Abstract
The synthesis of organic–inorganic composite materials was performed by the surface modification of mesoporous material type MCM-41 by chlorodimethylphenylsilane and dichloromethylphenylsilane. Applying IR spectroscopy, low temperature nitrogen adsorption/desorption (isotherms BET), thermogravimetric measurements and technique of competitive adsorption of toluene and water it was shown that the degree of silylation, hydrophobicity, surface and volume properties (pore size distribution, pore volume) strongly depends on the nature of silylation agent and the ratio of calculated amount of silanol groups to the modifier. Two types of condensation reaction take place: (1) the reaction of the modifier with surface silanol groups, and (2) an inter-molecular condensation of the modifier, resulting in additional pore blocking. Only 24–36 % of the surface silanol groups react with modifier agent. The materials are stable up to temperatures of about 170 °C that is higher than the corresponding polymeric resins. The TG/DTA data allowed concluding that the degree of grafting depends on the ratio silylation agent to SiOH groups. As shown by Fourier transform diffuse reflectance mode spectroscopy only free silanol groups react with modifier.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Organic–inorganic composites attract significant interest as polyfunctional materials. The combination of properties of various materials leads to an increase in the efficiency of material use. Changes in structural characteristics on a nanoscale and the use of more ordered structures allows for the development of more targeted and inexpensive processes.
Classic synthetic resins are widely used in liquid chromatography [1–3]. However, the use of such resins in high pressure liquid chromatography (HPLC) is generally unfeasible [4] due to swelling [5]. The porous materials used as the stationary phase in chromatography are characterized by low mechanical and thermal stability. Natural and modified inorganic materials based on alumosilicates are not widely used as stationary phases for chromatography because of low mechanical and chemical stability, low sorption capacity and low homogeneity [5, 6]. Moreover, such materials cannot be applied in aqueous media for chromatography because they could be hydrolytically destroyed. Composite materials that are widely used as chromatographic sorbents combine the properties of inorganic matrices and ion-exchange properties [7], leveling the disadvantages of synthetic polymeric ion exchangers.
In 1990, Kuroda and coworkers first reported the preparation of mesoporous silica with uniform pore size distributions, named FSM-16 [8, 9]. Beck et al. [10] synthesized a material with hexagonal geometry containing large uniform pore structures, high specific surface area and high specific pore volume (MCM-41). The ordered mesoporous material MCM-41 is of particular interest as an inorganic matrix. The pore system of MCM-41 consists of parallel tubes with a hexagonal structure with mesopores with diameter of 40 Å [10]. In addition, MCM-41 has a high surface area, enabling access of organo-functional groups during the modification.
The application of functionalized mesoporous materials with high pressure and chemical stability is very promising in catalytic [11], chromatographic [12, 13] and sorption [14–16] processes. The process of grafting is frequently used for mentioned purposes and could be reserved for adsorptive methods (e.g., the removal of toxic or environmentally relevant contaminants by adsorbent materials, or the separation of proteins and biocatalysts by restriction of the freedom of movement) [17].
The hydrolytic stability and the adsorption capacity of water are strongly influenced by the nature of the applied silylating agent [18]. In the case of silylation with trimethylchlorosilane (TMCS), its small molecular size can aid in achieving high coverage on material surfaces, while the large size of the chlorodimethylphenylsilane (CDMPS) molecules could produce steric hindrance effects that may retard the reaction of the silylation compound with silicate backbone [18]. Obviously, the conditions of modifications applied to the material can change surface properties, thermal stability, adsorption properties [19] of the final material consequently changing their possible applications. The hydrophobing of the microenvironment of acid sites on a material is an important challenge to reduce poisoning/deactivation by water molecules. In all cases, it is necessary to choose appropriate synthesis methods of property-defined materials. It is important to study the influence of the nature and quantity of the modifier on the surface properties and the activity of the remaining silanol groups of the modified material.
The aim of this work is to synthesize and characterize composite inorganic–organic materials based on MCM-41 modified by phenylsilanes to control the degree of grafting, hydrophobicity, thermal stability, sorption ability and permeability of the material. The desired sorbent should have high surface area, pore volume and a uniform pore size distribution.
