Abstract
Stephanodiscus niagarae Ehrenberg is currently restricted to specific regions of central Mexico, however, during the late Pleistocene, it had a wider distribution in the country. This change in distribution is similar to those observed for several organisms that migrated southwards during cold, glacial climates, supporting the hypothesis that central Mexico acted as glacial refugia for these species. This study aims to support this hypothesis for S. niagarae as well as to analyze its ecological distribution in modern environments in central Mexico. For this purpose we studied 18 samples from 16 lakes located around Mexico City, selected among 46 lakes along the Trans-Mexican Volcanic Belt. Diatom assemblages in superficial sediments, and climatic, hydrochemistry, and nutrient parameters of each lake were analyzed by means of canonical correspondence analyses. Additionally, we created an ecological niche model (ENM) with modern occurrence data (n = 47) and environmental variables (WorldClim) to produce potential distribution maps of S. niagarae during the present time and under the LGM conditions in the Nearctic realm. S. niagarae was recorded only in 4 sites in central Mexico (abundances < 10%) associated with temperate, subhumid conditions in freshwater lakes with [Mg2+] − [Ca2+] − [HCO3−] ionic dominance and high turbidity, mesotrophic to hypertrophic systems (based on chlorophyll a values), but with a tendency to P-limitation. In our study sites S. niagarae showed low abundances in diatom assemblages dominated by Aulacoseira spp. Temperature (annual mean, coldest and warmest quarters means) was identified by ENM as the main environmental variable controlling its distribution, with its highest modern support in the USA, southern Canada, and a restricted distribution in the highlands of western and central Mexico. Whereas, the LGM scenario (− 5.5 °C) identified the western and central highlands in Mexico and southern USA as the highest probability distribution areas supporting the approach that the Sierra Madre Occidental could have acted as a migration corridor offering suitable habitats for a southward migration into central Mexico during colder (glacial) periods. In conclusion, S. niagarae distribution in the central and western mountains of Mexico is controlled by temperature changes and its presence may be associated with colder (glacial) periods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stephanodiscus niagarae Ehrenberg (1845) is a diatom species that has been widely studied in North America, in both modern environments and fossil records (Theriot and Stoermer 1984a; Stoermer et al. 1989). In modern environments it is common in all the Great Lakes and surrounding areas of Canada and the USA (Theriot and Stoermer 1984b; Håkansson and Kling 1989; Julius et al. 1998; Pla et al. 2005; Lashaway and Carrick 2010; Reavie et al. 2014a) and it has also been reported from sites in north-western USA (Oregon, Washington, Montana and Wyoming) (Edmondson et al. 2003; Bradbury et al. 2004; Theriot et al. 2006). It is recognized as a good ecological indicator by several authors that consider it a planktonic diatom in freshwater bodies with circumneutral pH (Theriot and Stoermer 1984a; Fritz et al. 1993; Bradbury 2000). The presence of S. niagarae is also associated with temperate to cold conditions in modern (Stoermer and Ladewski 1976; Brugam 1983; Bradbury et al. 2004; Colman et al. 2004; Reavie et al. 2014b) and paleolimnological studies (Bradbury 1971, 2000). Reavie et al. (2014a, b) observed that in the Great Lakes, particularly in lake Erie, S. niagarae was most abundant during spring associated with cool, high-nutrient, and turbid environments. This diatom has been found in a wide range of trophic conditions, even though it seems to have a preference for eutrophic lakes (Rawson 1956; Theriot and Stoermer 1984a), however, its abundance decreases in areas with heavy industrial pollution and habitat disturbance (Stoermer and Jang 1970; Julius et al. 1998).
In contrast to its relatively wide modern distribution in northern USA and Canada, living populations of this species in Mexico have only been reported from two sites in the central highlands of the Trans-Mexican Volcanic Belt (TMVB) (Oliva-Martínez et al. 2005; Valadez et al. 2005). Nevertheless, this diatom was a much more abundant taxon in the region during the late Pleistocene (Davies et al. 2002) and its abundance reduced greatly during the early Holocene (Valadez et al. 2005). We have documented 9 sites (Fig. 1a, ESM1) where S. niagarae was abundant in late Pleistocene to early Holocene sediments. In six of them it was present in sediments dating to the last glacial (~ 70–12 ka BP): Babícora (Metcalfe et al. 2002), Pátzcuaro (Bradbury 2000), Zacapu (Metcalfe 1995), Cuitzeo (Israde-Alcántara et al. 2002, 2010b, 2018), Tecocomulco (Caballero et al. 1999) and Chalco (Caballero and Ortega Guerrero 1998; Caballero et al. 2019). And in three it was also abundant in sediments dating to the previous glaciation (> 120 ka BP): Cuitzeo (Israde-Alcántara et al. 2002), Texcoco (Bradbury 1971), and Chalco (Avendaño et al. 2018).
