Abstract
Wettability of solid surfaces is an important property, which depends on both the surface chemistry and surface roughness. The present paper describes the room temperature synthesis of dip coated water repellent silica coatings on glass substrates using phenyltrimethoxysilane (PTMS) as a co-precursor with two-step sol–gel process. Silica sol was prepared by keeping the molar ratio of tetraethylorthosilicate precursor, methanol solvent, acidic water (0.001 M oxalic acid) and basic water (12 M NH4OH) constant at 1:11.03:0.17:0.58 respectively, throughout the experiments and the PTMS weight percentage was varied from 0 to 15 %. It was found that with an increase in wt% of PTMS, the roughness and hydrophobicity of the films increased. However, the optical transmission decreased from 93 to 82 % in the visible range. The hydrophobic silica films retained their hydrophobicity up to a temperature of 386 °C and above this temperature the films became hydrophilic. The hydrophobic silica thin films were characterized by taking into consideration the surface roughness studies, Fourier transform infrared spectroscopy, percentage of optical transmission, scanning electron microscopy, thermogravimetric–differential thermal analysis and contact angle measurements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ultrahydrophobic surfaces with static water contact angle higher than 130° and a sliding angle close to 8° generated a lot of research interest both in academia and in industry because of the self-cleaning properties. In 1997, Barthlott and Neinhuis showed that the self-cleaning property of lotus leaves was due to their specialized surface morphology and hydrophobicity [1]. Hydrophobicity is a property that provides water repellency and non-wettability of a solid surface. The fabrication of hydrophobic coatings is an active area of research in recent years because of its wide range of applications, such as windshields of automobiles, dust-free and self-cleaning surfaces for solar panels, satellite dishes, building walls and roof glasses, exterior architectural glass and green houses, heat transfer surfaces in air conditioning equipment and so on [2–4]. Various methods have been employed to generate engineering surfaces that can mimic the structure and chemistry of natural superhydrophobic surfaces [5]. These days, Sol–gel derived coatings have been found to be useful for several applications mainly due to the ease of solution based processing and the synthesis flexibility, which can be used for preparing a wide range of thin films and coatings [6, 7].
In general, the sol–gel process involves the transition of a system from a liquid “sol” (mostly colloidal) into a semi-solid “gel” phase. The dip coating method is a novel and facile route for the synthesis of transparent and uniform films by the sol–gel process. The sol–gel technique with simple dip coating method is used to prepare silica films on glass substrates [8–11] and the formation of nonfluorinated superhydrophobic surfaces at low temperatures is important for the fabrication of environment friendly coatings. Xiu et al. [12] prepared superhydrophobic durable self-cleaning silica films by the incorporation of isobutyl-trimethoxysilane into tetramethoxysilane causing hydrophobic isobutyl groups to be present on the film surface, thus generating surface hydrophobicity. Tadanaga et al. [13] reported the formation of transparent superhydrophobic films on glass plates through the sol–gel method by the combination of microstructural and chemical approaches. The hydrophilicity of silica films is due to the presence of a large number of Si–OH groups on the surface of the films. Replacement of the H from the Si–OH groups by the hydrolytically stable Si–R (where, R = alkyl or aryl) groups through the oxygen bonds prevents the adsorption of water and hence results in the hydrophobic silica surfaces [14, 15].
There are many reports available on the synthesis of hydrophobic silica films by sol–gel co-precursor method [16–19]. But till date, only a few reports are available on the preparation of water repellent surfaces using TEOS as a precursor [20–23]. In this paper, the conversion of the hydrophobic surfaces (CA >120°) by using PTMS as co-precursor is reported. The aim of this study is to prepare water repellent silica films via the sol–gel process using non-fluorinated additives and to achieve quite similar water repellence like fluorine compounds.
2 Experimental
2.1 Preparation of silica coatings
The hydrophobic silica coatings are generally produced by two methods:
-
1.
Co-precursor method
-
2.
