solution, mean diameter is about 2–4 nm and after growth average size of Co nanoparticle is about 10–20 nm with highly uniform size distribution, it is clearly showed that CTAB serves as a excellent capping agent and provide good stability at nano-sized cobalt metal atom.
3.2 Catalytic activity study
In the past few years, we have devoted much of our attention to this field. Herein, we wish to report that a cobalt-based catalyst also have ability to form C–C bonds in a commercial methodical manner. The nano-cobalt catalyzed Heck-type arylation of allyl acetate with derived arylhalides in presence of a base is summarized in Table 1. In all these cases, reactions conducted under air and nitrogen atmosphere. In coupling reactions we have used, electron-donating, electron-neutral and electron-withdrawing, substituents connected at ortho, meta and para position of arylhalides (Scheme 1).
The initial goal was to optimize reaction conditions, since in previous studies of C–C and C–hetero atom coupling reactions, N-methyl-2-pyrrolidone (NMP) and potassium carbonate were found to be a preferred solvent and base respectively, we used these conditions first. In the preliminary reaction we have used nano-Co (10 mol%) as a catalyst subsequently with K2CO3 as base for the coupling of 1 equivalent bromobenzene (3aa) with 2 equivalent of allyl acetate (4aa) in NMP solvent at 120 °C under air and N2 atmosphere. After 8 h, the coupling reaction provided 90 and 92% yield respectively (entry 2, Table 1), similar reactions were conducted at same experimental condition in the absence of nano-cobalt catalyst did not yield any coupling reaction (entry 1, Table 1).
A series of solvents, such as triethylamine (Et3N), isobutyronitrile, dimethylformamide (DMF), acetonitrile (MeCN), toluene, acetone and tetrahydrofuran (THF), were used, but it was found that they were less effective than NMP (Table 2). The yield was reduced to 20% when Et3N was used as the base as well as the medium (entry 3, Table 2). In isobutyronitrile solvent, reactivity was slightly sluggish (entry 2, Table 2). The yield of 5a was suddenly reduced to 20% when Et3N was used as a base (entry 3, Table 3). It has been found that CH3COONa, NEt3, NaHCO3, KOH, and NaOH displayed less efficient, among these inorganic bases, K2CO3 gave quantitative results for the coupling reaction (entries 1, Table 3) hence NMP and K2CO3 was found to be an extremely efficient combination for heck reaction. It is noteworthy that the identical results are observed under air and nitrogen atmosphere. To finding the influence of the amount of the loaded catalyst on the product yield, the reaction performed under the same experimental condition and deferent amount of the catalyst loaded it was found that loading of 0.5 mmol/L catalyst give below 50% yield on the other hand extremely low quantity say 0.001 mmol/L produced a moderately good yield therefore an intermediate quantity say 0.01 mmol/L of the catalyst produced very high yield (entries 2, Table 4). Typically exhalent yield observed under N2 atmosphere than under air which leads to variation of 5–10% yield in most of the reactions (Table 1). With optimized reaction conditions, we have probed the scope of allyl acetate with different aryl halide employing 2% mol of nanocobalt catalyst in NMP at 120 °C under N2 atmosphere.
The scope of both ally lactates and aryl halides was explored for the arylation reaction under the standard conditions (Table 2). A variety of aryl halides (1a–e) were first examined by reacting with allyl acetate (2a) (entries 1–10, Table 2). The results indicated that both electron-rich and electron-deficient aryl iodides were suitable for the arylation reaction, but aryl bromides had lesser activity. For example, aryl bromide (1aj), p-methyl aryl bromide (1ak), p-methoxy aryl bromide (1al) reacts with allyl acetate under standard condition, furnishes the arylation product 3am, 3an, 3ao but they were slightly shifted towards lesser yield. It was interesting to perceive that the reaction between iodobenzene substituted by methyl group at para position as well as ortho position and allylacetate which give exceptional yields (entries 3 and 4), but it gives small variation in the product yield. Ortho-substituted iodobenzene gives quite lesser yield compared with para-substituted iodobenzene hence; we deduced that little steric factor comes into play. In particular, 4-iodobenzaldehyde 1ae and 4-iodobiphenyl 1af, react with allylacetate 2aa results 3ae and 3af individually quite lesser yield (entry 6, 7, Table 1) in another side p-methoxyiodobenzene 1ab give excellent yield (entries 5) with just 2 mol% of the Co nano-catalyst. The substituted aryl halide with electron-withdrawing substituents exhibits little sluggish nature than with electron-donating substituent’s, 3ag, 3ah, (entries 8, 9, Table 1; Schemes 2).
The reductive coupling with substituted allylacetate and aryl halide under similar catalytic condition was given unusually less product yield. Pent-1-en-3-ylacetate 2ab, methyl prop-2-en-1-yl carbonate 2ac and prop-2-en-1-yl benzoate 2ad react with aryl iodide 1aa offered 73, 78 and 74% yield respectively (entry 11, 12, 13). The remaining present is the formation of side product where it was confirmed by TLC but could not be isolated and characterized. The result advised that any substitution at the terminal alkene affected the product yield significantly (Schemes 1, 2, 3).
