Abstract
The present paper deals with preparation of silica aerogel granules by two step acid–base sol–gel process involving ambient pressure drying of alcogels with additional use of mechanical shaker to accelerate the solvent exchange process and characterization of the yielded aerogels granules to study their physical properties. The conventional ambient pressure drying of alcogels is crucial since it needs tedious repetitive gel washing and solvent exchanges (10 times) which consumes total process time of 4 days. We have succeeded to synthesize aerogels within 2 days by making use of alcogels shaking. To get good quality aerogels in terms of low density, high optical transparency, low volume shrinkage, various base catalysts and their combinations were used. The optimal molar ratio of precursor chemicals Tetraethoxysilane (TEOS): Methanol (MeOH): Oxalic acid: NH4OH: NH4F: Trimethylchlorosilane (TMCS) found to be 1: 16.5: 0.49: 0.58: 0.60: 0.98, respectively. Among six catalyst studied, combination of NH4OH and NH4F resulted in low density and transparent aerogels. Hydrophobicity was achieved by surface silylation using TMCS silylating agent but lead to decrease in transparency due to chloride precipitation. We have improved transparency of aerogels by methanol washing of alcogels prior to silylation. The hydrophobicity has been studied by FTIR analysis and contact angle measurements. The thermal analysis indicates thermal stability of hydrophobicity up to 318 °C and the Surface morphology of aerogel has been studied by FESEM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Silica aerogel is a fascinating class of solid derived by sol–gel technique which possess unique properties such as high porosities (~99%), high specific surface area (~1,000 m2/g), low thermal conductivity (~0.04 W m−1 k−1) and low density (<50 kg m−3) [1, 2]. These interesting characteristics of aerogel lead to several applications like thermal insulator and oil spill cleanup [3]. The ambient pressure dried granular silica aerogels find applications for solar energy windows because of its interesting thermal and optical properties, especially in the field of thermal insulation [4, 5].
Aerogels are usually synthesized by hydrolysis and condensation of a silicon alkoxide followed by supercritical drying of the solvent from a wet gel. In supercritical drying method of silica aerogels, the surface tension of solvent is reduced to zero at the supercritical state of the solvent and hence the resulting aerogels found to be monolithic [6]. However, supercritical process is expensive and dangerous because of the high pressures (5–10 MPa) and high temperatures (250–270 °C) involved, which puts the restriction on the commercial scale processing of the aerogels [7]. In order to overcome this drawback, attempts have been made to synthesize the aerogel granules by atmospheric pressure drying (APD) method. This method is commercially attractive as a safer process compared to the supercritical drying method as it does not involve very high temperature and pressure. In the ambient pressure drying, the effect of capillary forces can be minimized by exchanging the alcohol solvent with a low surface tension solvent, but the solvent exchange process is time consuming and needs the repetitive exchanging of the solvent. This increases the production cost of silica aerogels and also the processing period. These are the main disadvantages of the ambient pressure drying method [8].
In the present work, we intended to reduce the total processing period by at least half and enhance the optical transmission of the aerogels that are chemically modified with TMCS. The processing time is reduced to less than 2 days which is the half of the minimum reported process time for the aerogels prepared by ambient pressure drying method reported by Rao et al. [9]. The detailed studies of the process parameters like the drying conditions, type of catalyst, methanol washing prior to silylation have been studied and yielded aerogels have been characterized by density measurements, FTIR spectroscopy, contact angles measurement, thermal conductivity and surface morphology by scanning electron microscopy and observed results have been reported in the present research paper.
