Abstract
The present paper deals with the synthesis and characterization of low density and hydrophobic silica aerogels dried at ambient pressure using low cost sodium silicate precursor. The hydrogels were prepared by sol–gel processing of sodium silicate precursor and acetic acid catalyzed water followed by vapour passing and solvent exchange with methanol. The mixture of MeOH:trimethylchlorosilane (TMCS):hexane was used for the end capping of the silanols present on the silica surface. The process was optimized by varying vapour passing time, gel ageing time, molar ratios of H2O/Na2SiO3, CH3COOH/Na2SiO3 and TMCS/Na2SiO3 and silylation period. The aerogels have been characterized by bulk density, % of volume shrinkage, porosity, Fourier transform infrared spectroscopy, thermogravimetric and differential thermal analysis and contact angle measurements. The best quality silica aerogels in terms of low density (0.073 g/cc), higher porosity (96%) and better hydrophobicity (θ = 146°) have been obtained with the molar ratio of Na2SiO3:H2O:CH3COOH:TMCS at 1:166.6:2.25:11.9.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Silica aerogels are a class of open-cell mesoporous solid materials with numerous unique properties such as high surface area (∼1,000 m2/g), low density (∼0.03 g/cc), high optical transmission (∼90%), small refractive index (∼1.01–1.1) and low thermal conductivity (∼0.02 W/m.K) [1–4]. Due to these unusual properties, silica aerogels have a variety of applications. Particularly, silica aerogels with low density and hence with low thermal conductivity are of great interest for various thermal insulation applications such as solar energy systems, refrigerators, thermos flasks and cold storages [5–7]. However, conventional production of silica aerogels includes the expensive raw materials such as tetramethoxysilane (TMOS) or tetraethoxysilane (TEOS) and supercritical drying which prohibit their commercialization.
Recently, for large scale production of silica aerogels, Schwertfeger et al. [8], developed an ambient pressure drying process for water glass based silica aerogels in which sodium silicate solution was passed through an ion-exchanger to obtain monosilicic acid by the replacement of Na+ ions with H+ ions. In this method, the formation of silicic acid is governed by molecular diffusion which is more time consuming and expensive for the large scale production of silica aerogels.
Recently, our group has produced the low density hydrophobic silica aerogels using sodium silicate precursor by an ion exchange method [9, 10] Therefore, in the present studies, we report the synthesis of low density silica aerogels dried at ambient pressure from sodium silicate precursor using a vapour passing technique. In this method, the solvent exchange was carried out using methanol solvent and, a mixture of MeOH:trimethylchlorosilane (TMCS):hexane was used for surface chemical modification. In addition, for the long term applications of silica aerogels even in humid atmosphere, attempts have been made to improve the hydrophobicity of the as produced silica aerogels.
2 Experimental
2.1 Sample preparation
Silica hydrogels were prepared by single step sol–gel process by hydrolysis and polycondensation of sodium silicate precursor in the presence of an acetic acid catalyst as per the following chemical reactions:
The preparation scheme for the synthesis of silica aerogels is shown in Fig. 1. In the present work, the as received sodium silicate of specific gravity 1.36 (Loba chemie, India, SiO2:Na2O = 3.3) was diluted with triple distilled water to have a sodium silicate solution of specific gravity 1.04. For the preparation of hydrogels, a sol was prepared by mixing the sodium silicate solution and acetic acid catalyzed water in the molar ratio of 2.4 × 10−2. The sol was then cast into cylindrical glass connector (2.8 cm in diameter and 9 cm in length), bottom sealed with aluminium foil. Following the gelation and ageing at 50 °C in a PID controlled oven, water vapour was allowed to pass through the sample for 0.5 h by replacing aluminium foil with a metallic (Aluminium) mesh. The Na+ ions were removed partially from the pores of silica network by passing water vapour through the hydrogel. Then, the wet gel was cut into pieces and subjected to pore fluid exchange with methanol solvent. The surface chemical modification was carried out by immersing the sample in MeOH:TMCS:Hexane mixture in the volume ratio of 1:1:1 at 50 °C. After 24 h, the mixture was decanted and the sample was dried at room temperature for 24 h followed by heat treatment each at 50 and 200 °C for 1 h, respectively. The temperature of the PID controlled oven was increased from 50 to 200 °C at a constant heating rate of 2 °C/min.
