Abstract
Carbon aerogels with densities ranging from 0.182 to 0.052 g/cm3, pore sizes ranging from 88 to 227 nm, and particle diameters ranging from 20 to 13 nm were prepared. Thermal conductivity measurements by laser flash method indicate that the lowest thermal conductivity can be obtained at a density of 0.066 g/cm3, in the temperature range from 100 to 300 °C in air. The lowest thermal conductivity is 0.0263 W/m K at 200 °C. The characteristic density, at which the lowest thermal conductivity can be obtained, is temperature dependent. At a higher temperature, a higher density carbon aerogel will be more efficient in the reduction of the total conductivity by reducing the radiative conductivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Because of their excellent thermal insulation properties, nanostructured aerogels are promising materials for thermal insulators [1]. In all kinds of aerogels with various chemical components that have been explored to date, carbon aerogels (CAs) [2–5] have the highest thermal stability and can maintain their mesoporous nanostructure at a high temperature even exceed 2,000 °C in an inert atmosphere [6]. For comparison, the surface area of SiO2–Al2O3 hybrid aerogels decreased sharply at above 1,300 °C [7]. In addition, the specific extinction coefficients of the CAs are much higher than those of other aerogels, e.g. 190 m2/kg for CAs [8] and 20 m2/kg for pure silica aerogels [1], thus the radiative thermal conduction transmitted through the CAs is very low [8]. These merits mentioned above make CAs to be the promising candidates for extreme applications such as the high temperature thermal insulations [9] for advanced industrial devices, space vehicles landing on other planets, and hypersonic vehicles, in which the heat flux and the temperature are extremely high and the weight and the thickness of the thermal insulation are strictly limited.
However, the thermal conductivities of aerogels are affected significantly by their densities. The smallest measured thermal conductivities were 0.012 W/m K at a density of 0.157 g/cm3 for organic aerogels, and 0.013 W/m K at a density of 0.120 g/cm3 for SiO2 aerogels [1]. Lu et al. [10] had determined the thermal conductivities of CAs with densities ranging from 0.082 to 0.637 g/cm3 at room temperature, and concluded that at a density of 0.082 g/cm3, which is the lowest density limit in their experiments, the smallest thermal conductivity can be obtained. There was no experimental data evidence for a lower density CA. The lowest densities of CAs that other authors consequently prepared for thermal conductivity measurements were 0.381 [11], 0.301 [8], 0.240 [12], and 0.225 g/cm3 [9], respectively. However, the solid thermal conductivity (λ s) of nonporous carbon materials is higher than those of the nonporous organic or the oxide bulk materials, because of the strong bonding of the light carbon atoms resulting in a large phonon contribution to the thermal conductivity [13]. We surmise that the density, at which the smallest thermal conductivity can be obtained for CAs, would be lower than 0.082 g/cm3 (in Ref. [10]). In order to verify our assumption, herein we introduce the fabrication of CAs with densities as low as 0.052 g/cm3, and the first characterization of their thermal conductivities in the temperature range from 100 to 300 °C in air by laser flash method.
2 Experimental section
2.1 Preparation
Organic aerogels were synthesized by using resorcinol (R) and formaldehyde (F) as precursors, deionised water (W) as solvent, and sodium carbonate (C) as catalyst [14]. Five series of RF aerogels with various R/C and W/R molar ratios were prepared. The F/R molar ratio was two, the R/C and the W/R molar ratios were shown in Table 1. After mixing the raw materials in together and magnetically stirring for 30 min, the RF solutions were sealed in glass test tubes with 18 mm diameter, and kept at room temperature for 1 day, then gelled in 50 °C water bath for 1 day, and cured at 80 °C for 3 days. The obtained aquagel columns were soaked in ethanol for 3 days, then exchanged with petroleum ether (boiling point: 30–60 °C) for another 3 days, the ethanol and the petroleum ether were exchanged one time for each day. The gel columns were placed in an autoclave (volume: 3 L) and supercritically dried with petroleum ether (240 °C, 7 MPa). The obtained RF aerogel columns were carbonized at 1,000 °C for 1 h in a tube furnace with flowing nitrogen (100 mL/min) to form CAs.
