Abstract
Porous hollow CeO2 microspheres were fabricated using negative-charged PS microspheres as templates by a facile method. The hollow CeO2 microspheres were characterized by X-ray diffraction (XRD), Fourier-transform infrared (FT-IR) spectroscopy, field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM) and N2 adsorption–desorption. The results showed that the as-synthesized hollow CeO2 microspheres are well monodisperse and uniform in size. The porous shells of hollow microspheres are relatively rough and composed of tiny nanoparticles. The external diameter, internal diameter, and shell thickness of hollow CeO2 microspheres are about 190, 160, and 15 nm, respectively. A possible mechanism for the formation of hollow CeO2 spheres was also discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hollow microspheres have a unique microstructure, characterized by a hollow center surrounded by a porous or non-porous shell [1]. In recent years, well-defined hollow inorganic microspheres (SiO2, ZnO, TiO2, ZnS, etc.) have attracted more attentions due to their unique properties such as low density, large surface area, excellent loading capacity, high permeability, and potential applications in catalysis, fillers, microreactors, waste removal, drug/gene storage, and controlled release [2–8]. Various methods including template-assisted synthesis and template-free routes are proposed to fabricate hollow microspheres. In comparison, the template-assisted method is more controllable. The core templates include solid particles, soft emulsion droplets, vesicles, aggregates, and gas bubbles. After removing the template cores, hollow microspheres can be finally achieved.
CeO2 is a technologically important rare earth material because of its wide applications as fast ion conductors, oxygen storage capacitors, catalysts, UV blockers, and polishing materials [9]. To date, considerable efforts have been focused on the CeO2 with special morphologies and microstructures. Hollow CeO2 microspheres have received considerable attention recently. Guo and coworkers [10] synthesized CeO2 hollow microspheres with controlled shell thickness using silica microspheres as templates. The as-synthesized CeO2 hollow microspheres exhibit strong absorption properties in the ultraviolet range. Wang and coworkers [11] prepared porous hollow microspheres of crystalline ceria via carbon sphere template method. The results indicated that the as-prepared ceria hollow microspheres possess porous structure and high surface area. Li and coworkers [12] prepared CeO2 hollow spheres via a layer-by-layer (LBL) method using carbon spheres as sacrificial templates, and the catalytic activity of the CeO2 hollow sphere was evaluated by CO oxidation. Yang and coworkers [13] fabricated CeO2 hollow microspheres using l-asparagine as both OH—provider and templates, and a hollowing growth mechanism was proposed. Besides, Zhang and coworkers [14] synthesized single/multiwall hollow CeO2 microspheres by self assemblage through hydrothermal method. Zhang and coworkers [15] synthesized CeO2 hollow spheres via sequential process of a hydrothermal reaction and heat treatment. Although many success examples of synthesis of hollow CeO2 microspheres are reported, whereas great care should be taken to avoid formation of unwanted and irregular aggregates of CeO2.
Polystyrene (PS) microspheres are typical solid cores for the preparation of various hollow microspheres. However, there are few reports on the fabrication of hollow CeO2 microspheres using PS microspheres as templates, except that Kartsonakis and coworkers [16] reported a complicated procedure to synthesize hollow ceria nanospheres using polystyrene lattices. Herein, we described a simple and efficient route to prepare hollow CeO2 microspheres using negative-charged PS microspheres as template. The whole process required neither surface treatment for PS microspheres nor additional surfactant or stabilizer.
2 Experimental
2.1 Materials
Styrene (St) was purchased from Shanghai Chemical Reagent Co. (China) and purified by treatment with a 5 wt% aqueous NaOH solution to remove the inhibitor.
Chemicals used in the synthesis were divinylbenzene, potassium persulfate (KPS), methylacrylic acid, cerium nitrate hexahydrate (Ce(NO3)3·6H2O), and hexamethylenetetramine (HMT). The above chemical reagents were of analytic grade and used without further purification. Deionized water was employed in the present study.
2.2 Synthesis of PS latex
The PS microspheres were synthesized by soap-free emulsion polymerization. The typical synthesis was introduced as follows. 80 mL water, 20 mL St, 2 mL divinylbenzene and 1 mL methyl acrylic acid were charged into a 250 mL flask equipped with a mechanical stirrer and a condenser at room temperature. The mixture was vigorously stirred at about 300 rpm for about 30 min to form homogeneous aqueous solution, and then the flask was heated to 125 °C gradually in an oil bath for 5 min. After that, 0.5 g KPS (dissolved in 20 mL water) was added to initiate polymerization. Finally, the polymerization was maintained for 2 h, and then the emulsion was cooled to room temperature to stop the polymerization.
