Abstract
In this study, the synthesis of poly(4-vinylpyridine) (P4VP) supported on mesoporous carbon (CMK-3) by in situ polymerization of 4-vinylpyridine in the presence of CMK-3 has been investigated. The structural properties of the P4VP/CMK-3 were investigated by FT-IR, XRD, BET, TGA, SEM and TEM techniques. The catalytic activity of this new heterogeneous basic catalyst was tested for Knoevenagel reaction. Excellent yields at room temperature in aqueous media and solvent-free conditions were obtained. The catalytic activity of this purely organic hybrid catalyst was compared with P4VP/SBA-15 to clarify the advantages of mesoporous carbon on mesoporous silica as support. The results showed that the stability of P4VP/CMK-3 was excellent and could be reused 10 times without much loss of activity in Knoevenagel reaction. Surprisingly, the composite prepared by mesoporous carbon showed much higher activity than that of P4VP/SBA-15. This unique result opens new perspectives for application of mesoporous carbons as structurally defined hydrophobic catalyst support in catalytic reactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There is a vast interest in the use of heterogeneous catalytic processes as a substitute for homogeneous catalysis, which suffers from several environmental drawbacks [1]. Recently, heterogenization of homogeneous catalysts has become an important strategy for the preparation of supported catalysts which retain the active catalytic sites of the homogeneous analogue and provide at the same time advantages of easy separation and recycling as well as reusability of the catalysts [1, 2]. Development of heterogeneous catalysts for C–C bond formation in organic synthesis following green approach always provides a challenge. One such example is the versatile Knoevenagel condensation reaction [3, 4], a useful method for the synthesis of α,β-unsaturated carbonyl compounds.
After the successful synthesis of mesoporous silica crystals, synthesis of mesoporous carbon crystals was developed by Ryoo et al. [5] using silica mesoporous crystals as hard templates. This new group of materials designated as CMK-n (Carbon Mesostructured by Korea Advanced Institute of Science and Technology) possess several advantages compared to mesoporous silica molecular sieves, i.e. high thermal stability up to 1,600 °C in N2 atmosphere, high stability in strong acids and bases, high mechanical stability, especially electric conductivity and many more due to their interesting properties such as high surface areas, narrow pore size distributions and regular frameworks [6]. Therefore, the mesoporous carbons are of great interest for, e.g. adsorption, catalyst, catalyst supports, host–guest chemistry and offer potential applications such as size and shape-selective adsorption media, chromatography separation systems [7, 8].
The most investigated carbon material of this series is the CMK-3 as it can be seen in a number of publications dealing with the synthesis, characterization and the properties of this material [9–11]. CMK-3 is synthesized by applying the mesoporous silica SBA-15 as a template and sucrose as the carbon source via an impregnation procedure [7, 12]. The pristine CMK-3 and the SBA-15 silica show couple of similarities. Both materials are 2-D hexagonal structured (space group: p6mm) and exhibit micro- and mesopores. The specific surface areas (BET) of the CMK-3 material up to 1,600 m2/g are higher compared to the SBA-15 (BET: 600 m2/g).
Nowadays, mesoporous carbon has been used as a carbon support for catalysts [13]. Carbon-based catalysts have several advantages as solid catalysts because of the high surface area and large porosity as well as hydrophobic surface nature. Because of these advantages, research using carbon as a catalyst has increased [14–18].
In our previous work, the effect of polymer-inorganic hybrid materials as heterogeneous basic catalyst for Knoevenagel condensation reaction using mesoporous silica materials such as SBA-15 was studied [19–21]. Here in, we will introduce a novel heterogeneous polymer-organic hybrid catalyst based on a mesoporous carbon material. Hydrophobic surface nature of CMK-3 is necessary and enables monomers to penetrate into the pore channels of CMK-3, which makes it possible for the formation of poly(4-vinylpyridine) layers inside the pores of CMK-3 via in situ polymerization. Therefore, the main goal of this catalytic synthesis was to introduce a novel and efficient organic composite to expand the use of these types of composites for organic reactions, and compared with our previous works.
