Abstract
Mesoporous silica particles of MCM-41 type were synthesized by sol–gel method from tetraethyl orthosilicate in 2-methoxyethanol and deionized water mixture in base conditions at room temperature. Ammonia or sodium hydroxides were used as catalysts and cetyl-trimethylammonium bromide and n-dodecyl-trimethylammonium bromide as structure directing agents. The porosities and the ordered structure have been analyzed using transmission and scanning electron microscopy, small angle neutron and X-ray diffraction, nitrogen adsorption, thermal analysis and FTIR spectroscopy. The samples consist of spherical particles of sub-micrometer size, with radially arranged pores. The comparison of the effect of the different surfactants and catalysts shows that by varying the surfactant type and their proportion, the pore sizes can be controlled. As compared to the commonly used ammonia catalyst, the use of NaOH as catalyst results in a much smaller porosity of the as-prepared materials. These materials are not resisting to the heat treatment at 700 °C used for the template removal, and the ordered porous structure is completely lost.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years the progress in nanotechnology has motivated researchers to develop nanostructured materials for different applications, including biomedicine where the different types of nanoparticles serve as controlled delivery systems [1]. The porous biomaterials with large surface areas and large pore volumes are good candidates for drug delivery systems [2–4]. Drug carriers require biocompatibility and well defined morphological characteristics, and here the sol–gel methods of mesoporous silica materials production offer the simplicity of preparation and ease of control of their pore sizes adapted to the different drug molecules [5, 6].
Ordered mesoporous spherical silica particles can be obtained by using an ionic surfactant, such as cetyl-trimethylammonium bromide, CTAB. This was the first and even now is the most frequently used templating agent, for acid or base catalyzed sol–gel process or under hydrothermal conditions. Various pore morphologies can be achieved with different templating agents by adjusting their concentrations in the reaction mixture [7, 8].
Surfactants with shorter or longer alkyl chains can be used to control the pore size [9, 10]. Thus, silica particles synthesized with dodecyl-trimethylammonium bromide, DTAB, have a very small pore size, which allows a slowing down of the diffusion process and therefore minimizes the leakage in the system [11]. Modified Stöber synthesis can be used for preparation of micrometer sized silica particles with ordered pore structure [12–14]. The size of silica nanoparticles can be tuned by changing the synthesis conditions, the proportion of the solvents and reactants [15]. The increase of template agent chain length from 12 to 16 methyl groups results in increasing of the pore mean diameter [12, 16]. The two dimension parameters may also correlate: in an ammonia catalyzed synthesis, the particle size decreased as the chain length of the surfactant increased [17].
The pore sizes and the lattice periodicity depend primarily on the type of the surfactant [12, 18], but also organic co-solvents can affect the pore size acting as spacers inside the micelles [19]. Thus, ethoxyethanol acts not only as solvent but also as co-surfactant, controlling the morphology and pore structure. In alkali-catalyzed reactions, the co-surfactants promote TEOS water solubility facilitating the hydrolysis reactions [4, 20]. It was found that, compared with ethanol (the most used solvent in this kind of synthesis), methanol shows less inhibition effect on the development of long-range order, favouring the formation of silica spheres with hexagonally ordered mesopores [21].
2-Methoxyethanol belongs to the class of solvents known as glycol ethers which are notable for their ability to dissolve a variety of different types of chemical compounds and for their miscibility with water and other solvents. It is a porogen and confers hydrophobicity [22] 2-methoxyethanol was used as a co-solvent facilitating to dissolve PEG [23]. 2-Methoxyethanol could be used like a capping agent to help particles formation over a gel structure; the approach would be to passivate the silica nanoparticles (i.e., the particles in the sol) so that they cannot interconnect to form a gel. Also, a homogeneous gel state can be achieved by hydrolysis enhancement, respectively by lowering of solution temperature and also by addition of solvents like 2-methoxyethanol [24].