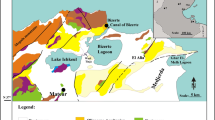
Location maps. a Map of Mexico with the Trans-Mexican Volcanic Belt (light gray shaded area) and the location of the 9 fossil records of Stephanodiscus niagarae: 1. Babícora, 2. Chapala, 3. Zacapu, 4. Pátzcuaro, 5. Cuitzeo, 6. Chignahuapan, 7. Texcoco, 8. Chalco, 9. Tecocomulco. b Research area with the location of study sites around Mexico City (black lines show state borders and blue line delimits the Basin of Mexico): San Juanico (SJN), La Huaracha (HUA), Danxhó (DXHO), Zumpango (ZUM91 and ZUM18), La Luna (LUN), El Sol (SOL), Zempoala (ZEM), Coatetelco (COA), Texcoco Artemia (ART), Texcoco Jalapango (JTX), Texcoco Recreativo (TXR), Texcoco Nabor Carrillo (NCTX), Chalco (CHA91 and CHA16), Acuitlapilco (ACUI), Atlangatepec (ATL), and Tecocomulco (TEC)
This change in the geographic distribution of S. niagarae between the late Pleistocene and present seems to be related to the extensive changes in climate and landscape conditions in North America during late Pleistocene compared to the Holocene (present). Certainly, there is wide evidence that some plants, insects and vertebrate species migrated southwards during cold glacial climates, when the continental ice sheets extended over North America (Graham et al. 1996; Hewitt 2000; Jackson et al. 2000; Waltari et al. 2007; Pardi and Graham 2019). For example, evidence of fossil Pleistocene mammals in Mexico suggest a southern displacement from the USA along corridors of suitable climates, with central Mexico acting as a glacial refugia for mammals as well as for other organisms (Ceballos et al. 2010; Pardi and Graham 2019). Even though such studies have seldom been undertaken with small, aquatic organism such as diatoms, here we propose that central Mexico was also a refugia for S. niagarae during past glacial periods. This is not the first time that the concept of glacial refugia has been applied for diatoms, as for example Spaulding et al. (1999, 2010) proposed that the presence of endemic lineages of diatoms in Antarctica responded to the continuous presence of ice-free habitats that acted as refugia during the repeated glacial cycles of the Cenozoic.
To support the refugia hypothesis, in this research we used ecological niche models (ENMs) to generate suitable habitat maps for S. niagarae under modern climatic conditions and also during the cooler, last glacial maximum (LGM, 26–19 ka cal BP). We also aimed to analyze the present distribution of S. niagarae in central Mexico and its relationship with environmental variables such as temperature, salinity, ionic dominance and nutrient levels in lakes located along the TMVB. This research could contribute to future paleolimnologic and paleoclimatic reconstructions of Pleistocene glacial intervals in Mexico, when this species was most abundant.
Study sites
Based on the high abundances (30%) reported for S. niagarae in modern samples from the Santa Elena canal (Oliva-Martínez et al. 2005; Valadez et al. 2005), we decided to resample this site to record the environmental variables where this species lives today in central Mexico. However, when we visited the site, the canal was dry and could not be sampled, but we realized that it drained a nearby dam (Danxhó) from where we took water and surface sediment samples. These samples confirmed the presence of S. niagarae in the water column and sediments from Danxhó, and we now believe that the S. niagarae population previously reported for the Santa Elena canal might ultimately have come from the nearby dam. This site (Santa Elena-Danxhó), located to the NW of Mexico City, was the only one among 40 lakes in the TMVB where relatively high abundances (> 10%) of S. niagarae were identified (Davies et al. 2002; Caballero et al. 2019), therefore for this study we decided to analyze in further detail the distribution of this species in other lakes around Mexico City, in an area defined by the following geographical coordinates: 18° 36′–20° 12′ N and 97° 53′–100° 18′ W (Fig. 1b). Within the defined study region, 10 of the 40 lakes from the Caballero et al. (2019) central Mexico modern diatoms database were included, and we add in this work data from 6 new sites and 2 sites that were resampled (Zumpango and Chalco) between 2016 and 2018, giving a total of 18 samples from 16 sites around Mexico City where the presence and distribution of S. niagarae was explored.