In order to find out the best composition for producing low density silica aerogels, several sol–gel parameters viz. vapour passing time, ageing time, molar ratios of H2O/Na2SiO3, CH3COOH/Na2SiO3 and TMCS/Na2SiO3 and silylation period, were varied systematically. The limiting values of above sol–gel parameters were decided from the observations of density, % of volume shrinkage, porosity and hydrophobicity of the as produced silica aerogels.
2.2 Characterization
The bulk density of the aerogels was measured from weight to volume ratio using a known volume of the silica aerogels and its weight measured with a microbalance (10−5 g accuracy). The % of volume shrinkage, porosity and pore volume were determined as described in our earlier paper [11]. Organic and inorganic bonds forming an aerogel structure were studied by Fourier transform infrared spectroscopy (FTIR) using a Perkin Elmer (Model No. 1760X in 400–4,000 cm−1 range) IR spectrophotometer. Thermal stability of the hydrophobic aerogels in terms of retention of hydrophobicity was observed using thermogravimetric and differential thermal analysis (TG-DTA). Hydrophobicity of the aerogels was tested by measuring the contact angle (θ) of the water droplet with the aerogel surface using travelling microscope (least count 0.001 cm) with the formula [12],
where ‘h’ is the height and ‘
’ is the width of water droplet touching the aerogel surface.
3 Results and discussion
3.1 Effect of vapour passing time (t v)
In order to study the effect of vapour passing time (t v) on the density and optical transmission, the hydrogels prepared by keeping the molar ratio of Na2SiO3:H2O:CH3COOH constant at 1:166.6:2.25 were aged at 50 °C for 3 h in an oven. The vapour passing time (t v) was varied from 0.5 to 2 h and the silylation was carried out in 24 h. It has been observed that the vapour passing treatment leads to an increase in the stiffness accompanied by an increase in the pore radius of the gel network [13]. Therefore, the permeability (D) of the gel network increases according to the Carmen–Kozeny equation [14].
where ρ b is the bulk density and r is the pore radius. For a fixed ρ b, larger r values increase the permeability of the gel network making solvent exchange and silylation mechanisms easier.
However, from the plot of density versus vapour passing time (t v) shown in Fig. 2, it has been observed that with the increase in t v value (>0.5 h), the density of silica network increases gradually. This is due to the fact that the extent of gel shrinkage increases with the t v value due to the pressure exerted on the walls of the silica network until the bulk modulus of the gel network increases enough to resist the pressure exerted on the walls of silica network. As the gel shrinks, the SiO2 particles pack together more closely leading to an increase in the bulk density of the aerogel. Also, it has been observed that the optical transmission of the aerogel increased with t v value. It might be due to the fact that the % of removal of Na+ ions trapped in the silica network increases with increase in t v value which in turn causes an increase in the optical transmission of the aerogel (Table 1).
3.2 Effect of gel ageing time (t a)
To study the effect of gel ageing time (t a) on the physical properties of silica aerogels, the gels were allowed to age for different periods ranging from 0 to 4 h by keeping vapour passing time, silylation period and the molar ratio Na2SiO3:H2O:CH3COOH:TMCS constant at 0.5, 24 h and 1:166.6:2.25:11.9, respectively. Figure 3 shows the variation in the density of the aerogel with ageing time. It has been observed that the density of unaged silica aerogels is higher (0.14 g/cc) and decreases to 0.073 g/cc for 2 h ageing and increases slightly with further increase in ageing time up to 4 h. This variation in density is due to the fact that the number of Si-O-Si bonds increases long after gelation. At the point of gelation only Q2 species (Q n = silicon atom linked to n oxygen atoms) are found, hence the gel network is weak and cannot withstand pressure exerted during the vapour passing treatment. This leads to the irreversible shrinkage of the gel during drying causing an increase in the bulk density of the aerogel. Whereas, the proportions of Q3 and Q4 species increase with time and causes coarsening of the gel network during ageing leading to the growth of necks due to dissolution and reprecipitation of smaller silica particles [15]. Thus, continued polymerization and coarsening of the gel stiffens and hence strengthens the gel network contributing to minimum shrinkage. Thus, reduction in volume shrinkage with increase in gel ageing time (t a) leads to low bulk density aerogels. However, for higher t a values (≥4 h) slight increase in the bulk density was observed due to syneresis, condensation and pore fluid evaporation from the gel network.