Surface derivatization method
In the present study, the co-precursor method was used because it is simple and time consuming as compared to second method. In order to study the effect of phenyltrimethoxysilane as a hydrophobic agent on the water repellent properties of silica coatings, the silica coatings were synthesized by the sol–gel co-precursor method. The chemicals used were Tetraethoxysilane, phenyltrimethoxysilane (Sigma-Aldrich Chemie, Germany), methanol (s.d.fine-chem ltd, Mumbai), oxalic acid (Qualigens Fine Chemicals, Mumbai) and ammonia (NH3, Loba Chemie, India). All the reagents were used as received. Double distilled water was used for all experiments. The glass substrates (from BlueStar®, India) of 1.5 cm × 5 cm were used as substrates in all experiments. To get a uniform coating, the glass substrates were soaked in chromic acid overnight, cleaned with detergent and labolene, followed by deionized water and acetone rinses.
The silica film was synthesized by keeping the molar ratio of TEOS, MeOH,oxalic acid and NH4OH constant at 1:11.03:0.17:0.58 respectively and the percentage of PTMS was varied from 0 to 15 %. In the first step of the experiment, TEOS was diluted in methanol along with the hydrophobic reagent, PTMS. The acidic water (oxalic acid, 0.001 M) was added to this solution, drop by drop, while stirring (∼2 h). In the second step, basic water (ammonium hydroxide, 12 M) was added to the solution, drop-by-drop, while stirring (∼10 min), after 12 h of hydrolysis reaction. The homogeneous alcosol thus obtained was transferred to airtight glass test tubes of 14 mm outer diameter and 85 mm height. No protective atmosphere was used for the deposition of silica films. Silica films were deposited at ambient conditions by dip coating (withdrawing speed 5 mm s−1) from freshly made sol prior to gel formation. The films were dried at room temperature (27 °C) for 30 min to produce chemical bonds between the deposited sol and the substrate. The prepared silica films were annealed at 120 °C for 2 h with a ramping rate of 1 °C min−1. The silica films so produced were taken out of the oven after it was cooled to the ambient temperature.
2.2 Methods of characterization
The roughness of the film was determined using a surface profilometer (Ambios XP-1 Model, India). The contact angle (θ) measurements were carried out to quantify the degree of hydrophobicity using a contact angle meter equipped with a CCD camera (Ramehart Instrument Co., USA) at ambient temperature. A water droplet of 10 μl was placed at three different places on the surface of the film under investigation and the average value was taken as the contact angle (θ) to increase the accuracy in the measurement. The films were also characterized by Fourier transform infrared spectroscopy (FTIR) (Perkin-Elmer, model no. 783, USA), which gave information about various chemical bonds responsible for hydrophobicity, such as O–H, Si–C, C–H, and Si–O–Si. The optical transmittance of the films was measured in the visible wavelength range using a optical spectrophotometer (Systronic 119, USA). Thermo gravimetric and differential thermal analyses (TG–DTA) were used to investigate the stability of the water repellent coatings against temperature. The surface morphology of the silica coatings was studied by means of scanning electron microscopy (JEOL, JEM 6360).
3 Result and discussion
3.1 Reaction mechanism
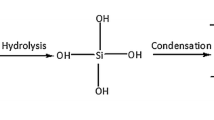
In the sol–gel process, initially TEOS is hydrolyzed and condensed as per the following chemical reactions:Hydrolysis:
Condensation:
Water condensation:
Alcohol condensation:
However, using PTMS as co-precursor in the sol–gel processing stage, the Hs from the OH groups on the silica clusters are replaced by the hydrolytically stable ≡Si–phenyl as per the following chemical reactions:

Here we used phenyltrimethoxysilane as a co-precursor for the preparation of silica coatings. The hydrophobicity of silica surfaces is due to the attachment of ≡Si–phenyl groups. Hence, as the percentage of co-precursor in TEOS increases, the hydrophobicity of silica coatings also increased. The photograph of water droplets on PTMS modified silica coating is shown in Fig. 1.