The study region is located in the central area of the TMVB, where topography defines closed lacustrine basins of tectonic and volcanic origin (Fig. 1a). Altitude ranges from 980 to 4280 m asl, and it is associated to a climatic gradient from warmer conditions in the lowlands, with annual average temperatures of ~ 24 °C, to high-altitude cold climates with annual averages of ~ 4 °C. Precipitation ranges from ~ 560 to ~ 1200 mm and it is concentrated during a summer rainy season, even though occasional rainfall also occurs during the winter (SMN 2019).
Materials and methods
Sampling and analytical methods for all sites followed those described in Sigala et al. (2017). In brief, geographical location (latitude, longitude, altitude) was determined using a handheld navigator (GARMIN GPSMAP 62 stc) and confirmed in Google Earth. Mean annual temperature (MAT, °C), precipitation (MAP, mm year−1) and evaporation (MAE, mm year−1) for all sites were taken from the closest meteorological stations (SMN 2019). Water depth (m) was measured in the field with a portable depth sounder (Speedtech instruments). Field measurements included Secchi disk depth (m) and depth profiles for temperature (°C), oxygen concentration (mg L−1), pH, and electric conductivity (EC, µS cm−1) determined using a multiparametric probe (Hydrolab Quanta G).
For water chemistry we took one sample at 0.5 m depth at a centrical location site of each lake. Cation samples were acidified in the field using concentrated (55%) HNO3 and all the samples were kept in refrigeration until they were analyzed. Standardized methods were used for the analysis of major ions (APHA 1995, 1998; APHA et al. 2005; Armienta et al. 2008). Total alkalinity and ions concentration units were meq L−1 and to determine ionic dominance concentrations were transformed to percentages. Salinity was expressed as total dissolved solids (TDS, mg L−1).
Nutrients determinations (APHA 1998) included ammonium, nitrites, and nitrates, which were added to express as dissolved inorganic nitrogen (DIN, µM), as well as total phosphorus (TP, µM), soluble reactive phosphorus (SRP, µM) and silica (SiO2, µM). In the field the ammonium and nitrates samples were acidified using concentrated H2SO4 (98%). The following nutrient ratios were calculated: DIN:TP, DIN:SRP, SiO2:DIN, and SiO2:SRP. For chlorophyll a (mg m−3) samples were extracted using (90%) methanol and measured using a spectrophotometer. Calculations were done based on Holden equations (Meeks 1974). Chlorophyll a and nutrients determinations were not performed for 7 of the 18 samples (Acuitlapilco, Chalco91, Coatetelco, Texcoco Artemia, Texcoco Jalapango, Texcoco Recreativo, and Zumpango91) which defined a reduced nutrient subset of 11 lakes. The information for each of the 16 studied lakes was summarized in a format similar to that presented in Sigala et al. (2017) and are presented as Electronic Supplementary Material (ESM2).
Diatom analysis
For diatom analysis superficial sediment from each site was collected with a Ekman dredge. 0.5 g of dry sediment was treated with (10%) HCl to eliminate carbonates and (30%) H2O2 to digest organic matter. If necessary, 5 ml of HNO3 was added to accelerate the elimination of organic matter. Permanent slides were prepared with 200 µl aliquots of final solution using Naphrax (refraction index of 1.66) as mounting medium. Each sample was analyzed using a light microscope (Olympus BX50) at 1000 ×. A minimum of 100 to 400 valves was counted for each sample and relative abundances were calculated as species percentages. Diatoms were identified based on Håkansson and Locker (1981); Theriot and Stoermer (1981); Krammer and Lange-Bertalot (1986, 1988, 1991); Gasse 1986; Krammer et al. (1991); Håkansson (2002); Yu (2011). To confirm the taxonomy of some species, including S. niagarae, valves were observed using a scanning electron microscope (SEM JEOL JCM-6000PLUS). A detailed description and illustrations of S. niagarae in the lakes from central Mexico is included in Electronic Supplementary Material (ESM3).