2 Experimental
2.1 Materials
Mesoporous material of type MCM-41 (Süd Chemie, Germany) was used as an inorganic matrix (200–300 μm particle size, SBET = 1,250 m2/g) for the synthesis of composite materials. Chlorodimethylphenylsilane (CDMPS) (Lancaster) and dichloromethylphenylsilane (DCMPS) (Aldrich) were used as silylation reagents. Mesoporous material modified by CDMPS and DCMPS will be denoted below as MP and MDC, respectively.
2.2 Surface modification of MCM-41 by CDMPS and DCMPS
First, samples of MCM-41 were activated in a nitrogen stream at 130 °C for 12 h. 5.0 g samples of activated MCM-41 were placed in 100 mL two-necked flasks under nitrogen. Solutions of 0.025 mol of imidazole and 0.0125, 0.025 and 0.050 mol of CDMPS or DCMPS dissolved in 50 mL of dry dichloromethane were added. To keep the temperature of modification constant all reactions were carried out in jacketed flasks at the boiling point of dichloromethane (T = 41 °C) under reflux for 4 h. The products of reaction were washed with 200 mL dichloromethane and were dried at 130 °C for 24 h. The amount of used silanes calculated from the ratio of surface OH groups (vide infra) to amount of modifier, 1:2 (0.0125 mol modifier), 1:1 (0.025 mol modifier) and 2:1 (0.05 mol modifier). Modified material is denoted as MP12 (MDC12), MP11 (MDC11) and MP21 (MDC21), respectively.
2.3 Characterization of modified MCM-41 samples
Nitrogen adsorption/desorption isotherms were obtained at 77 K and relative pressure range p/p0 = 0.10–0.99 using Autosorb—1 (Quantachrom, USA). Prior to each measurement, the samples were degassed at 130 °C K for 8 h under vacuum. The method of BJH [20] was used to determine the pore size distribution.
IR spectra were recorded using a Bruker Equinox 55 infrared spectrometer in Fourier transform diffuse reflectance mode (DRIFT) with the wave number range 400–4,000 cm−1 and a resolution of 4 cm−1. Samples were prepared for IR by mixing the sample with KBr (Merck, Germany) in a ratio of 1:4. The device “Harricks cell” for in situ heating was applied to remove adsorbed water from the samples and observe the structural changes of the sample during the heating process. Nitrogen passed over the sample with a flow rate of approximately 60 mL/min, to remove the products of desorption and decomposition. IR spectra were recorded after the samples were heated in situ for 90 min at 30, 150 and 250 °C.
The hydrophobicity index of the final materials (HI) was estimated according to the procedure proposed by Weitkamp [21, 22] applying competitive adsorption of toluene and water from the gas phase. The adsorbent was used as pressed at p = 40 N/mm2, crushed and sieved to a particles size fraction with diameter ranging from 200 to 315 μm The samples were activated at a temperature of 110 ± 5 °C for 13 h in the reactor under a continuous flow of nitrogen to remove adsorbed water. A mixture of water vapor (pw = 3.13 kPa) and toluene (pt = 3.82 kPa) was passed through a fixed bed of sorbent (1.0 g) with a bed height of 15–20 mm at a temperature of 55 ± 5 °C. Nitrogen was used as a carrier gas with a flow rate of ~11.0 mL/min. The carrier gas, saturated with a mixture of toluene and water, passed through the column and was analyzed using a Hewlett Packard 5,890 gas chromatograph (GC), equipped with capillary column and thermal conductive detector by taking samples via six-port valve every 3 min.
The amounts of adsorbed water and toluene were calculated as described in [23] from the breakthrough curves.
The mass of adsorbed water or toluene was calculated as
where m i , M i and P i —mass, molecular weight and partial pressure of water and toluene in mixture, respectively.
The hydrophobicity index was calculated as [21]:
where X tol and X w are the loading, i.e. the mass of adsorbed compounds per mass of dry adsorbent, determined from the competitive adsorption of gaseous toluene and water under specified conditions [21].
Determination of thermal stability of the modified samples and the total hydroxyl group content in MCM-41 and organic modifier groups content in organic–inorganic composites of MCM-41 were made with Mettler-Toledo GmbH TGA/SDTA 851e instrument using sample amount of 16–30 mg. Heating rate was 5 °C per minute in the temperature range of 25–800 °C in a nitrogen atmosphere.