2 Materials and methods
2.1 Experimental procedure
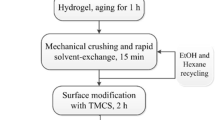
Silica aerogels were prepared by a two step acid base sol–gel process followed by ambient pressure drying. Figure 1 shows the flow chart for the preparation of the hydrophobic silica aerogels by ambient pressure drying method. Initially, the sol was prepared using tetraethoxysilane (TEOS) precursor diluted in methanol (MeOH). Oxalic acid (0.001 mol L−1 C2H2O4) was added to this solution. The molar ratio of TEOS: MeOH: C2H2O2 was kept constant at 1: 16.5: 0.49, respectively. The sol was stirred for 1 h and kept at room temperature for 12 h for hydrolysis. After 12 h, the base catalyst was added to the sol in a petridish and the dish was covered with aluminium foil. Six different base catalysts and their combinations viz. tetrabutylammonium fluoride (BAF), tetraethylammonium fluoride (EAF), tetramethylammonium fluoride (MAF), ammonium hydroxide (NH4OH), ammonium hydroxide + ammonium fluoride (NH4OH + NH4F) and ammonium fluoride (NH4F), were used. These alcogels were kept in the oven at 50 °C for 40 min for strengthening of the silica network. After aging of the alcogels, they were cut into small cubic pieces which were then kept in methanol solvent for 10 min. The methanol was decanted out and hexane was added. The methanol in the gel was exchanged with hexane at 50 °C. The system was shaked at 120 rpm for 4 h in a shaker (Remi instruments, Mumbai, India). To make the gels hydrophobic, the gels were immersed in TMCS for silylation in an oven for 16 h at 50 °C. The unreacted TMCS was exchanged with the solvent by keeping again in the shaker for 4 h at 50 °C with 120 rpm speed. Finally, the alcogel pieces along with a little solvent were kept in the bottle. The bottle was covered with aluminum foil with 8–10 small pin holes to allow the evaporation of the solvent. Such bottles were kept in the oven at 50 °C for 1 h and at 150 °C for 2 h. The resulting aerogels were cooled to room temperature. The total processing period for one experiment is less than 2 days (40 h). The combination of NH4OH and NH4F as the base catalyst resulted in low density (67 kg m−3) silica aerogels with high optical transmittance (90%). The transparent, low density and hydrophobic silica aerogels were obtained for the molar ratio of TEOS: MeOH: Oxalic acid: NH4OH: NH4F: TMCS as 1: 16.5: 0.49: 0.58: 0.60: 0.98, respectively.
2.2 Reaction mechanism
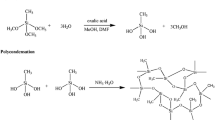
The hydrolysis and condensation of methanol diluted TEOS in the presence of oxalic acid, NH4OH and NH4F is shown in the following chemical reactions:
Hydrolysis:
Condensation:
In the present study, the surface chemical modification of hydrophilic aerogels was made with TMCS in hexane. The surface modification of the gel with TMCS is shown in the following reaction [10, 11].

2.3 Methods of characterization
The bulk density, % of volume shrinkage, % of porosity and pore volume of the as prepared silica aerogels were measured using following formulae [12].
where, V a and V g are the volumes of the aerogel and alcogel, respectively, in cm3, ρ s skeletal density (~1.9 g cm−3) and ρ b is the bulk density in g cm−3. Pore volume has unit of cm3 g−1.
Here density measurements of granules were conducted on the basis of mass of granules per unit volume in c.c. Volume shrinkage of aerogels is measured with respect to alcogel volume which is assumed as 100%. In the present case granules of size ~1 × 1 cm were cut from initial alcogel which were washed for solvent exchange and TMCS treated for surface modification and finally dried at ambient pressure conditions. The resulted aerogels were characterized by various techniques to know their physical properties. The thermal conductivity was measured using a ring probe sandwiched between two identical aerogel samples [C-T meter from Teleph Company, France]. The contact angle (θ) was measured using contact angle meter (Rame–Hart Instruments, New-Jersey, USA). The thermal stability of the aerogel samples was characterized by TGA and DSC analysis in air atmosphere. Surface modification of the aerogels was studied using Fourier Transform Infrared Spectroscopy (Perkin Elmer Instruments, Spectrum one, USA) and the surface morphology was studied by Field Emission Scanning Electron Microscopy (model—Carl Zeiss SUPRA 40 V Japan). The effect of humidity on aerogels was studied by putting the samples in the humidity chamber (Remi Instruments Ltd. India) in 90% relative humidity at 30 °C.