2.2 Characterization
The bulk densities (ρ) of the CAs were obtained by measuring the volumes and the weights of the aerogel columns. Nitrogen sorption measurements were performed to obtain pore properties such as the BET-specific surface area (S BET) and the external specific surface area (S ext, i.e. surface area including mesopore and macropore) with a QuadraSorb SI (Quantachrome, USA) analyzer. S ext was calculated by t-plot method. The mean pore size (D pore) was calculated by Eq. 1 [9, 12], and mean particle diameter (d particle) by Eq. 2 [9, 12].
S ext is the external surface area. ρ is the bulk density of the aerogel samples, and ρ s is the intrinsic density of the backbone particles formed the the aerogels, ρ s = 1,400 kg/m3 [9, 12].
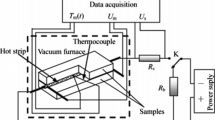
The morphologies of the CAs were investigated by Hitachi S4800 (Japan) scanning electron microscope (SEM) after coating with a thin platinum layer. The CA columns (Online Resource Fig. s1) were carefully cut into disk-shape with diameters of 12.7 mm and thicknesses of approximately 1.3 mm (as shown in Fig. 1) by an automatic diamond saw device with a low moving speed of 1 mm/min, and the thermal diffusivities of the disk-shape samples were determined by the laser flash method in air, using a Netzsch LFA 447 (German) apparatus. We found that, in the relatively low temperature range from 25 to 70 °C, the thermal diffusivities were much higher than the values in the temperature range above 100 °C, especially for the lower density CA (Online Resource Fig. s2). The reason may be that the adsorption of water and gas molecules enhanced the gas thermal transport. Therefore, to minimize the effects of the adsorbed water and other gas molecules, the measurement were performed under the temperature range from 100 to 300 °C. The Cowan method was selected to correct the diffusivity measurements. The total thermal conductivity λ t(T) was obtained according to Eq. 3 from the thermal diffusivity α(T), with the specific heat capacity c p(T) and the density ρ [9].
T is the temperature at which the measurement was performed. Literature values of c p(T) were used [9]. Standard deviation error for the thermal conductivity measurement was estimated to be 15%.
3 Results and discussion
3.1 Textural properties of the aerogels
Table 1 shows the linear shrinkage and the textural properties of the RF aerogels and the CAs during the synthesizing process. During the supercritical drying process, only a relatively low linear shrinkage (2–6.7%) occurs, the linear shrinkage decreases mildly with increasing the W/R value, due to the larger pore size and thus induces lower capillary pressure in the higher W/R value gels. Relatively large linear shrinkage (24–25.9%) occurs during the carbonization process, regardless of the R/C values or the W/R values. With the designed variation of W/R values from 44 to 177, the density of the CAs decreases from 0.182 to 0.052 g/cm3, which is exceeded the lowest density limit (0.082 g/cm3) in Ref. [10]. The S BET and the S ext of the CAs, obtained by nitrogen sorption analysis (the nitrogen sorption isotherms are shown in Fig. 2), are not changed obviously with the variation of R/C and W/R values. The d particle, affected mostly by the R/C value [2, 4], decreases mildly from 20 to 13 nm, with the decreasing of R/C values from 546 to 357. The D pore, affected mostly by the W/R value [2, 4], increases from 88 to 227 nm, with the increasing of W/R values from 44 to 177. The D pore is comparable to the mean free path of air (≈70 nm at room temperature) [1], it indicates that the Knudsen number (Kn) is of the order of 1 and the thermal transport is semi-ballistic, which is important in nanoscale heat transfer [15].
Figure 3 shows the SEM images of the CAs with different densities. The CAs are three dimension network nanostructures formed by pearl-necklace chains. The structure of the CAs with lower density is looser and the pore diameter is larger than the one with higher density, but the d particle which formed the network is not changed. The d particle is approximately 20 nm, this size is consistent with the data in Table 1. The porosity decreases as the density increases (W/R value decreases). This indicates that the gaseous thermal conductivity will decrease as the W/R value decreases.