2.3 Synthesis of hollow CeO2 microspheres
In a typical procedure, 4 mL PS colloids were dispersed into 100 mL water with ultrasonic vibration for about 10 min, and then 1.009 g Ce(NO3)3·6H2O (dissolved in 50 mL water) and 1.629 g HMT (dissolved in 50 mL water) were added. The resulting mixture was initially dispersed by means of an ultrasonic bath for 15 min to form homogeneous suspension. The obtained aqueous suspension was reacted at 75 °C for 2 h under magnetic stirring, and the CeO2 coated PS (PS/CeO2) composite microspheres were obtained. The resulting composite particles precipitates were separated by centrifugation and washed with water and ethanol, then dried at 80 °C in air for 2 h. Finally, the composite microspheres were calcined at 600 °C in air for 2 h. After these treatments, the hollow CeO2 microspheres were formed.
2.4 Characterization
The crystalline phases of the products were analyzed using a Rigaku D/max 2,500 X-ray diffractometer with Cu Kα radiation. The SEM images of the resulting samples were obtained on a Hitachi S-4800II field emission scanning electron microscope. The TEM images, as well as the selected area electron diffraction (SAED) patterns, were obtained via Philips Tecnai-12 and JEOL JEM-2,100 transmission electron microscope. Fourier-transform infrared (FT-IR) spectra of the samples were recorded on a Nicolet Avatar 370 spectrometer. The nitrogen adsorption–desorption analysis was carried out at on a Micromeritics TriStar II 3,020 apparatus. The analytical data were processed by the Brunauer–Emmett–Teller (BET) equation for surface areas.
3 Results and discussion
Fig. 1 shows the FESEM image of the PS microspheres that we used as templates. The spherical PS microspheres are reasonably uniform with an average diameter of 160 nm. As we know, the morphology and dispersibility of the core material are crucial to the final hollow spheres products. Herein, PS latex with narrow size distribution was successfully synthesized.
The IR analysis provide some insights into the compositions of the pure PS (a), PS/CeO2 composite microspheres (b), CeO2 hollow microspheres (c), and the results are presented in Fig. 2. The characteristic adsorption peaks of PS at ca. 3,100–2,900, 1,600–1,350, and 700 cm−1 can be attributed to the stretching vibrations of aromatic C–H in-plane, stretching vibrations of aromatic C–C, and bending vibrations of aromatic C–C out-of-plane, respectively [4]. Compared with the spectrum of PS, the PS/CeO2 composite microspheres (Fig. 2b) not only clearly exhibit characteristic absorption bands attributed to the PS core, but also show an adsorption bands at ca. 690 cm−1 correspond to the Ce–O–Ce stretching vibrations [17], which indicated that PS microspheres cores are fully encapsuled by CeO2 particles, and the PS/CeO2 composites are formed. As shown in Fig. 2c, the typical adsorption peaks of PS are not observed, which further demonstrate that PS template is thoroughly removed from the composite microspheres by calcinations.
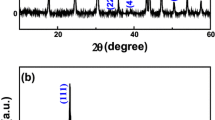
The XRD patterns of the PS microspheres (a), PS/CeO2 composite microspheres (b) and hollow CeO2 microspheres (c) are shown in Fig. 3. An obviously broadened diffraction peak is presented at about 20° (Fig. 3a), which is a typical XRD character of amorphous PS materials [18]. Based on Fig. 3a, characteristic diffraction peak at 2θ (Brag angle) of 28.6, 33.1, 47.5 and 56.3°, which are assigned to (111), (200), (220) and (311) (JCPDS 34-0394) lattice planes of CeO2. Furthermore, the spectrum of the composite particles reveals a little amorphous peak at around 2θ = 20°, attributes to the introduction of organic PS core. Based on the above analysis, it is confirmed that CeO2 particles are successfully coated on the surface of the PS microspheres. The formation of crystalline CeO2 with hollow microspheres is also confirmed by XRD analysis (Fig. 3c). The position of the peaks of the pattern, their intensity, and the fact that they are very narrow suggest that crystalline CeO2 are produced by calcining the composite particles at 600 °C. No other peaks appeared, indicating that the structured CeO2 microspheres are of high purity.