2 Experimental method
2.1 Catalyst characterization
The samples were analyzed using FT-IR spectroscopy (using a Perkin Elmer 65 in KBr matrix in the range of 4,000–400 cm−1). The BET specific surface areas and BJH pore size distribution of the samples were determined by adsorption–desorption of nitrogen at liquid nitrogen temperature, using a Series BEL SORP 18. The X-ray powder diffraction (XRD) of the catalyst was carried out on a Bruker D8Advance X-ray diffractometer using nickel filtered Cu Kα radiation at 40 kV and 20 mA. Moreover, the morphology of the catalyst surface was performed by scanning electron microscopy (SEM) (SERON, AIS-2100). The thermal gravimetric analysis (TGA) data were obtained by a Setaram Labsys TG (STA) in a temperature range of 30–650 °C and heating rate of 10 °C/min in N2 atmosphere. Transmission electron microscope (TEM) observations were performed on a philips, Cm-10, HT100KV.
2.2 Catalyst preparation
The employed mesoporous carbon (CMK-3) was synthesized following the method reported by Ryoo [5] using SBA-15 as template.
2.3 Preparation of SBA-15
Mesoporous silica SBA-15 was prepared using Pluronic P123 (EO20PO70EO20) template as a structure directing agent and tetraethylorthosilicate (TEOS) as the silica precursor. In a typical synthesis, Pluronic P123 (2 g) was dissolved at room temperature in H3PO4 (4.2 mL, 85%) and deionized water (75.4 mL), then TEOS (4.6 mL) was added to the solution and synthesis was carried out by stirring at 35 °C for 24 h in sealed Teflon breakers, and it was subsequently placed at 100 °C for 24 h. Then, the solution was filtered, washed with deionized water, and finally dried at 95 °C for 12 h in air. Template removal was performed by calcination in air using two successive steps; first heating at 250 °C for 3 h and then at 550 °C for 4 h.
2.4 Preparation of CMK-3
Mesoporous carbon CMK-3 was prepared using mesoporous silica SBA-15 as template and sucrose as the carbon precursor. 1.0 g SBA-15 was added to 5 mL aqueous solution containing 1.25 g (3.65 mmol) sucrose and 0.14 g (1.42 mmol) of H2SO4 (98%). The resulting mixture was heated in an oven at 100 °C for 6 h and then 160 °C for another 6 h. In order to obtain fully polymerized sucrose inside the pores of the SBA-15 template, 5 mL aqueous solution containing 0.8 g (2.33 mmol) sucrose and 0.09 g (0.917 mmol) of H2SO4 were added again, and the mixture was subjected to the thermal treatment described above one more time. Then, it was carbonized under N2 gas flow at 900 °C for 6 h with a heating rate of 5 °C min−1. Finally, the resulting solid was washed with 1 M NaOH solution (50 vol. % ethanol-50 vol. % H2O) twice to remove the silica template, filtered, washed with ethanol until pH = 7, and dried at 100 °C for 4 h. Thus, mesoporous carbon CMK-3 was obtained.
2.5 Preparation of P4VP/CMK-3
4-Vinyl pyridine (4VP) (0.5 mL, 4.6 mmol), and CMK-3 (0.5 g) in 7 mL tetrahydrofuran (THF) were placed in a round bottom flask. Benzoyl peroxide (0.034 g, 3% mol) was added and the mixture was heated to 65–70 °C for 5 h while being stirred. The resulting white fine powder composite was collected by filtration, washed several times with THF, and finally dried at 60 °C under reduced pressure.
P4VP/SBA-15 was prepared with the same procedure in order to compare the effect of mesoporous materials on the catalytic activity of the catalysts.
Basic content of P4VP/CMK-3 and P4VP/SBA-15 were estimated by back-titration using NaOH [22, 23]. 5 mL of HCl (0.2 N), was added to 0.05 g of the composites and stirred for 30 min. The catalysts were removed and washed successively with deionized water. The excess amount of HCl was titrated with NaOH (0.1 N) in the presence of phenolphthalein as an indicator. Basic sites contents of P4VP/CMK-3 and P4VP/SBA-15 were 7.35 and 7.24 mmol g−1, respectively.