2-Methoxyethanol was previously used for the synthesis, by the sol–gel methods, of uniform spherical shape of silica nanoparticles [25]. 2-Methoxyethanol was also used as solvent and complexing agent in the synthesis of mixed silica–titania [26]. Moreover, the 2-methoxyethanol allows, in one-step reaction, the preparation of silica–titania gels in the full composition range for very short gelation times. This is possible because of the use of 2-methoxyethanol, a protic polar solvent, which plays two different specific roles: it acts as a solvent as well as a stabilizer of titanium alkoxide towards the hydrolysis-precipitation reaction, by an accurate adjustment of the quantity of methoxyethanol in the mixture, controlling therefore the reactivity of the titanium precursor [27]. To our best knowledge, the use of 2-methoxyethanol as co-solvent in ordered porous silica synthesis has not been reported to date.
In the present study MCM-41 type materials have been prepared by using room temperature sol–gel hydrolytic route starting from tetraethyl orthosilicate (TEOS) as unique precursor, hydrolyzed in deionized water and 2-methoxyethanol mixture. Ammonia or sodium hydroxide was used as catalyst while CTAB or DTAB or their equimolecular mixture were used as templates. The effects of the two surfactants and of two catalysts on the sample morphologies have been studied.
2 Experimental
2.1 Sample synthesis
All chemicals were commercially available: tetraethyl orthosilicate (TEOS), (99 %, for analysis, Fluka); 2-methoxyethanol (Sigma-Aldrich, 99.8 %); NH4OHaq (25 %, SC Silal Trading SRL); NaOH (Chemopal); hexadecyl-trimethylammonium bromide (cetyl-trimethylammonium bromide) (Sigma-Aldrich); n-dodecyl-trimethylammonium bromide (Merck).
Silica samples with ordered porous structure we prepared by adapting earlier reported procedures [4, 14]. Szegedi et al. [14] synthesized silica ordered matrix by using CTAB, TEOS in ethanol and water mixture and base catalyst NH3. Tan et al. [4] synthesized porous silica nanoparticles by using CTAB, TEOS in 2-ethoxyethanol and water mixture and base catalyst NH3.
In the present work we used 2-methoxyethanol as co-solvent. The molar ratios of the reactants were TEOS/2-methoxyethanol/H2O/NH3(NaOH)/surfactant = 1:58:144:11 (0.31):0.3 respectively. The base (catalysts) concentration was calculated as 5.25 mol L−1, in order to keep constant the ionic strength in all reactants mixtures.
The synthesis procedure has been previously presented [28]. Six xerogel samples were prepared in sol–gel synthesis by using CTAB, DTAB or their equimolecular mixture, either in presence of ammonia or NaOH. Typically, CTAB (4.93 mg), or DTAB (4.17 mg), or their mixture, CTAB + DTAB (2.465 + 2.08 mg), were dissolved in a mixture of 117 mL of distilled water and 206.6 mL of 2-methoxyethanol. Subsequently 37.087 mL of ammonia (or 0.56 mg NaOH) were added. The mixtures were vigorously stirred in a closed vessel at room temperature for 30 min. Then, 10 mL of TEOS sol–gel precursor was slowly dripped in, and vigorous stirring was continued for 24 h. The resulted suspension was washed several times with distilled water in ultrasonic bath, and then centrifuged at 3,500 rpm until the pH of the supernatant was close to neutral.
Ethanol was added to the separated precipitate and left for 24 h. After that, the material was dried at 40 °C for 3 h, then heated up to 60 °C and dried during 9 h. These samples were labeled as 60. A part of the each dried sample has been further heated up to 500 °C, another part to 700 °C for 6 h, and the corresponding samples were labeled as 500 and 700 respectively (see Table 1).
2.2 Sample characterization
FTIR spectra were taken on KBr pellets with a JASCO-FT/IR-4200 apparatus. Textural properties of the composite silica samples were analyzed using nitrogen adsorption/desorption measurements at liquid nitrogen temperature (77 K) with a Quantachrome Nova 1200e apparatus. Prior to the measurements the samples were degassed during 4 h at room temperature. The specific surface area (SBET) was calculated from the BET equation, the total pore volume (Vp) determined from the last point of adsorption and the pore diameter (Dp) determined by Barrett, Joyner and Halenda (BJH) method, from the desorption branches of the isotherms using a NovaWin software.