Calculation of the mass of desorbed water was performed by the formula:
where m (H2O), the weight of water (mg); m0, mass of the sample at 25 °C; mt, mass of the sample at upper range of temperature (135, 280, 800 °C respectively); m800, mass of the sample at 800 °C.
The amount of adsorbed water:
where \(n_{{H_{2} O}}\) the amount of water (mol), \(M_{{H_{2} O}}\) the molar mass of water (g/mol).
Number of OH groups was calculated according to Ek et al. [24] and Kozlova et al. [25]:
where, W 25 − W 800, is the weight loss, (wt%) in the temperature region 25–800 °C; n OH, the number of OH groups (mol).
The number of water molecules and OH groups per 1 nm2 (N), respectively, was calculated according to Kozlova et al. [25]:
where, n, is the number of moles of water or OH groups per gram mole sorbent; NA, Avogadro number (mol−1); S, surface area of the sample; m2/g.
3 Results and discussion
3.1 Modification of MCM-41
Post-synthesis grafting assumes that at the first stage of synthesis, the hydrophobic groups of aromatic organosilanes are covalently attached to the surface of the mesoporous material, utilizing the surface hydroxyl groups of the silica support (Schemes 1, 2).
Preliminary calculations of the optimum quantity of silylation agent were carried out. As shown by Das et al. [26], three types of SiOH groups are distributed over siliceous MCM-41 surfaces, single, hydrogen-bonded and geminal SiOH groups with a total density of 2.5–3.0/nm2. From the density of SiOH groups and the surface area from BET, the specific quantity of OH-groups was calculated. As shown later, the surface area of parent MCM-41 used in this work was 1,250 m2/g, according to nitrogen adsorption/desorption. For this mesoporous material the specific quantity of OH-groups was estimated as 5–6 mmol/g assuming a density of silanol groups of 2–3 per nm2 [26]. Thus, it was necessary to add the equivalent quantity of the silylation reagent, to totally cover the organic groups.
Using IR-spectroscopy it was shown [24] that silanol groups cannot completely be replaced by organic modifier. The band corresponding to OH-stretching (3,200–3,600 cm−1) is still present after modification and remains at the same intensity. It is possible that the hydrogen-bonded SiOH groups remain inaccessible for silylation. Concentration of attached organosilane groups becomes 0.7/nm2, corresponding to quantity of free SiOH groups [24]. Thus, the quantity of silanol groups participating in hydrogen-bond formation can then be readily calculated to be 1.8–2.3/nm2. The degree of silylation of SiOH groups on MCM-41 surface can, therefore, be estimated.
3.2 Surface properties
The quantity of the modifier depends on the quantity of OH-groups of mesoporous material. The quantity of CDMPS in the reaction mixture ranged from 5 to 10 mmol/gram of MCM-41. As can be seen from the pore-size distribution data (Fig. 1), the identity of the modifier can change the surface area (SBET) and pore volume. In Table 1 and Fig. 2, the results of the calculations of SBET, pore volume (VP) and pore-size distribution with use of various silylation agents and their quantities [20, 27–30] are presented. The analysis of the data shows that average diameter sizes of the pore have the same value for MCM-41 (32.8 Å) and material modified by CDMPS (32.4 Å). After CDMPS modification the surface area decreases from 1,250 m2/g (MCM-41) to 830 m2/g (MP11). Average diameter of pore D h , was calculated assuming existence of a cylindrical pores by the formula D h = 4V p /S BET [28].
Silylation of the mesoporous material by dichloromethylphenylsilane takes place in various ratios of theoretically calculated quantities of silanol groups and modifier agent (DCMPS) molecules (2:1—MDC21, 1:1—MDC11, 1:2—MDC12). As it is shown in Table 1, there is a significant decrease in SBET for MDC21, MDC11 and MDC12 (from 750 to 515 and 590 m2/g correspondingly). It seems that the greatest extent of surface modification, the greatest decrease in surface area and pore volume is observed at a ratio 1:1 of quantity of silylation reagent to SiOH groups. The observed variation of surface properties indicates a surface grafting of a layer of the modifier. The higher degree of silylation is observed when DCMPS is used as the modifier in equimolar ratios to the SiOH groups present on the mesoporous material. The grafting (Scheme 3) can be accompanied by a parallel process of intermolecular condensation of additional dichloromethylphenylsilane molecules. This could also contribute to a pore filling and pore narrowing.