By using the same protocol, we have been able to carry out the reductive coupling with substituted aryl bromide which was complete in 6–8 h and produced moderately good yield with just 2 mol% of the nano Co catalyst (entries 14–18, Table 1). Thus, bromobenzene 1aj reacted with allylacetate forming 3am 70% yield (entry 14). Similarly, p-methyl 1ak and p-methoxy 1al substituted aryl bromide reacted with 2aa to afford the substituted aryl product 3an and 3ao relatively with moderate yield (entry 15, 16 in Table 1).The results were concluding that, the change of halogen in aryl substrate is quite effective in product yield. Excitingly, an extremely efficient reaction were observed in the case of 1aa, 1ab, 1ac and 1ai, producing their corresponding product’s 3aa, 3ab, 3ac and 3ai being obtained in high yield after 7 h, 7 h, 6 h and 6 h respectively [91, 90, 83 and 88% yield (entries 2–4, 10, Table 1)].
4 Conclusion
In this work, we prepared spherical cobalt nanoparticle by using a seed mediated growth method and this synthesised cobalt nanoparticle are successfully used as a catalyst in the Heck coupling reaction of iodo and bromo arenes with allylacetate. More importantly, the synthesised catalyst was cheaper compared to the palladium catalyst. This coupling reaction emphasizes the potential of using cobalt as a very user-friendly, inexpensive, and efficient catalyst for coupling reaction of carbon–carbon bonds. Such work is on-going in our laboratory.
References
V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara, J.M. Basset, Chem. Rev. 111, 3036 (2011)
K. Esumi, K. Tano, K. Meguro, Chem. Mater. 2, 564 (1990)
J.S. Bradley, E.W. Hill, B. Chudret, A. Duteil, Chem. Mater. 5, 254 (1993)
N.R. Jana, Z.L. Wang, T. Pal, Langmuir 16, 2457 (2000)
A. Henglein, J. Phys. Chem. B 97, 5457 (1993)
Y. Yonezawa, T. Sato, M. Ohno, H. Hada, J. Chem. Soc. Faraday Trans. 1 83, 1559 (1987)
K. Torigoe, K. Esumi, Langmuir 8, 59 (1992)
H. Hirai, Y. Nakamura, N. Toshima, J. Macromol, Sci. Part A 13, 727 (1979)
K. Torigoe, Y. Nakajima, K. Esumi, J. Phys. Chem. 97, 8304 (1993)
S.O. Obare, N.R. Jana, C.J. Murphy, Nano Lett. 1, 601 (2001)
T.K. Sau, C.J. Murphy, Langmuir 21, 2923 (2005)
B. Nikoobakht, M.A. El-Sayed, Langmuir 17, 6368 (2001)
X. Ye, L. Jin, H. Caglayan, J. Chen, G. Xing, C. Zheng, V. Doan-Nguyen, Y. Kang, N. Engheta, C.R. Kagan, C.B. Murray, ACS Nano 6, 2804 (2012)
H.Y. Wu, W.L. Huang, M.H. Huang, Cryst. Growth Des. 7, 831 (2007)
X. Ma, J. Feng, F. You, J. Ma, X. Zhou, M. C. Wang, Chin. Phys. B 23, 087807 (2014)
T. Mizoroki, K. Mori, A. Ozaki, Bull. Chem. Soc. Jpn. 44, 581 (1971)
R.F. Heck, J.P. Nolley, J. Org. Chem. 37, 2320 (1972)
A. de Meijere, F. Diederich, Metal-Catalyzed Cross-Coupling Reactions, 2nd edn, Chap. 3. (Wiley-VCH Verlag GmbH, Weinheim, 2004)
F. Diederich, P.J. Stang (eds.), Metal-Catalyzed Cross-Coupling Reactions, Chap. 3. (Wiley-VCH, Weinheim, 1998)
J.T. Link, L.E. Overman, in Metal-Catalyzed Cross-Coupling Reactions (Wiley-VCH, Weinheim, 2007), p. 230
F. Diederich, P.J. Stang, (eds.), Metal-Catalyzed Cross-Coupling Reactions, Chap. 6. (Wiley-VCH, Weinheim, 1998)
C. Amatore, E. Carre, A. Jutand, Organometallics 14, 5605 (1995)
A. de Meijere, F. Diederich, (eds.), Metal-Catalyzed Cross-Coupling Reactions, Chap. 5. (Wiley-VCH, Weinheim, 2004)