3 Results and discussion
3.1 Influence of different base catalysts
The silica aerogels prepared by using various base catalysts viz. tetrabutylammonium fluoride (BAF) [(C4H9)4NF], tetraethylammonium fluoride (EAF) [(C2H5)4NF], tetramethylammonium fluoride (MAF) [(CH3)4NF], ammonium hydroxide (NH4OH), ammonium fluoride (NH4F) and combination of NH4OH + NH4F. Table 1 shows the effect of various base catalysts on the physical properties of retrieved silica aerogel materials. Base catalysts such as BAF and EAF resulted in formation of transparent and very hard granules of the aerogels which might be due to steric effects. This happens because of bulky groups like butyl and ethyl groups are located in the vicinity of the reaction site. This retarded the rates of hydrolysis and condensation reactions, leads to the hard and transparent gel. MAF catalyst resulted in the formation of opaque aerogels which might be due to gelation occurs very fast (time is in fraction of seconds) and because this molecules are coagulated, particle size increases which leads to the decrease in the optical transparency of the aerogels. It has been observed that with the use of HF catalyst, the fluorine ion (f) act as smallest catalytic cation which results in shifting of the acid–base catalyst to acid-acid one. Thus resultant acidic pH of the precursor mixture accelerates the hydrolysis rate of the TEOS precursor and ultimately retards the condensation reaction rates. This result in the formation of smaller size silica (SiO2) particles and their entanglements results in relative high density HF catalyzed TEOS based silica aerogels. In case of NH4OH catalyst resulted in transparent as well as shrinked aerogels which may be due to very weak SiO2 network. Of course up to some extent shrinkage can be reduced by strengthening of SiO2 network by gel aging. In case of NH4F catalyst, it was observed that yielded aerogels are slightly translucent but relatively no shrinkage was observed compared to NH4OH catalyst. To avoid the demerits of these both individual catalysts, we used combination of NH4OH and NH4F as catalyst. The NH4 +/NH3 acid base couple has an acidity constant of 9.25 and dissociates as \( {\text{NH}}_{4}^{ + } \rightleftharpoons {\text{NH}}_{3} + {\text{H}}^{ + } . \) The HF/F− couple has an acidity constant of 3.17 and dissociates as \( {\text{HF}} \rightleftharpoons {\text{F}}^{ - } + {\text{H}}^{ + } . \) In the first step of two step acid–base catalysis method adopted in the present work, oxalic acid is used for partial hydrolysis of TEOS. In NH4OH catalyzed TEOS, the gelation rate is extremely low due to the dominant nucleophilic reaction mechanism whereas NH4F result in reduced gelation time. The combination of NH4OH and NH4F might have played an important role in controlling the condensation rate and allowing re-polymerization, syneresis and uniform pore formation [13] resulting in the aerogel with low density (67 kg m−3), high porosity (96.47%), large pore volume (14.39 cm3 g−1) and high optical transmission (~90%). Therefore, we have selected the combination of base catalyst NH4OH + NH4F for further studies.
3.2 Effect of shaking on the physical properties of the aerogels
In case of ambient pressure drying method, the alcohol in the gel pores needs to be exchanged using high vapour pressure (highly volatile) solvent like hexane. The binary phase diagrams for alcohol—hexane system have been discussed in details in the literature [14–18]. For total exchange of alcohol solvent by hexane there is requirement of repetitive gel washing for at least 10 times in each experiment. The demerit of this process is that it requires large process time of ≥4 days and there is wastage of lot of chemicals that are required for solvent exchange in gels. This increases the cost of the aerogels and limits the commercial production of the aerogels.
With an objective of reduction in process time, we introduced the process of shaking of alcogel to accelerate the solvent exchange process. The shaker revolution speed found to be optimal at 120 rpm at 50 °C since all solvent found to be exchanged within 4 h. Moreover, the optimal time required for silylation process found to be 16 h. Silylation period <16 h resulted in formation of aerogel materials in powdered form. This may be due to incomplete silylation. The shaking of the gel enhances the diffusion mechanism that leads to the reduction in the solvent exchange period. The overall or total processing time for solvent exchange found to be only 40 h and also observed that about three cycles of solvent exchange are adequate for total replacement of alcohol by hexane. Present procedure reported herewith requires lowest processing time of 40 h in the history of literate reported for ambient pressure drying [19].
3.3 Influence of drying parameters
Table 2 shows the effect of variations in drying parameters on the physical properties of the aerogels. The optimal heating rate was found to be 2 °C/min to attain the temperature of 150 °C from initial set temperature of 50 °C. The evaporation rate of solvent was controlled using perforated aluminium foil (2 pin holes/cm2) used for covering the opened top of the bottle in which sample under test is present. Table 2 indicates that drying of sample under test with the aluminum foil and with solvent resulted in good quality aerogels in terms of low density (~75 kg m−3), high porosity (96.05%) and large pore volume (12.79 cm3 g−1). The perforated aluminium foil helps in controlling the evaporation rate of solvent which results in minimization of the cracking of the aerogels and also shrinkage of alcogels which leads to yield aerogel granules with low density and large pore volume.