Small-angle neutron scattering (SANS) measurements were performed on the Yellow Submarine instrument at the BNC in Budapest (Hungary) [29]. Neutrons from a cold neutron source were monochromated by a mechanical velocity selector of 0.2 FWHM wavelength spread [30]. Sample-detector distances between 1.3 and 5.5 m, and mean neutron wavelengths of 0.47 and 1.14 nm allowed to cover a q-range 0.6–4.2 nm−1. The fine powder samples were filled in optical quartz glass cuvettes of 2 mm light path. Beam size at the sample was 8 mm in diameter. Measurements were carried out at room temperature. The raw data have been corrected for attenuation, scattering contributions from the empty cuvette and the room background.
The morphological characterization of the samples was done by scanning and transmission electron microscopy (SEM and TEM). For SEM an INSPECT S (FEI Company, Olanda) instrument has been used. TEM investigations have been carried out with Hitachi High-Tech HT7700 transmission electron microscope, operated in high contrast mode at 100 kV accelerating voltage. Samples have been prepared by drop casting from diluted dispersions of the nanoparticles in ethanol on 300 meshes holey carbon coated copper grids (Ted Pella) and vacuum dried.
X-ray powder diffraction (XRD) measurements were performed on Panalytical X’Pert Pro MPD diffractometer equipped with Cu-anode (Cu Kα; λ = 0.15418 nm) and X’Celerator detector. Measurements were done at room temperature in transmission mode using Mylar foil over an angular range from 0.65° to 10° in 2θ.
The thermogravimetric analysis was carried out between 25 °C and 700 °C using a 851-LF 1100-Mettler Toledo apparatus in air flow and a heating rate of 5 °C min−1.
3 Results and discussion
3.1 Thermal analysis
For all xerogel samples three stages of weight loss could be observed (Figs. 1, 2). The first weight loss is accompanied by an endothermic effect on the DTA curves (Fig. 2), due to desorption of the physisorbed and chemisorbed water [31–33]. CTAB starts to decompose above 200 °C [34]. The surfactant transformations with temperature during calcination are well recognized up to ~250 °C. Trimethylamine is a product of the template degradation at lower temperatures. In the case of siliceous MCM-41 an intensive release of trimethylamine was observed within the temperature range 200–300 °C [31]. At a relatively low temperature 250–300 °C fragmentation of the alkyl chain takes also place producing shorter hydrocarbons [35, 36]. In our case the second weight loss accompanied by an endothermic effect was assigned to partial decomposition and reduction of organics to carbon. The last weight loss is accompanied by an exothermic effect on DTA, because of the low molecular weight fragments of the surfactant and carbonaceous material removal by oxidation to carbon oxides [37, 38]. The weight losses for all samples are presented in Table 2.
3.1.1 The ammonia synthesized samples
According to the TGA and DTA curves, the temperature domains for the weight losses were: 25–180; 180–375; 375–700 °C. As it was expected the highest total weight loss (53.18 %) occurs in case of the samples synthesized with C16TAB. The total weight loss of the samples was increasing in the series: DTAB(C12) < Mix < CTAB(C16). The exothermic maxima of DTAB, Mix, CTAB (Fig. 2a) were clearly distinct, at 324, 329 and 330 °C respectively [28].
In the case of samples synthesized with NH3, two elimination steps of the surfactant were observed on DTG plot. Similar thermal behavior was observed by Denoyel et al. [39] in synthesis of ZnO nanostructures using mix of CTAB and sodium dodecyl sulfate. In the first step the surfactants started to degrade right after dehydration of water, followed by a second step when the residual surfactants decomposed until complete degradation. It can be assumed that, also in the present case, the two-step weight loss process can be due to decomposition and elimination first of the alkyl chain and then, at higher temperature, elimination of the head group. Finally, a rapid elimination by an auto combustion-like effect takes place (see the narrow TGA maximum in Fig. 2a).
3.1.2 The sodium hydroxide synthesized samples
For the samples synthesized with sodium hydroxide the weight loss temperature domains shifted to higher temperatures: 25–215; 215–450; 450–700 °C (compared to the ammonia synthesized samples). The total weight loss of the samples increases in the order: CTAB(C16) < DTAB(C12) < Mix. The exothermal maxima for CTAB, DTAB, Mix, (Fig. 2a) were distinctly noted at 337, 343 and 355 °C respectively [28]. For each sample the total weight losses have lower values (Table 2), compared to the samples synthesized with ammonia.