3.3 DRIFT spectra characterization
The proposed mechanism of modification, presented in this work can be revealed and confirmed by IR-spectroscopy. In the IR-spectra of mesoporous material MCM-41 (Fig. 2) there is a band corresponding to vibrations of free OH groups in Si–OH (3,734 cm−1—OH stretching) [31, 32]. Also, in the same spectra, in the region of 3,500–3,100 cm−1 there is a wide band of hydrated and hydrogen-bonded OH-groups. The bands at 1,626 and 954 cm−1 correspond to bending (scissoring) vibrations (in-plane and out-of-plane) of OH-groups [31, 32].
The appearance of the bands 2,842, 2,969, (ν C–H groups in CH3) 3,058, 3,075 cm−1 (ν C–H groups in aromatic ring) 1,583–1,588 cm−1 (ν C=C stretching in aromatic ring) and 1,430 см−1 (δ C–H groups in Si–Ar) [31] in spectra (Fig. 2) confirms the successful modification of mesoporous material MCM-41. The broad absorption bands at 1,630 and 3,200–3,400 cm−1 (Fig. 2) can be assigned to the vibrations of adsorbed water associated with the silanol groups by hydrogen bonds. The bands appearing at 3,640 cm−1 (Fig. 2) are assigned to hydrogen bonded SiOH groups (Scheme 4) [18].
The fact that replacing chlorine atoms by methyl groups in the molecule of CDMPS can result in a higher degree of silylation in the final material was previously shown by low temperature hydrogen adsorption/desorption data. The IR spectra of composites after modification by CDMPS and DCMPS (Fig. 2) at various ratios of OH-groups and silylation agent show not changes in comparison to the analogous pre-modification spectra. The optimum ratio (1:1) for modification of the SiOH groups is confirmed by the IR-spectra of the MP11 and MDC11 samples, which show the absence of an O–H stretching band at 3,737 cm−1 associated with free—OH groups. Absence of bands in the range maxima at 3,750–3,730 cm−1 in the IR-spectra of these composites (see Fig. 2) shows that practically all free SiOH groups reacts with molecules of modifier agents after modification of the mesoporous material, unless they are participating in hydrogen bond formation.
Processing IR spectra using the baseline correction method allowed comparing the intensities of free silanol groups of the different modified mesoporous materials. Silylation by CDMPS in the ratio 1:1 results in increasing intensity of a band at 1,583 cm−1 (stretching of C=C in aromatic ring) and an increase in the absorbance correspondent of the band to 1,430 (δ C–H groups in Si-Aryl) (Fig. 2). Introduction of a hydrophobic aromatic ring results in the elimination of water molecules, which were adsorbed on the surface of initial mesoporous material. The presented fact of modification is accompanied by the decrease of intensity of a band at 1,620–1,630 cm−1 (δ OH) [31, 32] assigned to bending vibration of water.
In situ heating of the samples MP12, MP11, MDC12, MDC11 (Fig. 3) to temperatures in the range 200–400 °C (Fig. 3) leads to a reduction of intensity of bands at 3,500–3,100 cm−1, 1,626 and 954 cm−1, which could be explained by dehydration of the sample. At the same time intensity of a band at 3,734 cm−1, which is affiliated with free Si–OH groups, also increases. IR spectra (Fig. 3) of samples MP12, MP11, MDC12, MDC11, heated in situ, confirm a higher degree silylation of MCM-41 surface with quantitative ratio 1:1 of silanol groups to modifier agents. Furthermore, the relative intensity of bands at 3,665 cm−1 is higher than for the case of modification with CDMPS. The hydrophobic aromatic rings are shielding the OH-groups, forming hydrogen bonds with neighboring OH groups. In situ heating of samples to 250 °C leads to splitting of a band at 1,583 cm−1, 1,575 and 1,591 cm−1 (C=C stretching).