3.4 Effect of methanol washing on silica aerogels
As TMCS is used as a silylating agent for surface modification of the wet gels, it has been observed that white fumes are produced during silylation process and leads to turn transparent alcogel into opaque one. This may be due to the formation of hydrochloric acid (HCl) and ammonium chloride (NH4Cl) precipitated in the gel as a result of reaction between TMCS and NH4OH. The proposed probable chemical reaction is as follows:

The ammonium chloride (NH4Cl) is white salt with melting point of 338 °C. Therefore, even on drying of alcogels at temperature of 150 °C, the ammonium chloride found retained in the aerogel network in the precipitate form. Photograph of silica granules clearly shows such white spots in aerogel granules (Fig. 2a). The resulted white spots decrease the optical transmittance and also lead to increase in density of the yielded aerogels.
In order to improve the optical transmittance of the aerogels, alcogels were washed with methanol before addition of the mixture of TMCS and hexane. The NH4OH gets dissolved in the methanol and resulted mixture of methanol and ammonium hydroxide has been decanted out. After methanol washing, when TMCS was added, it has been observed that the gel retains optical transparency without any localized whitish spots. The resulting transparent aerogels granules due to adaptation of methanol washing step in which white spots of ammonium chloride vanishes totally. The transparent silica aerogel granules, prepared using methanol washing method, are shown in Fig. 2b. Such resulting aerogels were transparent as well as retaining low density. Attempts were also made with use of hexamethyldisilazane (HMDZ) as a silylating agent without methanol washing which resulted in translucent aerogels without any white spots. This might be due to the absence of chlorine in the HMDZ and hence it does not form the white fumes of HCl. The advantage of using TMCS as a silylating agent is that it is cheaper by almost half the cost of HMDZ [20].
3.5 Thermal conductivity
The thermal conduction in silica aerogel is mainly due to two effects that are lattice vibrations and heat conducted by radiations. The Fig. 3 shows the variation in thermal conductivity and density for the aerogels prepared using different base catalysts. The variation in thermal conductivity follows the trend of variation in the density of the aerogels i.e. lower is the density lower is the thermal conductivity and vice versa. It is observed from Fig. 3 that the combination of base catalyst of ammonium hydroxide (NH4OH) and ammonium fluoride (NH4F) resulted in aerogels with low thermal conductivity of 0.056 W m−1 K−1. The decrease in thermal conductivity of the aerogels prepared using the combination of NH4OH and NH4F as the base catalyst can be attributed to its large pore volume (14.39 cm3 g−1) and low density (67 kg m−3). Due to such low thermal conductivity, the obtained aerogel granules possess potential applications in thermal insulation systems [21].
3.6 Fourier transform infrared spectroscopy
The wetting behavior of hydrophobic surfaces is governed mainly by chemical composition of surface. Figure 4 shows the Fourier transform infrared (FTIR) spectrum of the silica aerogels prepared using NH4OH and NH4F base catalyst combination with unmodified surface. Figure 5 shows that FTIR spectra of the silica aerogels prepared with different base catalysts with surface modification using TMCS. The absorption peak at 1,080 cm−1 which is characteristic of Si–O–Si bond is present in all samples [22]. The absorption bands observed at 1,440 and 2,900 cm−1 in Fig. 5 are due to bending and stretching mode of C–H bond, respectively [22]. The peaks at 3,450 cm−1 arise from the –OH bonding of physically adsorbed water molecules and the peak at 1,600 cm−1 arise from the bending modes of –OH bond. Figures 4 and 5 indicate that the intensity of Si–OH peak at 600 cm−1 in the surface modified aerogels is less as compared to the aerogels with unmodified surfaces. The absorption peaks at 850 and 1,250 cm−1 are due to the bending modes of Si–C bond. The intensity of these peaks at 850 and 1,250 cm−1 is found to be increased in surface modified aerogels. The reduction in relative intensity of Si–OH peaks and increase in intensity of Si–C peaks in the surface modified aerogels confirms the surface modification with replacement of Si–OH by the non hydrolysable –Si–(CH3)3 groups. The intensity of the peak at 3,450 cm−1 due to free water adsorption is also reduced in the surface modified aerogels. This is due to the increased moisture resistance of the surface modified hydrophobic aerogels.