3.1.3 Comparison of the NH3 and NaOH synthesized samples
The thermal analysis results show that the samples synthesized with NaOH are more stable according to the broadening of the second weight loss domain which starts at somewhat higher temperature than for samples with ammonia. It can be attributed to the higher reactivity of NaOH, with both, the templating agent and the matrix. In the case of samples synthesized with NaOH, an overlap of the two elimination steps is observed, so there is a partial overlapping in the decomposition and elimination of the alkyl chains and the core structure.
The carbon elimination by oxidation is slower in the NaOH samples than in the NH3 ones, probably due to the reduced porosity hindering of the surfactant in the matrix. In general, disordered structures, like mesoporous silica material prepared using polymerization of silicate anions surrounding surfactant micelles in the presence of organic salts have higher thermal stability than the ordered ones, such as MCM-41 [40].
3.2 FT-IR spectra
FT-IR spectra of the xerogels dried at 60 °C (NH3 catalyst) are presented in Fig. 3. No obvious differences could be observed between IR spectra of the ammonia and sodium hydroxide synthesized samples.
As expected, all samples (xerogels and thermally treated materials) show the main specific vibration bands for the silica skeleton at 1,050, 800 and 450 cm−1. They can be assigned to the asymmetric stretching [41, 42], symmetric stretching [42, 43] and its bending rocking mode vibration of the Si–O–Si network [42, 44–46], respectively.
For all the thermally treated samples the position of asymmetric stretching vibration band is shifted to higher values by approximately 20 cm−1, which is related to the densification of the silica network [47].
The presence of the silanol groups were confirmed by the presence of the band centered about 960 cm−1, which is associated with the stretching mode of the Si–OH groups [45].
The 1,640 cm−1 vibration band belongs to the molecular water [45]. The broad band centered at around 3,470–3,450 cm−1 corresponds to the overlapping of the O–H stretching bands of hydrogen-bonded water molecules (H–O–H···H) and SiO–H stretching of surface silanols hydrogen-bonded to molecular water (–SiOH···H2O) [43]. This can be attributed to the higher hygroscopicity of the calcined samples, due to the fact that in the xerogels the template molecules fill the pores, but after the calcination the pores are freed and the water molecules have more space to occupy.
The third group of characteristic bands is due to the surfactant molecules. In the non-calcined samples the bands at 2,920 and 2,850 cm−l (see Fig. 4) are the asymmetric and symmetric stretching vibration) groups of the methylene chain, respectively [48–50]. The band around 1,480 cm−1, is ascribed to C–H bond stretching vibration of the alkyl chains [6, 51]. These bands almost completely disappear in the calcined samples.
3.3 Nitrogen adsorption
3.3.1 Ammonia synthesized samples
In Fig. 5 are presented the nitrogen adsorption/desorption isotherms of the samples prepared with the NH3 catalysts. The xerogel samples have IV type isotherm with H3 characteristic hysteresis loops attributable to the materials with the slit shaped pores [52, 53]. After templating agent removal at 500 °C all isotherms are of IV type, characteristic for MCM-41 materials with elongated pores [54, 55]. At 700 °C the DTAB and Mix samples retain the IV type isotherm, while the CTAB-NH3-700 exhibit a hysteresis shape H4, indicating partial degradation of the pores. Very similar isotherm has been shown in base catalyzed MCM-41 materials after partial disintegration at high temperature [55].
For the xerogel samples the average pore sizes increases in the following order: DTAB < mix < CTAB (Table 3). All samples possess porosity in the microporous region also; the average pore size is 2–2.3 nm. In the calcined samples the average pore size is lower compared to the corresponding xerogel samples. Total pore volume evolution with temperature is indicating the densification of the sodium hydroxide derived silica network. In the case of ammonia catalyzed samples, the pore volume increased by 10–25 times when the xerogels were heated up to 700 °C. The consolidation of the ordered network by thermal treatment was also proved by the FT-IR, SANS and XRD measurements.