Fig. 4 shows the FESEM and TEM images of the as-synthesized PS/CeO2 composite microspheres. Compared with the smooth surface of PS particles, the outer surfaces of the composite microspheres exhibited very rough appearance (Fig. 4a), indicating that tiny particles appear to evenly deposit on the surface of the PS spheres. As shown in Fig. 4b, it was clearly observed that the strong contrast between the PS core and the shell. The grey part in the center represents the organic PS cores, while the outside black part is considered to be the aggregation of inorganic the CeO2 particles.
Fig. 5 shows the FESEM image of the hollow CeO2 microspheres. As shown in Fig. 5a, the as-prepared hollow CeO2 microspheres exhibit a regular spherical shape with the average diameter about 200 nm. From the higher-resolution image (Fig. 5b), it is clearly observed that there are partial cave-in on the surfaces of some hollow microspheres as indicated by arrows in Fig. 5b, indicating the hollow structure of the samples.
More detailed structural characterization of the hollow CeO2 microspheres was performed by TEM analysis. Fig. 6a shows a TEM image of the hollow CeO2 microspheres fabricated by using the PS microspheres in Fig. 1 as templates. Removal of the PS cores gives the hollow microspheres transparent look. The shells are sufficiently rigid to largely maintain their spherical shape after removal of the core and escape of gases. Practically no separate tiny CeO2 particles are observed outside the hollow microspheres. The strong contrast between the core and edge indicates that the CeO2 microspheres are hollow. These hollow microspheres were with an average external diameter of about 190 nm and their internal diameter and shell thickness were about 160 and 15 nm, respectively. The corresponding selected area electron diffraction (SAED) pattern of the hollow CeO2 microspheres is presented in Fig. 6b. The polycrystalline diffraction rings correspond to the (111), (200), (220), and (311) peaks of the XRD pattern, which indicates that the shell of the hollow microsphere is made up of CeO2 nanoparticles. Further higher-resolution TEM analyses on individual hollow CeO2 microspheres were conducted. As shown in Fig. 7a, a rough shell surface is evident, and the wall thickness is about 15 nm. It can also be clearly observed that the shell was composed of a lot of tiny particles and constituted a porous framework. Furthermore, all the microspheres exhibited loose state and even some interparticle voids can be clearly observed. To get further insight into the atomic order of the CeO2 particles in the shell, high-resolution TEM image was recorded. Fig. 7b shows an edge portion of a hollow microsphere. The HRTEM image shows well-developed lattice fringes, which are randomly oriented with respect to each other, confirming the SAED results. The average size of the CeO2 nanoparticles composing the shells of the hollow microspheres is about 5–8 nm as estimated from the HRTEM image. Two sets of lattice fringes with d spacings of 0.31 and 0.27 nm are measured, which can be corresponded to the (111) and (200) planes, respectively, of cubic CeO2 [13, 19, 20].
Fig. 8 shows the nitrogen adsorption–desorption isotherms of the hollow CeO2 microspheres samples. A distinct hysteresis loop can be observed between the adsorption and desorption branch, indicating the porous structure of ceria hollow microspheres. N2-sorption indicates that the as-synthesized hollow CeO2 microspheres exhibit a type II isotherm. The total surface area and pore volume are 65.76 m2/g and 0.1875 ml/g, respectively. As suggested by the large uptake at high relative pressures in the isotherm, the detected pore volume is mainly contributed by the interparticle voids and the hollows [21].
A possible mechanism of the formation of the hollow CeO2 microspheres is described in Scheme 1. The synthesis of the hollow CeO2 microspheres consists of three experimental steps. The first step involves the preparation of negative-charged polystyrene microspheres. For this reason, the reagent KPS is used as the initiator in order for the styrene monomers to be polymerized [16]. During the second step, Ce3+ cations can be absorbed onto the surfaces of the negatively charged PS microspheres. Under slow hydrolyzing of HMT, the opposite-charged OH− is released in the solution. Ce3+ combined with OH− slowly hydrolyzed from HMT driven by electrostatic attraction. Then, Ce3+ hydrolyzes to form gelatinous hydrous cerium hydroxide (Ce(OH)3·xH2O) on the surface of the PS microspheres, forming a core–shell structure [11]. Followed by dryness process in air, Ce3+ was oxidized to Ce4+ due to its intrinsic crystallization tendency [13]. The third step involves the formation of the hollow CeO2 microspheres, which are prepared by calcinations where the internal PS cores are burnt out. During a calcination process, the PS microspheres are removed in the form of carbon dioxide and vapor, which depart from the core through the shell. Meanwhile, the osmotic pressure may result in the formation of the hollow microspheres along with a number of pores on the shell.