3.2 Thermal conductivities of the aerogels
Figure 4 shows the thermal diffusivities of the CAs with various densities at the temperatures ranging from 100 to 300 °C in air. The thermal diffusivities measured at the relatively low temperature range from 25 to 70 °C are much higher than the values measured at the temperature range above 100 °C, especially for the lower density CA (Online Resource Fig. s2). This phenomena may be attributed to the adsorption of water and gas molecules on the nanostructured network of the CAs. This adsorption can be classified into two mechanisms: chemisorption and physisorption. The chemisorption of gas molecules may decrease the surface energy of the solid backbone of the CA and thus decrease the solid conductivity [16, 17]. But the chemisorption can not be vented out when it is heated up to a relatively low temperature (100 °C), thus the higher thermal diffusivity in the relatively low temperature (25–70 °C) may be attributed to the physisorption, which is desorbed when it is heated up to 100 °C. The physisorption of gas molecules in the nanopores of the CA will increase the possibilities of collisions between the gas molecules and thus increase the gaseous conductivity [18]. For the lower density CA, the pore size is larger and thus the gaseous conductivity is higher than that of the CA with higher density. Therefore, the physisorption of gas molecules shows more significant effects on the thermal diffusivity in a low density CA than in a high density one. To minimize the effects of physisorption, only the thermal diffusivities in the temperature range from 100 to 300 °C are used to calculate the thermal conductivities (Fig. 4). For the higher density (0.097–0.182 g/cm3) CAs, the thermal diffusivities is almost constant with increasing the temperature. While for the relatively lower density (0.052–0.066 g/cm3) CAs, the thermal diffusivities increase with increasing the temperature, especially for the CAs with the lowest density (0.052 g/cm3). At a certain temperature, the thermal diffusivities increase with decreasing the CA densities.
Figure 5 shows the λ t(T) of CAs with various densities versus measuring temperature in air. The λ t(T) of CA with the highest density (0.182 g/cm3) is the highest in the whole temperature range, because of its relatively high solid conductivity λ s(T). For the CA with the lowest density (0.052 g/cm3), the λ t(T) increases much more rapidly with temperature, than those of other CAs. This indicates that the radiative conductivity λ r(T) contributes a large component in the λ t(T). Because the λ r(T) is proportional to the reciprocal of density (1/ρ), the lower the density, the higher the λ r(T).
The λ r(T) of the aerogel can be described by [1, 12]
where n is the mean index of refraction, n = 1.1 for carbon aerogel [12]. σ B is the Stefan-Boltzmann constant. e(T) is the specific extinction coefficient. Because the d particle is not changed too much for CAs with various densities in this present work, the e(T) is estimated to be the same. Thus, at a certain temperature T, the λ r(T) can be simplified as R(T)/ρ. R(T) is the fit parameter.
The λ s(T) of low density aerogel can be written as [1, 8]
where C(T) is the fit parameter.
The gaseous conductivity λ g(T) can be described as [1, 12]
where П is the porosity calculated by
where ρ s is the density of the carbon particles, ρ s = 1,400 kg/m3 [12]. λ g0(T) is the gaseous conductivity of a free gas. In air, λ s0(300 °C) is approximately 0.045 W/m K. β ≈ 2 for air in aerogel [1]. The Knudsen number is
where l g(T) describes the mean free path of the gas particles, and is a function of the temperature according to Eq. 9 [12]. l g(300 °C) is approximately 110 nm for air.
To calculate the Kn for CAs with various densities, the D pore is obtained by fitting the experimental D pore data with an exponential function as
A and t is the fit parameters. The fitting results are shown in Fig. 6.
Pore sizes of the CAs versus their densities. The solid line is the fitting result of the experimental data (open diamond) with Eq. 10
The total thermal conductivity of CA thus becomes
The relationships between the λ t(T) and the densities of the CAs and their fitting lines by Eq. 11 are shown in Fig. 7. With the decreasing of the density from 0.182 to 0.066 g/cm3, the λ t(T) decreases significantly and shows a strong density-dependence relationship. When the density continues to decrease from 0.066 to 0.052 g/cm3, the λ t(T) of the CAs increases, especially for the values at 300 °C. This gives a concrete evidence for that the lowest λ t(T) can be obtained at a density of 0.066 g/cm3. Because when the density is lower than 0.066 g/cm3, the λ r(T) will increase rapidly and contributes a large component into the λ t(T). The smallest thermal conductivity is 0.0263 W/m K at 200 °C. This value is lower than the value (0.029 W/m K) for a CA with a density of 0.082 g/cm3 in air at room temperature in Ref. [10]. We consider that the reason may be the lower density in our CA and the prevention of the effects of adsorbed gas molecules in our measurements. From the fitting line in Fig. 7 for various temperatures, it can be seen that the characteristic density, at which the smallest thermal conductivity can be obtained, increases when the temperature increases. Because the λ r(T) is proportional to three power of temperature (T 3), as the temperature increases, the λ r(T) increases more rapidly than the λ s(T) or the λ g(T). Thus a higher density will be more efficient in reducing the λ r(T) at high temperature and results in reduction in the λ t(T).