Statistical analyses
Canonical correspondence analyses (CCAs) were performed with each of the two data subsets, identified as hydrochemistry (n = 18) and nutrients (n = 11). The environmental variables used were selected to avoid high correlation between them, correlation between variables was tested by means of an exploratory principal component analysis (not shown). The hydrochemistry CCA was done using temperature (MAT), precipitation (MAP), TDS, percentage of major ion concentrations ([CO32− + HCO3+], [Cl−], [Ca2+], [Mg2+] and [Na+ + K+]), and pH. Caballero et al. (2019) found that the two high altitude sites El Sol and La Luna represented outliers along the temperature gradient in the central Mexico data set, therefore we excluded these lakes from this analysis. The nutrient CCA was performed using DIN, SRP and Secchi disk depth. All variables (except pH) and diatom abundances were transformed (log10 + 1) to stabilize their variance. All of the analyses were carried out using the R software (R Development Core Team 2009), especially the “vegan” package (Oksanen et al. 2019).
Ecological niche modeling
Modern occurrences in the USA and Canada for S. niagarae were documented through a bibliographic review using online scientific literature databases. One occurrence point for each lake was taken, except for larger lakes with several sampling sites, when different points were considered if they were at least 20 km apart. Our final list of modern occurrences for S. niagarae consisted of 47 sites, 43 for the USA and Canada (ESM1) and four of our locations in central Mexico (Acuitlapilco, Danxhó, La Huaracha and San Juanico, ESM2). The nine late Pleistocene occurrences of S. niagarae in Mexico were also identified through a bibliographic review (ESM1).
To characterize the ecological niche of S. niagarae we used bioclimatic variables (2.5 arc minute spatial resolution, ~ 5 km) from the WorldClim database (version 1.4 data for 1960–1990, Hijmans et al. 2005). These variables were used as environmental predictors, and clipped to adjust to the Nearctic realm, which extends from Canada to central Mexico. We calculated Pearsons’s correlation coefficients for all pairs of bioclimatic variables to avoid collinearity between them and 11 out of the 19 bioclimatic variables were chosen (ESM1) by their low correlation (|r|< 0.25) using the ‘NicheToolbox’ package (Osorio-Olvera et al. 2020). The potential distributional area during the LGM of S. niagarae was modeled using analogous data layers from the Community Climate System Model (CCSM4, Gent et al. 2011). These layers were also clipped for the Nearctic realm.
The ENM was built using Maxent (Phillips et al. 2006) via the ‘kuenm’ package (Cobos et al. 2019). For model calibration, we created candidate models by combining the 11 selected bioclimatic variables into 9 sets of environmental predictors (ESM1) all sets including annual mean temperature (BIO1). Model calibrations were performed with 17 combinations of regularization multipliers (0.1–1.0 at intervals of 0.1, 2–6 at intervals of 1, 8 and 10) and all possible combinations of linear, quadratic and product feature classes. We selected the best model according to the ‘kuenm’ criteria of statistical significance based on low partial receiver operating characteristic (ROC) values, predictive power calculated by low omission rate at 5% and model complexity using Akaike information criterion (AICc), delta AICc ≤ 2 and high AICc weights, in that order of priority (Cobos et al. 2019). The final model was created with the best selected parameter set using 10 replicate bootstrap resamplings and final model transfers to the LGM scenario using an extrapolation with clamping (extrapolation which considers a constant response in areas with different environments from those in the calibration area) (Cobos et al. 2019) onto past-climate CCSM4 for the Nearctic zone. The projected LGM scenario was also validated using the distribution of S. niagarae late Pleistocene sites in Mexico.
Results
Salinity and ionic dominance
The 18 samples included in this study ranged from freshwater to mesosaline, and they had a wide range of ionic dominances (Fig. 2). In the anion field (Fig. 2a) most of the freshwater lakes (TDS < 500 mg L−1) had a [HCO3−] + [CO32−] dominance, the subsaline lakes (500–3000 mg L−1) had a higher proportion of sulfates and chlorides and those with the highest salinities (TDS > 3000 mg L−1, hyposaline to mesosaline) showed [Cl−] dominance. On the cation diagram (Fig. 2b) there is also a clear gradient from the freshwater lakes dominated by [Ca2+] − [Mg2+] to the higher salinity lakes (hyposaline to mesosaline) dominated by [Na+] + [K+].