2.6 General procedure for Knoevenagel condensation
In the typical procedure for Knoevenagel condensation, a mixture of a benzaldehyde (1 mmol), malononitrile (1 mmol, 0.066 g), and catalyst (0.12 g, P4VP/CMK-3) was ground at room temperature (25 °C) in a glass mortar and pestle. The progress of reaction was monitored by Thin Layer Chromatography (TLC) using n-hexane/ethylacetate (16:4) as eluent. After completion of the reaction (monitored by TLC), for the reaction work-up, 5 mL of hot ethanol was added to the mixture and the catalyst was removed from the mixture by filtration. The filtrate was cooled to 10 °C, the precipitated product was separated and recrystallized from ethanol to afford pure product. The products were identified with 1H NMR, 13C NMR and FT-IR spectroscopy techniques. Additionally, by using the reused catalyst, the selectivity (100% to α,β-unsaturated carbonyl compounds) was not changed.
3 Results and discussion
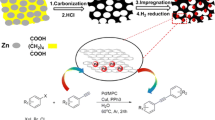
Mesoporous carbon CMK-3 has a (2-D) hexagonal (p6mm) structure [24]. The encapsulation of P4VP chains in the 2-D interconnected pore channels of CMK-3 can be explained by Scheme 1. As can be seen in the Scheme 1, interaction of P4VP with CMK-3 surface is a van der waals-london interaction between alkyl chain of P4VP with hydrophobe surface of CMK-3.
3.1 Catalyst characterization
Figure 1 shows the powder XRD patterns of the carbon CMK-3 (a) and P4VP/CMK-3 (b). It can be seen that CMK-3 shows three diffraction peaks in the 2Ө range of 0.5°–2°, which can be assigned to (100), (110) and (200) reflections. The result indicates that CMK-3 has an ordered two dimensional (2D) hexagonal structure [12]. The P4VP/CMK-3 sample shows the same pattern, indicating that the structure of the CMK-3 (100) was retained after the support of the surface of the CMK-3 with P4VP (Fig. 1b). However, the intensity of the characteristic reflection peaks of the P4VP/CMK-3 (2Ө = 0.6–5) sample is found to be reduced (Fig. 1b). This may be attributed to the symmetry destroyed by the hybridization of CMK-3 which is also found in the ordered mesoporous carbon loading with guest matter [25]. In addition, composite contains much less CMK-3 due to the dilution of the carbon material by P4VP; therefore, this dilution can also account for a decrease in the peak intensity. However, the XRD patterns of CMK-3 and P4VP/CMK-3 are almost similar to that of SBA-15, indicating that CMK-3 is a true replica of the mesoporous silica SBA-15 and the hybridization process almost does not damage the structure of CMK-3.
The FT-IR spectra of CMK-3 (a) and P4VP/CMK-3 (b) are shown in Fig. 2. A broad band at around 3,400–3,450 cm−1 was observed in both samples. It was mainly caused by the O–H stretching vibration of the small adsorbed water molecules. In the FT-IR spectrum of CMK-3 (Fig. 2a), there aren’t any signals of the organic bonds, resulting from the complete carbonization of sucrose (Fig. 2a). In the FT-IR spectrum of P4VP/CMK-3 (Fig. 4b), the appearance of the new bands at 1,604, 1,526 and 1,420 cm−1 is due to the characteristic absorptions of pyridine ring [26]. The band at 1,604 cm−1 corresponds to the stretching vibration absorption of C–N bond and the bands at around 1,562 and 1,420 cm−1 are attributed to the stretching vibrations absorption of C = C bond. Moreover, the presence of peaks at around 2,800–3,100 cm−1 corresponds to the aromatic and aliphatic C–H stretching in P4VP/CMK-3. The appearance of the above bands shows that P4VP has been attached to the surface of CMK-3 and P4VP/CMK-3 composite has been obtained.