3.3 Effect of H2O/Na2SiO3 molar ratio (S)
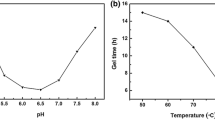
The effect of H2O/Na2SiO3 molar ratio (S) on the physical properties of silica aerogels was studied by varying the S value from 83.3 to 333.3 while keeping all other sol–gel parameters same as earlier, and ageing the hydrogels for 2 h at 50 °C. It has been observed that with an increase in S value, the gelation time of the sol increases from 1 min to 6 h (Fig. 4). This increase in gelation time with S value is due to the fact that for lower S value, the spacing between reacting silica species is small which leads to formation of 3-D silica network within a short time. However, with an increase in S value, the spacing between reacting species increases due to decrease in SiO2 content of the sol which leads to decrease in condensation reaction rate and hinders the formation of gel network resulting in a longer gelation time.
Figure 4 shows that with an increase in S value, the bulk density of the aerogels decreases first and then increases with further increase in S value. This variation in the density is due to the fact that for lower S value, due to large number of silica particles present in the sol, particles link with each other almost instantaneously and cannot grow further to larger sizes. Therefore, due to smaller pore and particle sizes, the capillary pressure exerted on the silica network is large which leads to shrinkage and cracking of the aerogels resulting in high density. Moreover, with an increase in S value (up to 166.6), due to more hydrolysis of the monomers and less silica content of the sol, particles grow to larger diameters, which leads to increase in pore size leading to minimum volume shrinkage and hence low density of the aerogels. However, further increase in S value (>166.6), leads to decrease in the bulk modulus of the gel network due to very less SiO2 content of the sol. Therefore, more shrinkage occurs during vapour passing and drying of the samples leading to dense silica aerogels.
3.4 Effect of CH3COOH/Na2SiO3 molar ratio (N)
The effect of CH3COOH/Na2SiO3 molar ratio (N) on the physical properties of silica aerogels was studied by varying the N value from 0.75 to 3.75 by keeping the molar ratio of Na2SiO3:H2O:TMCS constant at 1:166.6:11.9, respectively. Furthermore, the values of t v, t a and silylation period were kept constant at 0.5, 2 and 24 h, respectively. From the plot of gelation time versus CH3COOH/Na2SiO3 molar ratio, as shown in Fig. 5, it has been observed that the catalyst concentration strongly affects the gelation time. As the N value increases, t g value first decreases sharply, attains a minimum value at N equal to 1.5 (2 min) and again increases with further increase in N value. This can be attributed to the fact that for lower and higher values of N, the condensed species are more likely ionized and therefore mutually repulsive, causing longer gelation time [16].
The variation in the CH3COOH/Na2SiO3 molar ratio has strong influence on the density of the aerogel samples. At very low N values (<1.5), the hydrolysis and condensation reactions remain incomplete due to under stoichiometric conditions and therefore the particles cannot grow sufficiently, leading to the formation of weak network [17]. Hence, the network cannot withstand the stresses exerted during vapour passing treatment and drying of the samples, leading to increase in volume shrinkage which results in an increase in the density of the aerogel. With an increase in the CH3COOH/Na2SiO3 molar ratio, the rate of condensation reaction also increased and it was found to be maximum for N value equal to 1.5. At this stage, as the clusters have very little time to consume the available silica, the smaller clusters of silica grow in the sol and get linked to each other almost instantaneously to form a gel which leads to an increase in the density of the aerogel. Further increase in the catalyst concentration leads to the formation of a gel network with pores and particles of larger size due to mutual repulsion between the likely ionized condensed species. Therefore, the capillary pressure (P r ) exerted on the network will be low according to Laplace equation [18],
where, γ is the surface tension of the pore liquid, the negative sign is due to the negative radius of curvature, θ is the contact angle between the liquid and the pore wall and r is the pore radius. Thus, with an increase in pore and particle sizes, the capillary pressure decreases. Therefore, reduction in percentage of volume shrinkage and cracking of the sample was observed leading to decrease in the bulk density of the aerogels.
3.5 Effect of TMCS/Na2SiO3 molar ratio
To have silica aerogels with low density and better hydrophobicity, the surface hydroxyl groups of silica surface are to be replaced by organosilicon groups [(CH3)3-Si group] that are nonfunctional to participate in the further condensation reactions, using trimethylchlorosilane as a surface modifying reagent. During the surface modification the following reactions may occur [19].