Specific surface area of xerogel samples was of ~10–13 m2 g−1, probably due to hindered surface of the pores by confined surfactants. The thermally treated samples showed largely increased pore volume and specific surfaces. The maximum specific surface area (1,431 m2 g−1) was obtained for the sample obtained with CTAB and NH3 and calcined at 500 °C. On further heating it decreased to 1,060 m2 g−1. These values are consistent with the typical values reported for ordered porous materials prepared with CTAB [9, 14]. In a previous study, BET surface areas were reported, as 1,157 and 1,099 m2 g−1 for MCM-41 with CTAB and with DTAB, with a total volume of pores of 0.98 and 0.84 cm3 g−1, respectively [9]. In our synthesis, the DTAB-NH3-700 sample had somewhate smaller surface areas, 990 and 504 m2 g−1 after calcination at 500 and 700 °C, respectively.
When mixing the two surfactants, the characteristic porosity values are in between those of the single surfactant templated samples. Actually they are closer to those of the samples prepared with CTAB. Hence, it can be assumed that for fine tuning the porosity by using mixed surfactant template, one should balance the shorter chain surfactant and the longer chain surfactant fractions.
3.3.2 Sodium hydroxide synthesized samples
In Fig. 6 the nitrogen adsorption/desorption isotherms are shown for the xerogels and calcined samples obtained in the NaOH catalyzed synthesis. Both the dried and the 700 °C treated samples showed type II isotherms, characteristic for nonporous materials, though their structures were different: the dried samples were nonporous because of the pore filling by the surfactants, while in the calcined samples the pores were collapsed during the high temperature thermal treatment. The DTAB and CTAB templated samples calcined at 500 °C show IV type isotherm with specific surfaces 518 and 918 m2 kg−1, respectively, while the sample prepared with mixed surfactants collapsed, showing II type isotherm.
3.4 Electron microscopy
Scanning and transmission electron microscopy reveal the shape and inner structure of the synthesized particles. Two representative SEM images of the CTAB templated samples are shown in Fig. 7. All samples show spherical particles with sizes 0.5–0.8 μm.
The TEM images of the calcined CTAB-NH3-700 samples are shown in Fig. 8. Particles are perfectly spherically shaped, their diameters vary between 200 and 800 nm. Higher magnification reveals the ordered nanometer size channels, dominantly arranged in radial directions. The channel distance as calculated from the images was 4.1 nm for CTAB-NH3-60 samples and 3.2 nm for CTAB-NH3-700.
In contrast to the ammonia synthesized samples, the CTAB-NaOH-700 submicron-spheres are homogeneous at the studied magnification; TEM does not reveal the characteristic long channel porosity in these particles (Fig. 9). Particles with porous structure are very rare, practically absent on the studied parts of TEM grids. All of them are highly dense and have smooth surfaces.
3.5 Small angle neutron scattering (SANS) and X-ray diffraction
Small-angle scattering allows to study the structure of the materials at length scales 1–100 nm, providing a volume-average of the inner structure of the materials. SANS scattering curves of the dry silica gels treated at 60 and 700 °C are shown in Figs. 10, 11 and 12.
In Fig. 10 the scattering curves of samples made with CTAB are compared. The curves of the 60 °C dried materials show the typical structure of the ordered porous silica gel—an interference peak at q0 = 1.77 nm−1, corresponding to 2π/q0 = 3.55 nm interplanar distance. After calcination at 700 °C, the interference peak disappears for the CTAB-NaOH sample, while it shifts to q0 = 2.05 nm−1 for the other sample corresponding to a decrease of the characteristic distance to 3.06 nm.
The silica gels prepared using the DTAB templating agent, show the same structural features, see Fig. 11. For both non-calcined samples the peak position at q0 = 1.94 nm−1 corresponds to repeat distance of 3.24 nm, which decreases to 2.74 nm (q1 = 2.29) after calcination for the NH3 catalyzed sample, and the ordered structure completely disappears for the NaOH sample.
For the samples prepared with the mixture of the surfactants, the situation is overall similar, and the peak positions are 1.72 and 2.01 nm−1, before and after calcination (Fig. 12).
For the calcined samples, the scattering at small angles follows the I(q) ~ q−4 behavior, which displays in the log–log representation as a straight line with slope −4, as can be seen in Fig. 11. It corresponds to Porod’s law, and shows that surface of the silica microspheres is smooth [56]. For the non-calcined samples, the slopes are slightly lower, about −3.8, which can be attributed to their less smooth surface [57]. More precise measurements extending to lower q values would be necessary to resolve these differences.