3.7 Surface morphological studies
The field emission scanning electron microscopy (FESEM) image of the two-dimensional surface morphology of the retrieved silica aerogels is as shown in Fig. 6. From FESEM it is clear that there is uniformity and homogeneity of SiO2 particle and porous network. Such uniform and homogeneous nano-structured particle and pores results in high optical transparency of the aerogels. From few selected areas of the material under scan shown statistical average colloidal particle size of the silica found around 30–40 nm and the pore size around 80–100 nm. The silylation process might be leading to increase in pore sizes of the silica network [23]. The surface morphology is important as the wettability of a surface is dependent on its chemical composition and surface topography.
3.8 Effect of humidity on the aerogels
The TMCS modified silica aerogel samples prepared at optimized conditions were exposed to 90% relative humidity at 30 °C for 60 days and after each 2 days the contact angle was measured. Figure 7a shows the image of water droplet kept on silica surface modified with TMCS before exposing to the humid atmosphere, and Fig. 7b shows the water droplet kept on TMCS modified silica surface after exposing 2 days in 90% humid atmosphere. After exposing the sample to humidity for 2 days, the contact angle decreased from 150 to 142º and there after remained nearly constant for 60 days observation. The decrease in the contact angle might be due to a slight initial adsorption of moisture from the atmosphere by the polar –OH bonds present on the silica aerogel surface [24]. The samples exposed to humid atmosphere for 60 days were dried at 50 °C in oven and again contact angle was measured for this dried sample. The contact angle measured was nearly same to that of the measured before exposing to the humid atmosphere. This indicates that there was no significant effect of humidity on the surface modified aerogels.
3.9 TGA-DSC analysis
Figure 8 shows that TGA and DSC profiles of the TMCS modified silica aerogel sample. The TGA curve has small weight loss around 1.314% due to evaporation of the hydroxyl groups and physically adsorbed water molecules from room temperature to 318 °C. The DSC curve shows an endothermic peak at 40 °C due to the desorption of physically adsorbed water. The TGA curve shows noticeable weight loss at around 318 °C accompanied with an exothermic peak in the DSC curve. The sudden weight loss in TGA curve around 318 °C is due to the decomposition of alkyl (–CH3) groups. The exothermic peak in DSC curve indicates the oxidation of alkyl groups [25]. It indicates that the thermal stability of TMCS modified silica aerogels is up to 318 °C and above this temperature the aerogel becomes hydrophilic.
4 Conclusions
The ambient pressure dried TEOS based hydrophobic silica aerogels were obtained by the two-step sol–gel process using TMCS as the hydrophobic reagent for surface chemical modification. The total processing period for ambient pressure dried TEOS has been reduced to 2 days (40 h) which is the least process time ever reported in the literature for ambient pressure dried aerogels. The low processing time was achieved due to mechanical shaking of the wet gel during solvent exchange process. The reduced processing time minimizes the use of chemicals leading to the lower cost of aerogel synthesis which is useful for commercialization. The good quality monolithic aerogels in terms of low density (67 kg m−3), high porosity (96.47%) and large pore volume (14.39 cm3 g−1) were obtained for the base catalyst combination of NH4OH and NH4F at optimal molar ratio of precursor chemicals TEOS: MeOH: Oxalic acid: NH4OH: NH4F: TMCS found to be 1: 16.5: 0.49: 0.58: 0.60: 0.98, respectively. The ammonium chloride precipitate formed during TMCS silylation has been prevented by adopting methanol washing step prior to silylation. The drying conditions with solvent and with aluminum foil strongly affect the physical properties of silica aerogels resulting in low density silica aerogels. The hydrophobic nature can be retained up to temperature of 318 °C as indicated by the thermal analysis. The thermal conductivity of silica aerogels found to be low ~0.056 W m−1 K−1. The transparent, hydrophobic aerogels with low thermal conductivity and low density could find applications in industrial and technological areas for thermal insulating systems.