3.2 Gelation time and optical transparency
Gelation time of the sol is determined by a simple laboratory method [24]. Table 1 shows the effect of the PTMS weight percentage in TEOS on the gelation time of the silica alcosols. As the percentage of co-precursor have been increased from 0 to 15 %, the gelation time considerably increased from 5 min to 5 h 20 min for PTMS modified coatings. It has been reported that the gelation time increases with the increase in the quantity of the hydrophobic reagents [25, 26]. This is because, under the basic conditions, co-precursor, PTMS, hydrolyses slowly than the TEOS precursor. Hence, with the increase in PTMS co-precursor the hydrolysis and the subsequent condensation reactions take place slowly, leading to longer gelation. Figure 2 shows the percentage of optical transmission of the silica films for pure and PTMS modified silica coatings. The unmodified silica films were most transparent (93 %) while the transparency gradually reduced to 82 % for 15 wt% PTMS modified silica coatings.
3.3 Roughness and contact angle measurement
According to Young’s equation [27], the contact angle needs to increase accordingly to balance the increased surface energy between the solid substrate and the liquid droplet.
where, \( \gamma_{sl} \), \( \gamma_{lv} \) and \( \gamma_{sv} \) are the interfacial tensions between the solid and the liquid, the liquid and the vapor, and the solid and the vapor, respectively.
Two different models were suggested to explain the increase in contact angle on the rough surface. Wenzel had modified the Young’s equation as in the following [28]:
where r is a roughness factor, which is defined as the ratio of the actual area of a rough surface to the geometric projected area. This law is applicable if there is no air entrapped in the pits of the rough surface below the liquid.
In contrast, Cassie and Baxter [29] proposed an equation:
where, f is the area fraction of the liquid–solid contact to the projected surface area. This law describes the contact angle θ′ for a surface trapping air below the water droplet in the hollows of a rough surface so that droplet essentially rests on a layer of air.
Table 1 shows an increase in the roughness of the film surface with increase in percentage of co-precursors, as well as in case of thickness, same relation is followed. The phenyl groups present in the PTMS contributed to the enhancement of surface roughness of the film. The water contact angle values are also increased with an increase in the surface roughness of the films.
To evaluate the hydrophobic properties of the silica films, the contact angle (θ) of the water droplet with surface of the films prepared with various percentage of co-precursor have been measured as shown in Fig. 3. It is found that θ increased with increase in percentage of co-precursor. The as prepared silica films with 0 wt% of PTMS contain a large number of hydroxyl and alkoxy groups which are responsible for the hydrophilic character (θ = 30°) of the film surface. However, using PTMS as coprecursor in the sol–gel processing stage, the OH groups are replaced by hydrolytically stable O–Si–phenyl group as shown in the condensation reactions (3) and (4). The film surface becomes hydrophobic because of the hydrolytic stability of Si–C bonds. The water drop displayed a comparatively large contact angle (133°) on the PTMS modified silica films compared with the contact angle (30°) on unmodified silica films with increase in surface roughness. This result can be explained by the Wenzel equation, which indicates that the water contact angle of the surface increases with increasing surface roughness when the surface is composed of hydrophobic materials. The effect of humidity on the wetting properties of PTMS modified (15 wt%) film was carried out at a relative humidity of 85 % at 30 °C over 40 days. After 30 days of storage in humidity, the values of the water contact angle remained essentially constant, indicating that the surface has long-term durability.