As described in reaction (6), the reaction between TMCS and pore water is so rapid to yield hexamethyldisiloxane (HMDSO) and HCl, it may cause cracking of the gel. Therefore, to avoid the reaction between TMCS and pore water, prior to the surface modification, the pore water of silica hydrogel was exchanged with methanol solvent. Furthermore, hexane used in the reaction mixture helps to decrease both the reaction rate of TMCS with the pore water and capillary stress during drying due to its low surface tension. Therefore, associated drying shrinkage and cracking of the gels were avoided up to some extent. To modify the surface of silica aerogel, TMCS/Na2SiO3 molar ratio was varied from 4.74 to 11.9 by keeping all other sol–gel parameters same as earlier and CH3COOH/Na2SiO3 molar ratio constant at 2.25. Figure 6 shows the change in density of the aerogel with the variation of TMCS/Na2SiO3 molar ratio. At lower TMCS/Na2SiO3 molar ratio (4.74), the cluster surfaces are covered with a few Si-(CH3)3 groups leading to less hydrophobicity and higher density due to the condensation of surface OH groups. However, with increase in the TMCS/Na2SiO3 molar ratio, the number of Si-(CH3)3 groups attached to silica surface increases, minimizing the condensation reactions which lead to an increase in pore volume (Table 2) and hence decrease in the density of the aerogels.
Figure 7 shows the FTIR spectra of the aerogels with the variation of TMCS/Na2SiO3 molar ratio. The IR absorption peak at 1,095 cm−1 corresponds to Si-O-Si bonding and the absorption peaks at around 3,000 and 1,400 cm−1 correspond to different modes of vibrations (stretching and bending) due to C–H bonding [20, 21]. For lower TMCS/Na2SiO3 molar ratio, it has been observed that (curve a of Fig. 6) the C–H absorption peaks at 3,000 and 1,400 cm−1 are negligible while the O–H peaks at around 3,500 and 1,600 cm−1 are stronger, indicating hydrophilic nature of the aerogel. However, the intensity of O–H absorption peaks decreased and that of C–H peaks increased with an increase in the TMCS/Na2SiO3 molar ratio clearly confirming an increase in the hydrophobicity of the aerogels.
3.6 Effect of silylation period
In order to study the effect of silylation period on the density of the aerogels, the silylation period was varied from 12 to 24 h by keeping the vapour passing time and ageing time constant at 0.5 and 2 h, respectively, with the molar ratio of Na2SiO3:H2O:CH3COOH:TMCS constant at 1:166.6:2.25:11.9, respectively. From the plot of density versus silylation period (Fig. 8), it has been observed that with an increase in silylation period from 12 to 24 h, the density of silica aerogel decreases from 0.120 to 0.073 g/cc. This might be due to the fact that, as the silylation mechanism proceeds by diffusion of silylating mixture (MeOH:TMCS:Hexane) into the pores of the gel network, the replacement of H from the surface hydroxyl groups with non-polar Si-(CH3)3 groups increases with time. For the lower silylation period, the replacement of H by Si-(CH3)3 groups remains incomplete which leads to a decrease in the volume of the aerogel during drying due to the condensation of non-modified surface Si-OH groups causing an increase in the bulk density of the aerogel. However, with an increase in the silylation period, the number of non-polar Si-(CH3)3 groups attached to the silica surface increases resulting in decrease in the volume shrinkage and hence resulting in low density aerogels.
The FTIR spectroscopic investigations shown in Fig. 9 indicate that for lower silylation period the absorption peak at 1,600 and 3,500 cm−1 corresponding to O–H is dominant and the absorption peaks corresponding to C–H at 2,900 and 1,450 cm−1 are negligible. However, with an increase in silylation period the absorption peaks due to C–H at 2,900 and 1,450 cm−1 become stronger and O–H bond peaks at 1,600 and 3,500 cm−1 decreased indicating an increase in the surface chemical modification. This fact reflects in the contact angle measurements as shown in Table 2. With an increase in silylation period from 12 to 24 h, the contact angle increased from 138° to 146°.
The TG-DTA curves for a low density and hydrophobic sample are shown in Fig. 10. It can be seen from the figure that up to a temperature of 451 °C there is a very little weight loss and further increase in temperature causes sudden weight loss along with a strong exothermic peak corresponding to the oxidation of surface organic groups. The sample became hydrophilic after heating it to a temperature of about 470 °C.