4 Conclusions
Porous hollow CeO2 microspheres were fabricated using PS spheres as templates via a facile and efficient method. The samples were characterized by XRD, FT-IR, FESEM, HRTEM and N2 adsorption–desorption. The results reveal that the as-prepared hollow CeO2 microspheres are complete and homogeneous, and the porous shells of the hollow microspheres are relatively rough and composed of tiny nanoparticles. The external diameter, internal diameter, and shell thickness of the hollow CeO2 microspheres are 190, 160, and 15 nm, respectively. The size of the CeO2 nanoparticles is about 5–8 nm, as demonstrated by HRTEM result. The approach presented here can be applied to fabricate hollow CeO2 microspheres with desired void size by selecting the PS sphere templates with suitable diameter.
References
M.Y. Wei, C.J.V. Oers, X.P. Hao, Q. Qiu, P. Cool, S.Q. Liu, Microporous Mesoporous Mater. 138, 17 (2011)
S.N. Wang, M.C. Zhang, D. Wang, W.Q. Zhang, S.X. Liu, Microporous Mesoporous Mater. 139, 1 (2011)
Z.M. Chen, S.J. Li, F.F. Xue, G.N. Sun, C.G. Luo, J.F. Chen, Q. Xu, Colloids Surf. A 355, 45 (2010)
C.L. Wang, J.T. Yan, X.J. Cui, H.Y. Wang, J. Colloid Interface Sci. 354, 94 (2011)
K. Shin, J.J. Kim, K.D. Suh, J. Colloid Interface Sci. 350, 581 (2010)
K.L. Lv, J.G. Yu, K.J. Deng, J. Sun, Y.X. Zhao, D.Y. Du, M. Li, J. Hazard. Mater. 173, 539 (2010)
Q.Y. Zhu, J. Chen, Q. Zhu, Y.M. Cui, L. Liu, B. Li, X.F. Zhou, Mater. Res. Bull. 45, 2024 (2010)
T.J. Kuo, C.L. Kuo, C.H. Kuo, M.H. Huang, J. Phys. Chem. C 113, 3625 (2009)
X. Feng, D.C. Sayle, Z.L. Wang, M.S. Paras, B. Santora, A.C. Sutorik, T.X.T. Sayle, Y. Yang, Y. Ding, X. Wang, Y. Her, Science 312, 1504 (2006)
Z.Y. Guo, F.F. Jian, F.L. Du, Scr. Mater. 61, 48 (2009)
S.R. Wang, J. Zhang, J.Q. Jiang, R. Liu, B.L. Zhu, M.J. Xu, Y. Wang, J.L. Cao, M.Y. Li, Z.Y. Yuan, S.M. Zhang, W.P. Huang, S.H. Wu, Microporous Mesoporous Mater. 123, 349 (2009)
Z.J. Yang, L. Liu, H. Liang, H.X. Yang, Y.Z. Yang, J. Cryst. Growth 312, 426 (2010)
X.Z. Li, F. Chen, X.W. LU, C.Y. Ni, X.B. Zhao, Z.G. Chen, J. Porous. Mater. 17, 297 (2010)
Y.J. Zhang, Q.X. Hu, Z.Y. Fang, T. Cheng, K.D. Han, X.Z. Yang, Chem. Lett. 35, 944 (2006)
D.E. Zhang, Q. Xie, M.Y. Wang, X.B. Zhang, S.Z. Li, G.Q. Han, A.L. Ying, A.M. Chen, J.Y. Gong, Z.W. Tong, Solid State Sci. 12, 1529 (2010)
I.A. Kartsonakis, P. Liatsi, I. Daniilidis, G. Kordasw, J. Am. Ceram. Soc. 91, 372 (2008)
M. Teng, L. Luo, X. Yang, Microporous Mesoporous Mater. 119, 158 (2009)
Y. Yang, Y. Chu, Y. Zhang, F. Yang, J. Liu, J. Solid State Chem. 179, 470 (2006)
K.L. Yu, G.L. Ruan, Y.H. Ben, J.J. Zou, Mater. Sci. Eng. B 139, 197 (2007)
G.F. Wang, Q.Y. Mu, T. Chen, Y.D. Wang, J. Alloys Compd. 493, 202 (2010)
J. Yuan, D. Wan, Z. Yang, J. Phys. Chem. C 112, 17156 (2008)
Acknowledgments
The work was supported financially by Youth Foundation of Changzhou University (JQ201005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Lu, J. Facile fabrication of porous hollow CeO2 microspheres using polystyrene spheres as templates. J Porous Mater 19, 289–294 (2012). https://doi.org/10.1007/s10934-011-9474-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-011-9474-9