The BET specific surface areas and the pore sizes of CMK-3 and P4VP/CMK-3 were calculated using Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) methods (Table 1). Both of the samples exhibit a type IV adsorption isotherm with an H1 hysteresis loop with capillary condensation at relative pressure around 0.3–0.7 (Fig. 3). The corresponding BJH pore size distribution curves for CMK-3 and P4VP/CMK-3 materials are shown in Fig. 3. It is known that calcined CMK-3 has a high BET surface area, a large pore volume and pore size, indicative of its potential application as a host in organic materials (Table 1). After hybridization with P4VP through in situ polymerization, P4VP/CMK-3 exhibits a smaller specific surface area, pore size and pore volume in comparison to those of pure CMK-3, which might be due to the presence of polymer on the surface of CMK-3. Although these textural properties are smaller than those found for mesoporous carbon CMK-3, P4VP/CMK-3 still has a mesoporous form. Therefore, it is suitable to act as a basic catalyst.
TGA curves of bulk P4VP and P4VP/CMK-3 composite are presented in Fig. 4 under N2 atmosphere. The weight loss (around 62%, w/w) of P4VP begins at 210 °C because of thermo degradation of P4VP polymer chains, and the degradation ends at 500 °C (Fig. 4a). Whereas for P4VP/CMK-3, the weight loss (around 9%, w/w) begins at 360 °C that is related to the degradation of the polymer chains and the degradation ends at 460 °C (Fig. 4b). Obviously, the hybrid P4VP/CMK-3 shows higher thermal stability and a slower degradation rate than P4VP. Therefore, after hybridization, the thermal stability is enhanced greatly, which is very important for the catalyst application.
The surface morphologies of the obtained CMK-3 and P4VP/CMK-3 were observed by SEM, as shown in Fig. 5. Both the SEM images of the mesoporous carbon CMK-3 and P4VP/CMK-3 showed rod-like morphology (Fig. 5a, b). Virtually no significant difference in surface morphology between CMK-3 and P4VP/CMK-3 composite was observed, which indicated that the most of polymerization of 4-vinylpyridine occurred in the pores of CMK-3, which was also supported by the decrease in surface area, pore diameter and pore volume as shown in Table 1. In addition, small angle XRD analysis and TEM images confirmed that CMK-3 carbons remained the ordered channel after hybridization with P4VP.
The TEM micrographs of P4VP/CMK-3 were depicted in Fig. 6. Although the images were smeared and dark after the encapsulation of P4VP chains inside the mesochannels (Fig. 6), the ordered hexagonal p6 mm mesostructure of CMK-3 was retained and no damage of the periodic structure of the carbon framework was observed.
3.2 Catalytic activity
This novel composite was synthesized and fully characterized with different methods. The main goal of this catalytic synthesis was to compare this polymer-organic composite (P4VP/CMK-3) with polymer-inorganic composite (P4VP/SBA-15) and to expand the use of these types of composites for organic reactions. This composite was prepared by a very simple method without using any pre-modification on CMK-3 (pre-modification of CMK-3 involves complicated synthesis and purification method).
We have chosen CMK-3 to support the P4VP because the structure of CMK-3 has some specific characteristics compared to mesoporous SBA-15:
-
High thermal stability up to 1,600 °C in N2 atmosphere, high stability in strong acids and bases, high mechanical stability, especially electric conductivity and many more due to their interesting properties such as high surface areas, narrow pore size distributions and regular frameworks [6].
-
The specific surface areas (BET) of the CMK-3 material up to 1,600 m2/g are higher compared to the SBA-15.
-
Hydrophobic surface nature of CMK-3 compared to SBA-15 will allow that the organic reactants have a higher tendency to be adsorbed on the surface of P4VP/CMK-3 and consequently the yield will be increased.
In order to investigate the basic properties of this catalyst, Knoevenagel condensation was chosen as a typical reaction. Reactions were carried out at room temperature under solvent-free conditions with 0.12 g of P4VP/CMK-3 as catalyst.
The catalytic activity of P4VP/CMK-3 was investigated for the Knoevenagel condensation by employing various aromatic and hetero aromatic aldehydes with malononitrile as an active methylene compound. All reactions were almost completed at room temperature (Table 2) [27–33]. These reactions produced the corresponding electrophilic alkenes in high yield with 100% selectivity to the condensation products. In addition, it should be mentioned that in the absence of the catalyst, the product was not observed after 1 h.