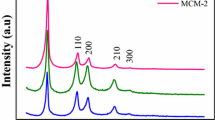
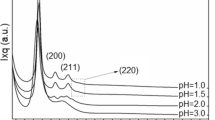
X-ray diffraction was performed for the samples prepared with the two templating agents and the two catalysts, before and after calcination at 700 °C (Fig. 13). The data reveal the same structural development as observed in SANS. The better angular resolution of XRD as compared to SANS allows detecting a very week and broad higher order peak for most of the samples. In MCM-41 type materials with well developed hexagonal structure of cylindrical pores, usually 3–5 reflections are visible, depending on the extent and quality of the long range order [9, 14, 40]. For the present samples only the weak second order peak can be discerned, indicating that the structure is not so perfect and does not show long range periodicity. In the NaOH synthesized samples, both the first and the second order peaks disappear after calcination.
Both diffraction experiments indicate that after calcination, the removal of the surfactant causes shrinkage of the distance between the pores. This shrinkage amounts in average to 15 % for the samples prepared with ammonia. In the samples prepared with NaOH, the interference peak completely disappears, indicating that the ordered porous structure is collapsed, and no characteristic interpore distance remains. Hence, those samples were not resistant to the calcination at 700 °C.
Although the use of long alkyl chain surfactants as templating agents leads usually to hexagonally arranged cylindrical pores, different structures may also form. Thus, in recent study of CTAB templated spherical silica particles, the electron microscopy revealed a complex core–shell structure, in which the core of the particle had apparently cubic structure, while the outer shell contained radially oriented nearly parallel channels [14]. Similar radial channels were also observed in some of our particles (Fig. 8a). Pauwels et al. [58] compared spherical and ordinary MCM-41 materials by high resolution TEM, and showed that in the spherical particles only short range hexagonal ordering exists, but it does not extend to the whole particle volume. As a result, an ill-ordered porous structure emerges, with an average spherical symmetry.
4 Conclusions
Spherical silica mesoporous particles of MCM-41 type have been synthesized by sol–gel method in base conditions using two templating agents, surfactants CTAB and DTAB. 2-Methoxyethanol was used as co-solvent, and the effects of two different bases used as catalyst, sodium hydroxide and ammonia have been compared.
Both templating agents and both catalysts lead to submicron sized spherical particles with elongated parallel pores. The pore sizes and the interpore distances were higher for the CTAB templated samples, in accordance with the longer alkyl chain of this surfactant. The thermogravimetric and differential thermal analysis indicated that the xerogels, catalyzed by sodium hydroxide were more stable than those prepared with ammonia. For the samples synthesized with sodium hydroxide the burning of the template surfactant takes place at higher temperatures, compared to the samples made with NH3. On the other hand the porous network in samples prepared with NH3 is more resistant to calcination: the ordered porous structure disintegrates in the NaOH catalyzed samples below 700 °C, while in the NH3 catalyzed samples the ordered pore structure remains, accompanied with shrinkage of the interpore distance.
Nitrogen adsorption showed low specific surfaces in the template-filled samples, while after calcination at 500 °C the specific surfaces in the NH3 samples increased to the level of 1,000 m2 g−1, typical for MCM-41 type porous materials. Further heating to 700 °C resulted in pore collapsing in the NaOH catalyzed materials, while the porosity in the ammonia catalyzed samples diminished by about 30 %.
When using mixture of two surfactants, the porosity became closer to the porosity of the CTAB templated samples, indicating that in case of an equimolar mixture of surfactants, the properties of the longer surfactant dominate the pore structure of the resulting material. The pore size and inter-pore distances can be varied changing the ratio of the templating surfactants.