References
C.J. Brinker, G.W. Scherer, Sol-Gel Science, 1st edn. (Academic Press Inc., London, 1990), pp. 12–36
M. Reim, G. Reichenauer, W. Korner, J. Manara, M. Arduini-Schuster, S. Korder, A. Beck, J. Fricke, J. Non Cryst. Solids 350, 358 (2004)
D. Richter, D. Lipka, Nucl. Instrum. Methods Phys. Res. A513, 635 (2003)
M. Reim, W. Ko¨rner, J. Manara, S. Korder, M.A. Schuster, H.-P. Ebert, J. Fricke, Sol. Energy 78, 131 (2005)
J.L. Gurav, A. VenkateswaraRao, J. Sol-Gel Sci. Tech. 50, 275 (2009)
A.V. Rao, S.D. Bhagat, H. Hirashima, G.M. Pajonk, J. Colloid Interface Sci. 300, 279 (2006)
P.M. Shewale, A.V. Rao, A.P. Rao, J. Appl. Sur. Sci. 254, 6902 (2008)
C.T. Wanga, C.L. Wua, I.-C. Chenb, Y.H. Huang, Sens. Actuators B107, 402 (2005)
A.P. Rao, A.V. Rao, G.M. Pajonk, J. Sol-Gel Sci. Tech. 36, 285 (2005)
A. Parvathy Rao, A. Venkateswara Rao, J. Mater. Sci. 45, 51 (2010)
D.J. Suh, T.J. Park, J.H. Sonn, J.C. Lim, J. Mater. Sci. Lett. 18, 1473–1475 (1999)
D. Vargas-Florencia, I. Furo, R.W. Corkery, Langmuir 24, 4827 (2008)
D.J. Suh, T. Park, J. Sonn, J. Lim, J. Mater. Sci. Lett. 18, 1473 (1999)
A. Zawisza, J. Chem. Thermodyn. 17, 941 (1985)
I.F. Hölscher, G.M. Schneider, J.B. Ott, Fluid Phase Equilib. 27, 153 (1986)
M. Iglesias, R. Gonzalez-Olmos, D. Salvatierra, J.M. Resa, J. Mol. Liq. 130, 52 (2007)
P. Sauermann, K. Holzapfel, J. Oprzynski, F. Kohler, W. Poot, T.W. de Loos, Fluid Phase Equilib. 112, 249 (1995)
B.C. Oh, Y. Kim, H.Y. Shin, H. Kim, Fluid Phase Equilib. 220, 41 (2004)
A. Parvathy Rao, G.M. Pajonk, A. Venkateswara Rao, J. Mater. Synth. Process. 9, 1 (2001)
C.E. Kim, J.S. Yoon, H.J. Hwang, J. Sol-Gel Sci. Techn. 49, 47 (2009)
J.L. Gurav, A. Venkateswara Rao, D.Y. Nadargi, H.-H. Park, J. Mater. Sci. 45, 503–510 (2010)
G.M. Pajonk, A.V. Rao, B.M. Sawant, N.N. Parvathy, J. Non-Cryst. Solids 40, 209 (1997)
N.D. Hegde, A. Venkateswara Rao, J. Mater. Sci. 42, 6965 (2007)
A. Parvathy Rao, A. Venkateswara Rao, J.L. Gurav, J. Porous Mater. 15, 507 (2008)
G. Lakshminarayana, Masayuki Nogami Electrochimica Acta. 55, 1160 (2010)
Acknowledgments
The authors are highly thankful to the Board of Research in Nuclear Science (BRNS) Mumbai, Department of Atomic Energy (DAE) for the funding this work under Project No. 2008/37/47/BRNS/2502 dated 28/01/2009. One of the authors, Dinesh B. Mahadik is highly grateful to the DAE-BRNS for the Junior Research Fellowship. The authors are highly thankful to Dr. Rajam, Dr. Harish, Dr. Selvakumar, Surface Engineering Division, National Aerospace Laboratory, Bangalore, for their help in FESEM characterization of aerogels.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahadik, D.B., Rao, A.V., Kumar, R. et al. Reduction of processing time by mechanical shaking of the ambient pressure dried TEOS based silica aerogel granules. J Porous Mater 19, 87–94 (2012). https://doi.org/10.1007/s10934-011-9451-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-011-9451-3