3.4 Hydrophobicity and sorption properties of composites
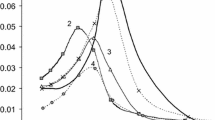
Grafting of the mesoporous material results both in change of surface properties and of the number of residual surface SiOH groups. In addition, linkage of phenylsilanes generally influences on the hydrophobicity of the material. As result, this fact changes the adsorption characteristics and permeability of the composites. One of the most useful methods to control adsorption properties and polarity of materials is probing the hydrophobic properties of the composite by the Hydrophobicity Index (HI) introduced by Weitkamp [21, 22]. The breakthrough curves presented by Gläser et al. [21] show that on MCM-41 water breaks through first and on its curves a maximum can be seen. Water is displaced by toluene from the adsorbent. Similar curves are observed for modified mesoporous material (Fig. 4, curves 2).
It has been noted [23] that for hydrophobicity interpretation it is convenient to use the relation of mol fraction instead of mass fraction of adsorbed water and toluene [21]. At equality (HI = 1) the material has identical affinity for both water and toluene molecules. Increasing HI will have the effect that toluene and other organic molecules are selectively adsorbed by the material. This effect becomes more significant when difference the between HI (mol fraction relations) and the value 1.0 is significant. Calculations of HI [21, 22] demonstrate that mesoporous material of type MCM-41 is hydrophobic and possess high adsorption for organic molecules. In the case of composite materials (MP11 and MP21) the hydrophobicity is close to the value of HI of MCM-41 (Table 2). However, as shown in Fig. 4 water and toluene break through earlier, which specifies a decrease in the general adsorption abilities of the material. Moreover, the value of HI remains almost constant (for MP21) and even decrease (for MP11) which seems to be unexpected. The calculation of the weight and mols both of adsorbed water and toluene show the general decrease of sorption ability of mesoporous composite. The decrease in the hydrophobicity index, mentioned above, is caused by the smaller size of water molecules and its higher mobility and permeability.
On one hand we can conclude that higher filling of mesopores is reached at equimolar ratio of the amount of modifier to number of SiOH groups. On the other hand it is important to note that the increase in degree of modification can sharply reduce sorption ability of the material. This is especially demonstrated for mesopores of MCM-41. It is also important to note that the sorption ability calculated from the data of water and toluene vapor adsorption can be changed considerably by the use of composites in liquid phases, e.g., for liquid chromatography.
3.5 Thermal stability
IR spectra recorded at high temperature show high thermal stabilities of the composites—presence of the bands 2,842, 2,969, (ν C–H groups in CH3) 3,058, 3,075 cm−1 (ν C–H groups in aromatic ring) 1,583–1,588 cm−1 (ν C=C stretching in aromatic ring) and 1,430 см−1 (δ C–H groups of aromatic ring linked to Si) up to 250 °C (Fig. 3). More in detail thermostability can be studied by thermogravimetric analysis.
Figure 5 and Table 3 show the weight loss curves of composites MP11 measured in nitrogen, MDC11 measured in nitrogen, and oxygen atmosphere.
Three maxima in the rate of weight loss can be observed. The first peak with a minimum at 50 °C corresponds to the removal of physically adsorbed water. This correlates to infrared spectra discussed earlier (Fig. 3). The layer of organic modifier is stable up to 250 °C. But, as specified by TGA data, the process of destruction starts at 170 °C and reaches a maximum at T = 235 °C in nitrogen and at T = 215 °C in oxygen atmosphere (Fig. 5). Those peaks could be assigned to elimination of aromatic ring from the surface. The third peak at T = 530 °C in nitrogen and T = 430 °C in oxygen atmosphere is assigned to the further decomposition of the grafted organic layer. Note that the oxidative decomposition proceeds in a smaller temperature range which indicates a similar composition of the combusted species, i.e. one can speculate that the organic layer is stable at least up to 400 °C. The figure shows that destruction of grafted modifier starts at T = 180 °C and ends at around T = 800 °C. The TG/DTA analysis combined with DRIFT data demonstrates the high stability of the grafted organic layer. Figure 5 shows that in oxygen atmosphere, the third peak shifts to lower temperatures. Under these conditions the organic layers have less stability as in inert nitrogen atmosphere.