3.4 FTIR studies and contact angle measurement
The chemical composition of the dip coated silica films on the glass substrates was investigated by the FT-IR spectroscopy using the KBr method in the transmission mode. Figure 4 shows the FT-IR spectra of unmodified, PTMS modified (15 %) silica films, respectively. In Fig. 4a, b the peaks at 1,084 and 1,091 cm−1 are associated with the transverse optical vibration mode corresponding to the asymmetric stretching of the intertetrahedral oxygen atoms in the Si–O–Si linkage [30]. The presence of this peak confirms the formation of a network structure inside the film. The relative intensity of this peak compared to densified glasses or sol–gels, suggests that the compressive stress in the sample is low, and that the silica network is comparatively stiff [30]. The PTMS modified silica film confirmed the absorption band at 2,983 cm−1 corresponding to asymmetric stretching vibrations of C–H bonds. The development of sharp and intense band at 1,430 cm−1, corresponding to symmetric deformation vibration of Si–phenyl bonds, represents phenyl groups attached to silicon atoms as shown in Fig. 4b [31]. The peaks at around 1,600 cm−1 and the broad absorption band at around 3,425 cm−1 are due to the –OH groups [32]. On the other hand, in Fig. 4b, the development of sharp band at 837 cm−1 corresponding to the rocking vibrations of Si–C bond which is reported elsewhere [33]. For unmodified silica films, less intense C–H absorption peak at 2,950 cm−1 is observed while at 1,600 and 3,400 cm−1 more broad O–H peaks are observed indicating the hydrophilic nature of the silica film. It can be seen from the FTIR spectra that with an increase in percentage of PTMS, the intensity of the C–H absorption peaks and the Si–C absorption peak increased, clearly indicating the replacement of surface H from the Si–OH groups by the nonhydrolyzable ≡Si–phenyl group and hence an increase in the hydrophobicity of the films.
3.5 Surface morphological and thermal stability studies
The morphological study of pure silica film and PTMS modified (15 wt%) silica film was carried out using SEM micrographs. Figure 5a, b shows the SEM images of unmodified and PTMS modified silica coatings at ×5,000 magnification. The microstructure of the unmodified silica film shows the crack like porous surface morphology, because thickness is low. As we increase the percentage of PTMS thickness of silica film goes on increasing and the silica film modified with PTMS shows, more porous nature. This porous morphology tends to trap the air in the pores of the film contributing to the easy rolling of water drop off the surface. This strongly implies that the contact model of a water droplet on the PTMS modified silica films follows the Cassie–Baxter’s model.
The Fig. 6 shows TGA-DSC curve for coating material. In such case, TGA-DSC analysis carried out at a rate of 10 °C/min under an oxygen atmosphere up to 1,000 °C. The exothermic peak is observed at 386 °C corresponding to the oxidation of surface –CH3 groups. The PTMS modified coating is hydrophobic up to 386 °C and after this temperature the coating becomes hydrophilic in nature.
4 Conclusions
The TEOS based uniform porous, ultrahydrophobic silica films were obtained using PTMS as a co-precursor by the two-step sol–gel process on a glass substrate. These films are optically transparent, thermally stable, and highly durable against humidity. The highest water contact angle of 133° was obtained for a PTMS modified silica coatings (15 wt%). The hydrophobicity of the as-prepared silica films was enhanced by increasing the roughness of the surface with increasing PTMS weight percentage in silica sol. The FTIR spectra showed an increase in the intensity of Si–C and C–H peaks and decrease in the intensity of O–H peaks with increase in percentage of PTMS, clearly indicating the increased chemical modification of the silica surface by the organic (silyl) groups. By modification of silica sols with PTMS, sol–gel coatings could be prepared on glass substrates at room temperature owing to excellent water repellent properties without any addition of fluorine containing compounds. Therefore, the films could find application as self-cleaning windshields of automobiles, in which a high transmission of visible light is essential.