Nutrients and trophic level
Trophic status of the lakes in this study was determined based on their chlorophyll a values (OECD 1982), and they ranged from ultra-oligotrophic (La Luna) to hypertrophic (Chalco16, La Huaracha, Texcoco Nabor Carillo, Zumpango18). Possible nutrient limitation in these lakes was identified according to their DIN and SRP concentrations compared to the nutrient starvation limits of 0.1 μM for SRP and 7 μM for DIN (Reynolds 1999), as well as whether their DIN:SRP ratios were above or below the 16N:1P Redfield ratio (Redfield 1958) or the 16Si:1P and 1Si:1N ratios (Xu et al. 2008) (Fig. 3a). According to these criteria, five sites have N:P ratios above the 16N:1P Redfield ratio (P limitation) but only four (Danxhó, La Huaracha, La Luna, and Zempoala) had SRP concentrations below the starvation limits and could therefore be considered to be limited by this element. On the other hand, one lake (El Sol) showed concentrations below the starvation values for both DIN and SRP and therefore was co-limited by both nutrients. Regarding SiO2, only Texcoco Nabor Carrillo had Si:SRP ratios below the 16Si:1P ratio suggesting silica limitation (Fig. 3b).
Nutrient concentrations comparisons of 11 lakes in central Mexico. a Variation of DIN:SRP stoichiometric ratios, diagonal line represents the Redfield (1958) N:P = 16:1 ratio, shaded areas represent nutrient limiting values according to Reynolds (1999) SRP < 0.1 µM y DIN < 7 µM. b Variation of Si:SRP and DIN:SRP stoichiometric ratio, diagonal line represents Si:N = 1:1 ratio according to Redfield (1958). Abbreviations correspond to Fig. 1. Trophic categories are based on annual maximum chlorophyll values according to OECD (1982)
Distribution of Stephanodiscus niagarae in central Mexico
Frustules of Stephanodiscus niagarae were identified only in four of the 18 analyzed samples: La Huaracha, San Juanico, Danxhó, and Acuitlapilco, even though relative abundances were low (< 10%). In fact these were the only lakes in which this species was present in percentages above 1% in the full set of 46 lakes in central Mexico (Caballero et al. 2019) for which we have data (Fig. 4). Scarce valves of S. niagarae were counted in two lakes (Burro and Teremendo), but they only reached relative abundances of ~ 0.5%. In other two lakes (Pátzcuaro and Atotonilco) some fragments of S. niagarae were also observed, but they were considered to represent reworked material as older lacustrine sediments outcrop within their basins (Davies et al. 2002; Israde-Alcántara et al. 2010a). The highest relative abundance was observed in La Huaracha with 6.0% followed by Danxhó 5.3%, San Juanico 4.1%; and the lowest was in Acuitlapilco with 1.1%. Total diatom abundance in these lakes ranged from 2.0 to 70.6 × 106 valves g−1 of dry sediment and therefore the lake with the highest concentrations of S. niagarae expressed as valves per gram of dry sediment was Danxhó (3.8 × 106), followed by La Huaracha (0.9 × 106), with low values in San Juanico (0.08 × 106), and Acuitlapilco (0.05 × 106).
Relative abundances of Stephanodiscus niagarae in 46 lakes studied along the Trans-Mexican Volcanic Belt, central Mexico (including new sites from this study and those from Caballero et al. 2019) in relation with altitude, mean annual temperature (MAT) and precipitation (MAP), total dissolved solids (TDS), Secchi disk depth, Chlorophyll a (Chl a), dissolved inorganic nitrogen (DIN), soluble reactive phosphorus (SRP), and SiO2. Dashed lines show average values of the variables for the lakes where Stephanodiscus niagarae was present
The diatom assemblages in these four lakes were dominated by Aulacoseira spp., either A. ambigua (Grunow) Simonsen (1979) in Huaracha and Danxhó or A. granulata (Ehrenberg) Simonsen (1979) and its variety A. granulata var. angustissima (O.Müller) Simonsen (1979) in San Juanico and Acuitlapilco. Other diatom species that were abundant in these sites were Cyclostephanos dubius (Hustedt) Round in Theriot et al. (1988) in Huaracha, Aulacoseira alpigena (Grunow) Krammer (1991) in San Juanico, Asterionella formosa Hassall (1850) and Fragilaria crotonensis Kitton (1869) in Danxhó, and Cyclotella meneghiniana Kützing (1844) in Acuitlapilco.