4 Conclusions
Low density and hydrophobic silica aerogels were prepared from waterglass precursor using trimethylchlorosilane as a surface modifying reagent. It was observed that the sol–gel parameters strongly affect the bulk density of the aerogels. The bulk density of silica aerogels was found to be minimum at medium values of ageing time (2 h), molar ratios of H2O/Na2SiO3 (166.6) and CH3COOH/Na2SiO3 (2.25). It has been observed that the density of the aerogels decreases with an increase in the molar ratio of TMCS/Na2SiO3 and silylation period, whereas a slight increase in density was found with an increase in vapour passing time. The replacement of H from the surface OH groups with Si-(CH3)3 make the surface non-polar and hence hydrophobic resulting in higher contact angle. The hydrophobicity of the aerogel increased with increase in TMCS/Na2SiO3 molar ratio. The FTIR investigations showed a clear evidence of Si-(CH3)3 bonding. TG-DTA studies showed that the aerogels are thermally stable up to a temperature of 450 °C in the air atmosphere. The best quality silica aerogels in terms of low density (∼0.073 g/cc), higher porosity (∼96%) and better hydrophobicity (θ = 146°) were obtained for the molar ratios of Na2SiO3:H2O:CH3COOH:TMCS at 1:166.6:2.25:11.9, respectively with the values of vapour passing time, ageing time and silylation periods constant at 0.5, 2 and 24 h, respectively.
References
L.W. Hrubesh, Chem. Ind. 17, 824 (1990)
G.C. Bond, S. Flamerz, A. Emmerling, Appl. Catal. 33, 219 (1987)
J. Fricke, A. Emmerling, Struct. Bond. 77, 27 (1992)
C.A.M. Mulder, J.G. Van Lierop, in Aerogels, ed. by J. Fricke (Springer, Berlin, 1986), p. 68
S. Svendson, J. Non-Cryst. Solids 145, 240 (1992)
V. Wittwer, J. Non-Cryst. Solids 145, 233 (1992)
Z. Deng, J. Wang, A. Wu, J. Shen, B. Zhou, J. Non-Cryst. Solids 225, 101 (1998)
F. Schwertfeger, D. Frank, M. Schmidt, J. Non-Cryst. Solids 225, 24 (1998)
A.P. Rao, A.V. Rao, G.M. Pajonk, Appl. Surf. Sci. 253, 6032 (2007)
A.P. Rao, A.V. Rao, G.M. Pajonk, P.M. Shewale, J. Mater. Sci. 42, 8418 (2007)
A.P. Rao, G.M. Pajonk, A.V. Rao, J. Mater. Sci. 40, 3481 (2005)
J.J. Bikerman, Surface Chemistry: Theory and Applications, 2nd edn. (Academic, New York, 1958), p. 343
G. Reichenaver, J. Non-cryst. Solids 350, 3482 (2004)
A.E. Scheidegger, The Physics of Flow Through Porous Media, 3rd edn. (University of Torento Press, Torento, 1974)
C.J. Brinker G.W. Scherer, Sol–Gel Science (Academic, San Diego, 1990), p. 358
C.J. Brinker G.W. Scherer, Sol–Gel Science (Academic, San Diego, 1990), p. 105
A.V. Rao, M.M. Kulkarni, D.P. Amalnerkar, Tanay Seth, J. Non-Cryst. Solids 330, 190 (2003)
P.C. Heimenz, Principles of Colloid and Surface Chemistry (Marcel Dekkar, New York, 1977)
C.J. Lee, G.S. Kim, S.H. Hyun, J. Mater. Sci. 37, 2237 (2002)
C.J. Pouchert (ed.) Aldrich Library of FTIR Spectra, vol 2 (Aldrich Chemical, Milwaukee, 1985)
M. Laczka, K. Cholewa-Kowalska, M. Kogul, J. Non-Cryst. Solids 287, 10 (2001)
Acknowledgements
The authors are grateful to the Condensed Matter Advisory Committee, Department of Science and Technology (DST), New Delhi, Government of India, for the financial support for this work through a major research project on “Aerogels” (No. SR/S2/CMP-67/2006). Dr. A. Parvathy Rao is highly thankful to the DST for the Senior Research Associateship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shewale, P.M., Rao, A.V., Gurav, J.L. et al. Synthesis and characterization of low density and hydrophobic silica aerogels dried at ambient pressure using sodium silicate precursor. J Porous Mater 16, 101–108 (2009). https://doi.org/10.1007/s10934-007-9173-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-007-9173-8