References
W. Barthlott, C. Neinhuis, Planta 202, 1 (1997)
A. Nakajima, K. Hashimoto, T. Watanabe, Monatsh. Chem. 132, 31 (2001)
H. Tada, H. Nagayama, Langmuir 11(1), 136 (1995)
R. Blossey, Nat. Mater. 2, 301 (2003)
X.J. Feng, L. Jiang, Adv. Mater. 18, 3063 (2006)
J.D. Mackenzie, E.P. Bescher, J. Sol–Gel. Sci. Technol. 19, 23 (2000)
M. Guglielmi, J. Sol–Gel. Sci. Technol. 8, 443 (1997)
H.M. Shang, Y. Wang, S.J. Limmer, T.P. Chou, K. Takahashi, G.Z. Cao, Thin Solid Films 472, 37 (2005)
G. Gu, H. Dang, Z. Zhang, Z. Wu, Appl. Phys. A 83, 131 (2006)
M.M. Viana, T.D.S. Mohallem, G.L.T. Nascimento, N.D.S. Mohallem, Brazilian J. Phys. 36, 1081 (2006)
S.D. Bhagat, Y.-H. Kim, Y.-S. Ahn, Appl. Surf. Sci. 253, 2217 (2006)
Y. Xiu, L. Zhu, D. Hess, C.P. Wong, Electronic Packaging Technology Conference 2005, in Proceedings of 7th Volume 2, 2005, p. 8
K. Tadanaga, N. Katata, T. Minami, J. Am. Ceram. Soc. 80(4), 1040 (1997)
A. Venkateswara Rao, D. Haranath, Microporous Mesoporous Mater. 30, 267 (1999)
H. Yokogawa, M. Yokoyama, J. Non-Cryst, Solids 186, 23 (1995)
S.S. Latthe, H. Hirashima, A.V. Rao, Smart Mater. Struct. 18, 095017 (2009)
W.A. Daoud, J.H. Xin, X. Tao, J. Am. Ceram. Soc. 87(9), 1782 (2004)
S.S. Latthe, D.Y. Nadargi, A.V. Rao, J. Appl. Surf. Sci. 255, 3600 (2009)
H.M. Shang, Y. Wang, K. Takahashi, G.Z. Cao, D. Li, Y.N. Xia, J. Mater. Sci. 40, 3587 (2005)
N. Khummalai, V. Boonamnuayvitaya, J. Biosci. Bioeng. 99, 277 (2005)
Y. Liu, X. Chen, J.H. Xin, Nanotechnology 17, 3259 (2006)
S. Smitha, P. Shajesh, P. Mukundan, T.D.R. Nair, K.G.K. Warrier, Colloids Surf. B Biointerfaces 55, 38 (2007)
B.R. Kim, J.W. Kang, K.Y. Lee, J.M. Son, M.J. Ko, J. Mater. Sci. 42, 4591 (2007)
D. Boschel, H. Roggendorf, J. Sol–Gel. Sci. Technol. 25, 191 (2002)
A.V. Rao, G.M. Pajonk, J. Non-Cryst, Solids 285, 202 (2001)
A.V. Rao, M.M. Kulkarni, J. Mater. Res. Bull. 37, 1667 (2002)
V.G. Parale, D.B. Mahadik, S.A. Mahadik, M.S. Kavale, P.B. Wagh, S.C. Gupta, A.V. Rao, Ceramics Inter. (2012). http://dx.doi.org/10.1016/j.ceramint.2012.05.079
A.B. Gurav, S.S. Latthe, C. Kappenstein, S.K. Mukharjee, A.V. Rao, R.S. Vhatkar, J. Porous Mater. 18(3), 361 (2011)
M.S. Kavale, D.B. Mahadik, V.G. Parale, P.B. Wagh, S.C. Gupta, A.V. Rao, H.C. Barshilia, Appl. Surf. Sci. 258, 158 (2011)
T.M. Parrill, J. Mater. Res. 7, 2230 (1992)
A.V. Rao, R.R. Kalesh, G.M. Pajonk, J. Mater. Sci. 38, 4407 (2003)
J.K. Hong, H.R. Kim, H.H. Park, Thin Solid Films 332, 449 (1998)
N.D. Hegde, H. Hirashima, A.V. Rao, J. Porous Mater. 14, 165 (2007)
Acknowledgments
The authors are highly thankful to the Board of Research in Nuclear Sciences (BRNS) and Department of Atomic Energy (DAE), Mumbai, India for funding this work under major research project No. 2008/37/47/BRNS/2502 dated 28/01/2009. The authors D. B. Mahadik and M. S. Kavale are highly grateful to the DAE-BRNS Mumbai for the Senior and Junior Research Fellowship. The author V. G. Parale is highly thankful to Department of Physics, Shivaji University, Kolhapur, for the fellowship as research assistant under DST, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parale, V.G., Mahadik, D.B., Kavale, M.S. et al. Sol–gel preparation of PTMS modified hydrophobic and transparent silica coatings. J Porous Mater 20, 733–739 (2013). https://doi.org/10.1007/s10934-012-9648-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-012-9648-0