Total thermal conductivities of the CAs versus their densities under various temperatures in air. The error bars indicate the standard error of twelve experimental results of three specimens, with four flash pulses for each specimen. The solid lines are the fitting results of the experimental data with Eq. 11
4 Conclusion
We prepared CAs with density as low as 0.052 g/cm3. Thermal conductivity measurements indicate that the lowest thermal conductivity can be obtained at a density of 0.066 g/cm3 for CAs in the temperature range from 100 to 300 °C. The characteristic density, at which the smallest thermal conductivity can be obtained, is temperature dependent. At higher temperature, higher density CAs will be more efficient in the reduction of the λ t(T) by reducing the λ r(T). Therefore, one should consider the working temperature of the given environment to design the density of the CAs for thermal insulation applications.
References
X. Lu, M.C. Arduini-Schuster, J. Kuhn, O. Nilsson, J. Fricke, R.W. Pekala, Science 255, 971 (1992)
S.A. Al-Muhtaseb, J.A. Ritter, Adv. Mater. 15, 101 (2003)
D. Wu, R. Fu, J. Porous Mater. 15, 29 (2008)
M. Mirzaeian, P.J. Hall, J. Mater. Sci. 44, 2705 (2009)
C. Scherdel, R. Gayer, T. Slawik, G. Reichenauer, T. Scherb, J. Porous Mater. 18, 443 (2011)
Y. Hanzawa, H. Hatori, N. Yoshizawa, Y. Yamada, Carbon 40, 575 (2002)
T. Horiuchi, T. Osaki, T. Sugiyama, K. Suzuki, T. Mori, J. Non-Cryst. Solids 291, 187 (2001)
M. Wiener, G. Reichenauer, F. Hemberger, H.P. Ebert, Int. J. Thermophys. 27, 1826 (2006)
M. Wiener, G. Reichenauer, S. Braxmeier, F. Hemberger, H.P. Ebert, Int. J. Thermophys. 30, 1372 (2009)
X. Lu, O. Nilsson, J. Fricke, R.W. Pekala, J. Appl. Phys. 73, 581 (1993)
V. Bock, O. Nilsson, J. Blumm, J. Fricke, J. Non-Cryst. Solids 185, 233 (1995)
F. Hemberger, S. Weis, G. Reichenauer, H.P. Ebert, Int. J. Thermophys. 30, 1357 (2009)
J.H. Seol, I. Jo, A.L. Moore, L. Lindsay, Z.H. Aitken, M.T. Pettes, X. Li, Z. Yao, R. Huang, D. Broido, N. Mingo, R.S. Ruoff, L. Shi, Science 328, 213 (2010)
R.W. Pekala, J. Mater. Sci. 24, 3221 (1989)
R. Carminati, Microscale and Nanoscale Heat Transfer, Topics in Applied Physics, Vol. 107. ed. by S. Volz (Springer-Verlag, Berlin Heidelberg, 2007), pp. 15–35
R. Prasher, Phys. Rev. B 74, 165413 (2006)
C.W. Padgett, D.W. Brenner, Nano Lett. 4, 1051 (2004)
W.D. Zhou, B. Liu, S.K. Yu, W. Hua, Phys. Rev. E 81, 011204 (2010)
Acknowledgments
The authors gratefully acknowledge financial supports from National Natural Science Foundation (51002187) and National Defense Preliminary Research Foundation (9140C8203051003) of China. Dr. Yonggang Jiang is thanked for helpful discussions and comments. The authors would also like to thank Dr. Shun Li and Dr. Meng Liu for the laser flash measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, J., Feng, J. & Zhang, C. Thermal conductivity of low density carbon aerogels. J Porous Mater 19, 551–556 (2012). https://doi.org/10.1007/s10934-011-9504-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-011-9504-7