References
A. Lodha, M. Lodha, A. Patel, J. Chaudhuri, J. Dalal, M. Edwards, D., J. Pharm. Bioallied Sci. 4(Suppl S1), 92 (2012)
W. Xia, J. Chang, J. Control. Release 110, 522 (2006)
Q. Yang, S.H. Wang, P.W. Fan, L.F. Wang, Y. Di, K.F. Lin, F.S. Xiao, Chem. Mater. 17, 5999 (2005)
S. Tan, Q. Wu, J. Wang, Y. Wang, X. Liu, K. Sui, X. Deng, H. Wang, M. Wu, Microporous Mesoporous Mater. 142, 601 (2011)
B. Gonzalez, M. Colilla, M. Vallet-Regi, Chem. Mater. 20, 4826 (2008)
G. Wang, A.N. Otuonye, E.A. Blair, K. Denton, Z. Tao, T. Asefa, J. Solid State Chem. 182, 1649 (2009)
J.I. Jung, J.Y. Bae, B.S. Bae, J. Sol Gel Sci. Technol. 31, 179 (2004)
H. Xu, Y. Li, Z. Yang, Y. Ding, C. Lu, D. Li, Z. Xu, Int. J. Appl. Ceram. Tec. 9, 1112 (2012)
M. Vallet-Regi, A. Rámila, R.P. del Real, J. Pérez-Pariente, Chem. Mater. 13, 308 (2001)
J. Goworek, A. Kierys, M. Iwan, W. Stefaniak, J. Therm. Anal. Calorim. 87, 165 (2007)
E. Johansson, E. Choi, S. Angelos, M. Liong, J.I. Zink, J. Sol Gel Sci. Technol. 46, 313 (2008)
K. Yano, R&D Rev. Toyota CRDL 40, 28 (2004)
A. Szegedi, Z. Kónya, D. Méhn, E. Solymár, G. Pál-Borbély, Z.E. Horváth, L.P. Biró, I. Kiricsi, Appl. Catal. A 272, 257 (2004)
A. Szegedi, M. Popova, I. Goshev, J. Mihaly, J. Solid State Chem. 184, 1201 (2011)
Y. Chen, X. Shi, B. Han, H. Qin, Z. Li, Y. Lu, J. Wang, Y. Kong, J. Nanosci. Nanotechnol. 12, 1 (2012)
C.T. Kresge, M.E. Leonowitz, W.J. Roth, J.C. Vartuli, J.S. Beck, Nature 359, 71012 (1992)
L.P. Singh, S.K. Bhattacharyya, G. Mishra, Appl. Nanosci. 1, 117 (2011)
Y. Yu-Xiang, Y. Hai-Ping, S. Jian-Guo, H. Zheng, L. Xiang-Nong, C. Ya-Ru, J. Am. Ceram. Soc. 90, 3460 (2007)
E.S.A. Safty, J. Porous Mater. 18, 259 (2011)
Y.B. Zhang, X.F. Qian, Z.K.J. Li, J. Solid State Chem. 177, 844 (2004)
H. Zhang, J. Wu, L. Zhou, D. Zhang, L. Qi, Langmuir 23, 1107 (2007)
J.M. Gladis, T.P. Rao, Microchim. Acta 146, 251 (2004)
J. Courtois, E. Bystrom, K. Irgum, Polymer 47, 2603 (2006)
T. Dobre, O.C. Parvulescu, G. Iavorschi, A. Stoica, M. Stroescu, U.P.B. Sci. Bull. Ser. B 71, 55 (2009)
H.N.T. Le, H.K. Jeong, Chem. Phys. Lett. 592, 349 (2014)
E. Pabon, J. Retuert, R. Quijada, J. Porous Mater. 14, 151 (2007)
A. Pirson, A. Mohsine, P. Marchot, B. Michaux, B. Michaux, O. van Cantfort, J.P. Pirard, A.J. Lecloux, J. Sol Gel Sci. Technol. 4, 179 (1995)
A.M. Putz, M.V. Putz, Int. J. Mol. Sci. 13, 15925 (2012)
L. Rosta, Appl. Phys. A 74(Suppl.), S52 (2002)
L. Almásy, A. Len, M. Markó, E. Rétfalvi, Z. Kristallogr. 226(Suppl. 23), 211 (2006)
J. Goworek, A. Kierys, W. Gac, A. Borówka, R. Kusak, J. Therm. Anal. Calorim. 962, 375 (2009)
Y.D. Wang, S. Zhang, C.L. Ma, H.D. Li, J. Lumin. 126, 661 (2007)
D. Zhao, Q. Huo, J. Feng, B.F. Chmelka, G.D. Stucky, J. Am. Chem. Soc. 120, 6024 (1998)
R. Kumar, H.T. Chen, J.L.V. Escoto, V.S.Y. Lin, M. Pruski, Chem. Mater. 18, 4319 (2006)