The four lakes where S. niagarae was present were preferentially located at altitudes around 2500 m asl (Fig. 4), associated to cool and humid conditions (MAT ~ 15 °C, MAP ~ 800 mm year−1). They were freshwater lakes (with TDS ≤ 500 mg L−1), with turbid waters (Secchi disk depth < 1 m) and mesotrophic to hypertrophic conditions according to their chlorophyll a concentrations (Fig. 4). They ranged from 0.40 to 1.69 mg L−1 in DIN, 0.01 to 0.05 mg L−1 in SRP, and SiO2 from 3.1 to 21.3 mg L−1 (Fig. 4).
The variance inflation factors (VIF) of all variables in the CCA performed with the hydrochemistry data set (Fig. 5a, b) were < 10 indicating low correlation between predictors. The CCA1 (λ = 0.78, p = 0.008 and proportion explained of 10%) correlated positively with TDS, [Na+ + K+] %, MAT, [Cl−] % and pH and negatively with precipitation, [Ca2+] % and [Mg2+] %. Whereas CCA2 (λ = 0.57, p = 0.5 and proportion explained of 10%) correlated positively with carbonates. The sites in the hydrochemistry CCA (Fig. 5a) follow a salinity gradient and its associated change in dominant ions from [CO32− + HCO3+] − [Ca2+] − [Mg2+] % to [Cl−] − [Na+ + K+] % dominated. The three lakes in which S. niagarae was most abundant are located to the left-bottom sector of the graph, at the low end of the [Na+ + K+] %, TDS, [Cl−] % and MAT gradients. In the species biplot (Fig. 5b) S. niagarae is also located to the left-bottom sector of the graph and it clusters with other species that were also present in the diatom assemblages of these lakes, mainly Aulacoseira ambigua, A. alpigena, and Cyclostephanos dubius, and more distantly Asterionella formosa, Aulacoseira granulata, A. granulata var. angustissima, and Fragilaria crotonensis.
Hydrochemistry canonical correspondence analysis (CCA1 vs. CCA2) of studied samples in central Mexico. a Hydrochemistry CCA (n = 16) including mean annual temperature (MAT), mean annual precipitation (MAP), mean annual evaporation (MAE), total dissolved solids (TDS), percentage of major ion concentrations ([CO32− + HCO3+], [Cl−], [Ca2+], [Mg2+] and [Na+ + K+]), and pH. b Diatom species distribution in the hydrochemistry CCA. Abbreviations correspond to Fig. 1. Squares represent sites where S. niagarae is present. Location of S. niagarae is the red star and blue words. The full names of the species and their codes are listed in Electronic Supplementary Material 1 (ESM1). (Color figure online)
The VIF of all variables in the CCA performed with the nutrients subset (Fig. 6a, b) were < 3 indicating low correlation between predictors. CCA1 (λ = 0.87, p = 0.001 and proportion explained of 16%) correlated positively with DIN and negatively with Secchi disk depth. CCA2 (λ = 0.81, p = 0.007 and proportion explained of 15%) corresponded negatively with SRP. This nutrient CCA shows that the sites are distributed along a trophic gradient (Fig. 6a) with the lower trophic level and clearer water (higher Secchi disk depth) sites located to the left of the diagram. In the species biplot (Fig. 6b) the diatom species clearly split in three groups, to the left those most abundant in clear (high Secchi disk depth) waters with low SRP and DIN, to the bottom-right, those present in turbid waters (low Secchi disk depth) with higher SRP and to the top-right, those preferring turbid waters with higher DIN values. S. niagarae is located in this last group of diatoms and it clusters with the same species as in the hydrochemistry CCA, mainly A. ambigua and, C. dubius, more distantly Asterionella formosa, Aulacoseira alpigena, A. granulata, A. granulata var. angustissima, and Fragilaria crotonensis.
Nutrient canonical correspondence analysis (CCA1 vs. CCA2) of studied samples in central Mexico. a Nutrient CCA (n = 11) including Secchi disk, dissolved inorganic nitrogen (DIN), soluble reactive phosphorus (SRP); b diatom species distribution in the nutrient CCA. Abbreviations correspond to Fig. 1. Squares represent sites where S. niagarae is present. Location of S. niagarae is the red star and blue words. The full name of the species and their codes are listed in Electronic Supplementary Material 1 (ESM1). (Color figure online)
Ecological niche modeling
We created 1071 candidate models by combining the 9 sets of environmental predictors. The results of the model evaluation showed the best model had in general a good performance with a partial ROC of 0.00, an omission rate at 5% of 0.04, delta AICc of 0.94 and weight AICc of 0.55. The best candidate model corresponded to environmental variables set 8 (ESM1), that included as environmental predictors mean annual temperature (BIO1) and the mean temperatures of the warmest and the coldest quarters (BIO10 and BIO11 respectively), and linear-product as feature class.