The catalyst reuse and stability were checked using Knoevenagel condensation of benzaldehyde with malononitrile at room temperature under solvent-free conditions. The catalyst was separated from the reaction mixture after each experiment by simple filtration, washed with diethylether and acetone and dried carefully before using it in the subsequent run. The results showed that this catalyst could be reused without any modification, 10 times and no significant loss of activity/selectivity performance was observed. It should be mentioned that there was very low catalyst leaching during the reaction and the catalyst exhibited high stability even after 10 recycles (Table 3). Compared to our previous work on composites prepared, using SBA-15 [19–21], P4VP/CMK-3 showed higher reusability. It can be related to the hydrophobic surface nature of CMK-3 that polymer chains will be more inclined to adsorb on the surface of CMK-3. Therefore, the polymer leaching will be very low and reusability will be very high.
The catalytic activity of P4VP/CMK-3 in Knoevenagel reaction was compared with P4VP/SBA-15 to investigate the effect of carbon mesoporous material on the activity and stability of the catalyst. The results are presented in Table 4. As it can be seen, P4VP/CMK-3 shows higher activity in Knoevenagel reaction at shorter time. Since, the basic sites content of P4VP/CMK-3 (7.35 mmol g−1) is almost similar to the P4VP/SBA-15.
(7.24 mmol g−1), so, it can be related to the hydrophobic nature of P4VP/CMK-3 compared to P4VP/SBA-15. It should be mentioned that CMK-3 is completely hydrophobe and SBA-15 is completely hydrophile. Therefore, the organic reactants have a higher tendency to be adsorbed on the surface of P4VP/CMK-3 and consequently the yield will be higher in the case of using P4VP/CMK-3 as catalyst.
4 Conclusion
In this summary, mesoporous carbon CMK-3, which can be replicated from mesoporous silica SBA-15 and whose surface is hydrophobic, was prepared and used as an ideal support for poly(4-vinyl pyridine) (P4VP). The catalytic activity of this novel hybrid composite was tested for Knoevenagel condensation under solvent-free conditions. This new heterogeneous catalyst is a practical alternative for application as an efficient catalyst in organic syntheses in view of the following advantages: high catalytic activity under very mild conditions, easy separation of the catalyst by simple filtration, high surface area, tunable porosity and hydrophobic surface, use of low toxicity, and chemical stability catalysts, involvement of environmentally benign processes and, reusability of catalyst. Comparison of catalytic activity of the composite was done with SBA-15 supported catalyst. A comparison between SBA-15 and CMK-3 supported catalysts showed superior activities and reusability of CMK-3 supported catalyst. This higher activity and reusability is attributable to high thermal stability and high surface area of CMK-3 compared to SBA-15. In addition, hydrophobic surface nature will allow that the organic reactants have a higher tendency to be adsorbed on the surface of P4VP/CMK-3 and consequently the yield will be increased. These unique results open new perspectives for application of these types of hybrid catalysts.