J. Wawryszczuk, J. Goworek, R. Zaleski, T. Goworek, Langmuir 19, 2599 (2003)
R. Zaleski, J. Wawryszczuk, J. Goworek, A. Borówka, T. Goworek, J. Colloid Interface Sci. 262, 466 (2003)
Q. Huo, R. Leon, P.M. Petroff, G.D. Stucky, Science 268, 1324 (1995)
D. Zhao, J. Feng, Q. Huo, N. Melosh, G.H. Fredickson, B.F. Chmelka, G.D. Stucky, Science 279, 548 (1998)
R. Denoyel, M.T.J. Keene, P.L. Llewellyn, J. Rouquerol, J. Therm. Anal. Calorim. 56, 261 (1999)
R. Ryoo, J.M. Kim, C.H. Ko, C.H. Shin, J. Phys. Chem. 100, 17718 (1996)
A. Duran, J.M. Fernandez-Navarro, P. Casariego, A. Joglar, J Non-Cryst Solids 82, 391 (1986)
R. Al-Oweini, H. El-Rassy, J. Mol. Struct. 919, 140 (2009)
C.J. Brinker, G.W. Scherer, Sol–gel Science, The Physics and Chemistry of Sol–gel Processing (Academic, New York, 1990), pp. 581–585
P.K. Ojha, S.K. Rath, T.K. Chongdar, N.M. Gokhale, A.R. Kulkarni, NJGC 1, 21 (2011)
R.F.S. Lenza, W.L. Vasconcelos, Mater. Res. 4, 175 (2001)
A. Bertoluzza, C. Fagnano, M.A. Morelli, V. Gottardi, M. Guglielmi, J. Non-Cryst, Solids 48, 117 (1982)
J. Gallardo, A. Durán, D. Di Martino, R.M. Almeida, J. Non-Cryst, Solids 298, 219 (2002)
K.H.S. Kung, K.F. Hayes, Langmuir 9, 263 (1993)
D.J. Neivandt, M.L. Gee, C.P. Tripp, M.L. Hair, Langmuir 13, 2519 (1997)
J.M. Berquier, L. Teyssedre, C. Jacquiod, J. Sol Gel Sci. Technol. 13, 739 (1998)
C.T. Kresge, M.E. Leonowicz, W.J. Roth, J.C. Vartuli, J.S. Beck, Nature 359, 710 (1992)
S. Lowell, J. E. Shields, M. A. Thomas, M. Thommes, Characterization of Porous Solids and Powders Surface Area, Pore Size and Density, vol 43 (Springer Science + Business Media, LLC, Originally published by Kluwer Academic Publishers, 2004), pp. 12–15
F. Rouquerol, J. Rouquerol, K.S.W. Sing, Adsorption by Powders and Porous Solids (New York, Elsevier Science, 1998), p. 441
M. Grün, I. Lauer, K.K. Unger, Adv. Mater. 9, 254 (1997)
M. Grün, K.K. Unger, A. Matsumoto, K. Tsutsumi, Microporous Mesoporous Mater. 27, 207 (1999)
C.J. Glinka, L.C. Sander, S.A. Wise, M.L. Hunnicutt, C.H. Lochmuller, Anal. Chem. 57, 2079 (1985)
T.R. Pauly, Y. Liu, T.J. Pinnavaia, S.J.L. Billinge, T.P. Rieker, J. Am. Chem. Soc. 121, 8835 (1999)
B. Pauwels, G. Van Tendeloo, C. Thoelen, W. Van Rhijn, P.A. Jacobs, Adv. Mater. 13, 1317 (2001)
Acknowledgments
Authors thank the Romanian Academy and the Inter-Academic Exchange Program between Academy of Sciences of the Czech Republic and Romanian Academy. This work was supported by Project KMR12_1_2012_0226 granted by the National Development Agency (NDA) of Hungary.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Putz, AM., Cecilia, S., Ianăşi, C. et al. Pore ordering in mesoporous matrices induced by different directing agents. J Porous Mater 22, 321–331 (2015). https://doi.org/10.1007/s10934-014-9899-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-014-9899-z