R.F. Heck Org. React. 27, 345 (1982)
I.P. Beletskaya, A.V. Cheprakov, Chem. Rev. 100, 3009 (2000)
G.T. Crips, Chem. Soc. Rev. 27, 427 (1998)
A. de Meijere, F.E. Meyer, Angew. Chem. Int. Ed. Engl. 33, 2379 (1994)
W. Cabri, I. Candiani, Acc. Chem. Res. 28, 2 (1995)
J.M. Gaudin, Tetrahedron Lett. 32, 6113 (1991)
L.E. Overman, D.J. Ricca, V.D. Tran, J. Am. Chem. Soc. 115, 2042 (1993)
L.F. Tietze, W. Buhr, Angew. Chem. Int. Ed. Engl. 34, 1366 (1995)
R.R. Bader, P. Baumeister, U. H. Blaser, Chimia (Aarau) 50, 99 (1996)
T.C. Wu, US Patent 5536870, (1996)
A. Eisenstadt, in Catalysis of Organic Reactions, ed. by F.E. Herkes (Marcel Dekker, New York, 1998)
T. Mino, Y. Shirae, Y. Sasai, M. Sakamoto, T. Fujita, J. Org. Chem. 71, 6834 (2006)
G. Zou, W. Huang, W.J. Xiao, J. Tang, New J. Chem. 30, 803 (2006)
J. Dupont, M. Pfeffer, J. Spencer, Eur. J. Inorg. Chem. 2001, 1917 (2001)
R.B. Bedford, Chem. Commun. 2003, 1787 (2003)
W.A. Herrmann, Angew. Chem. Int. Ed. 41, 1290 (2002)
R.B. Bedford, C.S.J. Cazin, D. Holder, Coord. Chem. Rev. 248, 2283 (2004)
D.J. Nielsen, K.J. Cavell, B.W. Skelton, A.H. White, Inorg. Chim. Acta 359, 1855 (2006)
C. Tubaro, A. Biffis, C. Gonzato, M. Zecca, M. Basato, J. Mol. Catal. A 248, 93 (2006)
R. Wang, B. Twamley, J.M. Shreeve, J. Org. Chem. 71, 426 (2006)
J. Masllorens, M. Moreno-Manas, A. Pla-Quintana, A. Roglans, Org. Lett. 5, 1559 (2003)
T.M. Gall, A. Birkner, G. Dyker, Organomet. Chem. 693(1), 1 (2008)
R. Narayanan, M.A. El-Sayed, J. Am. Chem. Soc. 125(27), 8340 (2003)
A.M. Doyle, S.K. Shaikhutdinov, S.D. Jackson, H.J. Freund, Angew. Chem. Int. Ed. 42, 5240 (2003)
M. Singla, P. Mathur, M. Gupta, M.S. Hundal, Trans. Met. Chem. 33, 175 (2008)
A.F. Littke, G.C. Fu, J. Org. Chem. 64, 10 (1999)
K. Selvakumar, A. Zapf, M. Beller, Org. Lett. 4, 3031 (2002)
D. Gelman, S.L. Buchwald, Angew. Chem. Int. Ed. 42, 5993 (2003)
W. Affo, H. Ohmiya, T. Fujioka, Y. Ikeda, T. Nakamura, H. Yorimitsu, K. Oshima, Y. Imamura, T. Mizuta, K. Miyosh, J. Am. Chem. Soc. 128, 8068 (2006)
Y. Ikeda, T. Nakamura, H. Yorimitsu, K. Oshima, J. Am. Chem. Soc. 124, 6514 (2002)
P. Gomes, C. Gosmini, J.Y. Nedelec, J. Perichon, Tetrahedron Lett. 43, 5901 (2002)
P. Gomes, C. Gosmini, J. Perichon, Tetrahedron 59, 2999 (2003)
Y.G. Li, P. Zhou, Z.H. Dai, Z.X. Hu, P.P. Sun, J.C. Bao, New J. Chem. 30, 832 (2006)
P. Zhou, Y. Li, P. Sun, J. Zhou, J. Bao, Chem. Commun. 2007, 1418 (2007)
W. Affo, H. Ohmiya, T. Fujioka, Y. Ikeda, T. Nakamura, H. Yorimitsu, K. Oshima, Y. Imamura, T. Mizuta, K. Miyoshi, J. Am. Chem. Soc. 128, 8068 (2006)
K.S. Jithendra kumara, G. Krishnamurthy, B.E. Kumara swamy, N.D. Shashi kumar, Satish naika, B.S. Krishna, Nagaraj naik. Appl. Organometal. Chem. 31(1), 3549 (2017). doi:10.1002/aoc.3549
Funding
The funding was provided by University Grants Commission (Grant No. UGC:MRP(S)-0098/12-13/KAKU023/UGC-SWRO/dated:01-10-2013).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jithendra Kumara, K.S., Krishnamurthy, G., Kumara Swamy, B.E. et al. Catalytic performance study of nano-cobalt: a catalyst for complement to the Heck coupling reaction. J Porous Mater 24, 1095–1103 (2017). https://doi.org/10.1007/s10934-016-0350-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-016-0350-5