References
M.H. Valkenberg, W.F. Hoelderich, Catal. Rev. 44, 321 (2002)
L. Martins, D. Cardoso, Quim. Nova 29, 358 (2006)
K.M. Parida, D. Rath, J. Mol, A. Catal, Chem. 310, 93 (2009)
L. Martins, W. Holderich, D. Cardoso, J. Catal. 258, 14 (2008)
T.W. Kim, R. Ryoo, K.P. Gierszal, M. Jaroniec, L.A. Solovyov, Y. Sakamotod, O. Terasaki, J. Mater. Chem. 15, 1560 (2005)
H. Darmstadt, C. Roy, S. Kaliaguine, S.J. Choi, R. Ryoo, Carbon 40, 2673 (2002)
R. Ryoo, S.H. Joo, M. Kruk, M. Jaroniec, Adv. Mater. 13, 677 (2001)
S.H. Joo, S.J. Choi, I. Oh, J. Kwak, Z. Liu, O. Terasaki, R. Ryoo, Nature 412, 169 (2001)
R. Ryoo, S.H. Joo, Stud. Surf. Sci. Catal. 148, 241 (2004)
H. Darmstadt, C. Roy, S. Kaliaguine, S.H. Joo, R. Ryoo, Micropor. Mesopor. Mater. 60, 139 (2003)
J. Roggenbuck, M. Tiemann, J. Am. Chem. Soc. 127, 1096 (2005)
S. Jun, S.H. Joo, R. Ryoo, M. Kruk, M. Jaroniec, Z. Liu, T. Ohsuna, O. Terasaki, J. Am. Chem. Soc. 122, 10712 (2000)
E. Antolini, Appl. Catal. B. Environ. 88, 1 (2009)
X. Wang, R. Liu, M.M. Waje, Z. Chen, Y. Yan, K.N. Bozhilov, P. Feng, Chem. Mater. 19, 2395 (2007)
R. Liu, X. Wang, X. Zhao, P. Feng, Carbon 46, 1664 (2008)
S. Tanaka, N. Nishiyama, Y. Egashira, K. Ueyama, Chem. Commun. 16, 2125 (2005)
R. Xing, Y. Liu, Y. Wang, L. Chen, H. Wu, Y. Jiang, M. He, P. Wu, Micropor. Mesopor. Mater. 105, 41 (2007)
L. Peng, A. Philippaerts, X. Ke, J. Van Noyen, F. De Clippel, G. Van Tendeloo, P.A. Jacobs, B.F. Sels, Catal. Today 150, 140 (2010)
R.J. Kalbasi, M. Kolahdoozan, A.R. Massah, K. Shahabian, Bull. Korean Chem. Soc. 31, 2618 (2010)
R.J. Kalbasi, M. Kolahdoozan, M. Rezaei, Mater. Chem. Phys. 125, 784 (2011)
R.J. Kalbasi, M. Kolahdoozan, K. Shahabian, F. Zamani, Catal. Commun. 11, 1109 (2010)
J. Alauzun, A. Mehdi, C. Reye, J.P. Corriu, J. Am. Chem. Soc. 128, 8718 (2006)
Y. Shao, J. Guan, S. Wu, H. Liu, B. Liu, Q. Kan, Micropor. Mesopor. Mater. 128, 120 (2010)
M. Yang, Q. Gao, Micropor. Mesopor. Mater. 143, 230 (2011)
H.I. Lee, Y. Jung, S. Kim, J.A. Yoon, J.H. Kim, J.S. Hwang, M.H. Yun, J.W. Yeon, C.S. Hong, J.M. Kim, Carbon 47, 1043 (2009)
R.J. Kalbasi, N. Mosaddegh, Catal. Commun. 12, 1231 (2011)
M. Hosseini-Sarvari, H. Sharghi, S. Etemad, Chin. J. Chem. 25, 1563 (2007)
B.M. Reddy, M.K. Patil, K.N. Rao, G.K. Reddy, J. Mol. Catal. A Chem. 258, 302 (2006)
G. Postole, B. Chowdhury, B. Karmakar, K. Pinki, J. Banerji, A. Auroux, J. Catal. 269, 110 (2010)
S.D. Sharma, P. Gogoi, D. Konwar, Indian J. Chem. 46, 1672 (2007)
M. Gupta, R. Gupta, M. Anand, Beilstein J. Org. Chem. 5, 68 (2009)
A. Kumar, M. Dewan, A. Saxena, A. De, S. Mozumdar, Catal. Commun. 11, 679 (2010)
M.B. Gawande, R.V. Jayaram, Catal. Commun. 7, 931 (2006)
Acknowledgments
The support by Islamic Azad University, Shahreza Branch (IAUSH) Research Council and Center of Excellence in Chemistry is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalbasi, R.J., Mosaddegh, N. Characterization and catalytic performance of poly(4-vinylpyridine) supported on mesoporous carbon: comparison with poly(4-vinylpyridine) supported on mesoporous silica. J Porous Mater 19, 557–565 (2012). https://doi.org/10.1007/s10934-011-9505-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-011-9505-6