The parent MCM-41 contains 3.4 % of physically adsorbed water, but modified samples contain approximately 8 % of adsorbed solvents. Heating of the sample results in desorption of solvent molecules in which the modification was made. The weight loss observed in the region of temperatures 135–800 °C, arises from the complete decomposition of the organic modifier. Using the TGA data, the amount of grafted modifier, per 1 gram of sorbent (νmod) and per 1 mol of OH groups (νmod/νOH) was calculated by the weight loss in the temperature range 135–800 °C (Table 3), taking into account the molar weight of the grafted group, i.e. molecular weight of modifier minus molecular weight of chlorine atom(s). The average amount of silanol groups on the surface of mesoporous materials is 2.5–3 groups/nm2 [33]. Taking into consideration the surface area of MCM-41 (1,250 m2/g), the amount of OH groups is 5.5–6.6 mmol/g [33].
Reported quantity of modifier per 1 gram of sorbent depends on the initial quantity of modifier. The highest value of grafting was obtained at a ratio 1:1 of silylation agent (CDMPS and DCMPS) to SiOH groups. But as seen from the table 3, only 24–36 % of the silanol groups react with modifier. As shown by DRIFT (see above) only free silanol groups react with modifier. Therefore, it is possible to estimate the quantity of free silanol groups of the parent mesoporous material to be also a quarter of all silanol groups.
4 Conclusion
Synthesis of organic–inorganic hybrid materials by chemical modification of MCM-41 by phenylsilanes (CDMPS and DCMPS) was carried out. Silylation of the mesoporous material depends on the nature of silylation agent and the ratio of theoretically calculated amount of silanol groups to the modifier. Using low temperature nitrogen sorption/desorption measurements it was shown that the most significant grafting takes place with DCMPS. It was concluded that higher filling of a mesopore is reached with equimolar relations of the modifier and SiOH groups. On the other hand an increase in the degree of modification can sharply reduce sorption ability and permeability of the material due to polycondensation reaction of silanol groups formed after leaving of the two chlorine atoms. DRIFT experiments confirmed the modification of MCM-41 and in situ heating of the sample shows the structural changes of the composites with stepped changes due to temperature. From the data obtained using TG/DTA we can conclude that the materials are stable up to temperatures of about 170 °C. The highest degree of grafting is 0.24 mol of modifier to 1.0 mol of OH groups of MCM-41.
References
V. F. Selemenev, G. Y. Oros, O. I. Stukalov, M. P. Tsurupa, V. A. Davankov, Patent of Russian Federation No 93032352/13(031326) 21.06.1993
V.A. Chirkin, S.I. Karpov, V.F. Selemenev, N.A. Belanova, Ind. Chem. 79, 23 (2013). (in Russian)
N.A. Udalova, S.I. Karpov, V.F. Selemenev, I.A. Sharmar, Rus. J. Phys. Chem. A. 83(6), 1006 (2009)
F. Hellferich, J. Chem. Educ. 40(4), 231 (1963)
O.B. Rudakov, Solution as a Means of Process Control in Liquid Chromatography (Voronezh State University Press, Voronezh, 2003). 302 p
M. Marhol, Ion Exchangers in Analytical Chemistry: Properties and Application (Mir, Moscow, 1982), p. 248
H. Xiao, N. Cezar, J. Colloid Interface Sci. 267, 343 (2003)
T. Yanagisawa, T. Shimizu, K. Kuroda, C. Kato, Bull. Chem. Soc. Jpn. 63, 988 (1990)
S. Inagaki, Y. Fukushima, K. Kuroda, J. Chem. Soc. Chem. Commun. 8, 680 (1993)
J.S. Beck, J.C. Vartuli, W.J. Roth, M.E. Leonowicz, C.T. Kresge, K.D. Schmitt, C.T.-W. Chu, D.H. Olson, E.W. Sheppard, S.B. McCullen, J.B. Higgins, J.L. Schlenker, J. Am. Chem. Soc. 114, 10834 (1992)
W.D. Bossaert, D.E. de Vos, W.M. van Rhijn, J. Bullen, P.J. Grobert, P.A. Jacobs, J. Catal. 182, 156 (1999)
T. Martin, A. Galarneau, F. Di Renzo, D. Brunel, F. Fajula, Chem. Mater. 16(9), 1725 (2004)
T. Yasmin, K. Müller, J. Chromatogr. A. May 14 1217 (20) (2010): 3362–3374. E pub 2010. Mar 10
M. Etiennne, A. Walcarius, Talanta 59, 1173 (2003)
Q. Gao, W. Xu, Y. Xu, D. Wu, Y. Sun, F. Deng, W. Shen, J. Phys. Chem. B. 112, 2261 (2008)
A.J. O’Connor, A. Hokura, J.M. Kisler, S. Shimazu, G.W. Stevens, Y. Komatsu, Sep. Purific. Tech. 48, 197 (2006)
F. Hoffmann, M. Cornelius, J. Morell, M. Froeba, Angew. Chem. Int. Ed. 45, 3216 (2006)
S. Chiarakorna, T. Areeroba, N. Grisdanurak, Sci. Tech. Adv. Mater. 8, 110 (2007)
E.V. Borodina, S.I. Karpov, V.F. Selemenev, F. Roessner, Nanotech 5(11), 808 (2010). (in Russia)
E.P. Barrett, L.G. Joyner, P.P.J. Halenda, J. Am. Chem. Soc. 73, 373 (1951)
R. Glaeser, R. Roesky, T. Boger, G. Eigenberger, S. Ernst, J. Weitkamp, Stud. Surf. Sci. Catal. 105, 695 (1997)
J. Weitkamp, S. Ernst, E. Roland, G.F. Thiele, Stud. Surf. Sci. Catal. 105, 763 (1997)
S.I. Karpov, F. Roessner, V.F. Selemenev, M.V. Matveeva, Russ. J. Phys. Chem. A 84(1), 58 (2010)
S. Ek, A. Root, M. Peussa, L. Niinistö, Thermochim. Acta 379, 201 (2001)
S.A. Kozlova, V.A. Parfenov, L.S. Tarasova, S.D. Kirik, J. Sib. Fed. Univ. Chem. 1, 376 (2008)
D. Das, J.F. Lee, S. Cheng, Chem. Commun. 21, 2178 (2001)
S. Brunauer, P. Emmett, E. Teller, J. Am. Chem. Soc. 60, 309 (1938)
M. Thommes, R. Koehn, M. Froeba, J. Phys. Chem. 104, 7932 (2000)
M. Thommes, R. Koehn, M. Froeba, Stud. Surf. Sci. Catal. 142, 1695 (2002)
M. Hesse, H. Meier, B. Zeeh, Spektroskopische Methoden in der Organischen Chemie (Thieme, Stuttgart, New York, 2002), p. 42
K. Kiss-Eröss, Comprehensive analytical chemistry: Analytical infrared spectroscopy, ed. by G. Svehla (Elsevier scientific publishing company, Amsterdam, 1976), p. 396
L.G. Wade, Organic Chemistry (Pearson Education, Upper Saddle River, 2006), p. 1024
X. S. Zhao, G. Q. Lu, A. K. Whittaker, G. J. Millar, H. Y. Zhu, J. Phys. Chem. B. 101. 6525 (1997). http://www.springer.com/chemistry/catalysis/journal/10934
Acknowledgments
This work was financially supported by the German Academic Exchange Service (DAAD) and Russian Ministry of Education and Science in the frame of program “Mikhail Lomonosov”. The authors are grateful to M. Wickleder and M.Ahlers (Institute of Chemistry, Carl von Ossietzky University, Germany) for TGA/DTA measurements. We also thank E. Borodina, F. Kirby (University of Utrecht, Department of Inorganic Chemistry and Catalysis) and E. Corker (Technical University of Denmark, Department of Chemistry) for valuable discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karpov, S.I., Roessner, F. & Selemenev, V.F. Studies on functionalized mesoporous materials—Part I: characterization of silylized mesoporous material of type MCM-41. J Porous Mater 21, 449–457 (2014). https://doi.org/10.1007/s10934-014-9791-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-014-9791-x