The modern potential distribution map of S. niagarae (Fig. 7a), showed a high probability of distribution in southern Canada and northern USA, with a more restricted probability of distribution along the western mountains of Mexico (Sierra Madre Occidental) and on the TMVB in central Mexico. Whereas ENM transfer to the LGM scenario (Fig. 7b) shows an expanded potential distribution in the southern part of the USA and along the highlands of western and central Mexico. For the LMG scenario, the late Pleistocene fossil observations (Fig. 7b) were located in the higher probability distribution areas, indicating a good performance of the model.
Ecological niche models (ENMs) of Stephanodiscus niagarae. a Potential distribution map of S. niagarae during present time. Points correspond to sites with reported living populations of S. niagarae (Electronic Supplementary Material 1, ESM1) used for the calibration and evaluation of the model. b Projected distribution under last glacial maximum (LGM) conditions. Squares show fossil locations during last glacial maximum (ESM1)
Discussion
Ecological distribution of Stephanodiscus niagarae in central Mexico
In this paper we examined the modern distribution of Stephanodiscus niagarae in central Mexico and its relationship with environmental variables. From a set of 46 lakes in the TMVB (new sites in this work and those in Caballero et al. 2019) it was only present with abundances higher than 1% in 4 of them (La Huaracha, San Juanico, Danxhó and Acuitlapilco). These lakes were located in surrounding areas of Mexico City, at altitudes of around 2500 m asl, with cool, humid conditions (MAT ~ 15 °C, MAP ~ 800 mm year−1). We studied in more detail the distribution of S. niagarae in 18 samples collected within a region around Mexico City, exploring its relationship with hydrological variables and trophic conditions.
The hydrochemistry CCA ordination (Fig. 5a, b) separates the three lakes where S. niagarae had its highest abundances (La Huaracha, San Juanico and Danxhó) because of their relatively low MAT, low TDS, low [Na] + [K] % and [Cl−] %, but relatively high MAP as well as a [Mg2+] − [Ca2+] % dominance. These results agree with Fritz et al. (1993, 2001), who reports that S. niagarae is rare or absent in lakes with salinity above 3000 mg L−1. In this CCA S. niagarae clusters with Aulacoseira ambigua, A. alpigena, and Cyclostephanos dubius, and more distantly with Asterionella formosa, Aulacoseira granulata, A. granulata var. angustissima, and Fragilaria crotonensis, all of which were abundant diatom taxa in these three lakes.
The nutrient CCA ordination (Fig. 6) separates the three lakes where S. niagarae had its highest abundances (La Huaracha, San Juanico and Danxhó) by their high turbidy (low Secchi disk depth), high DIN but relatively low SRP values. Even though according to their chlorophyll a concentrations these lakes ranged from mesotrophic (Danxhó) to hypertrophic (La Huaracha), all of them had high N:P ratios and two of them (La Huaracha and Danxhó) were identified as P-limited by their low SRP concentrations. These results are in agreement with Kilham et al. (1996), who reports moderate to high N:P ratios (OECD 1982) in Yellowstone lakes where S. niagarae is present. All this suggests that S. niagarae has a preference for high turbidity and relatively high productivity systems (high chlorophyll a values), but with high N:P ratios and a tendency to P limitation. Our results are also consistent with Rawson (1956) and Stoermer and Jang (1970), who consider S. niagarae as a eutrophic species. The preference of S. niagarae for relatively turbid waters can explain why this species was not present in other relatively cool, high-altitude lakes like Zempoala or El Sol, as these lakes had clear waters (high Secchi disk depths) and therefore plotted to the left of the nutrients CCA diagram. This conclusion agrees with Reavie et al. (2014b) who associated it to cold, high-nutrient and turbid environments in Lake Erie.
Current and past potential distribution of Stephanodiscus niagarae
The ENM developed identified that temperature (annual mean, coldest and warmest quarters means) was the most important environmental variable to define habitat suitability for S. niagarae in areas like northern USA and southern Canada (Fig. 7a), which are effectively the areas where S. niagarae is presently an abundant planktonic diatom (Theriot and Stoermer 1984a; Edlund et al. 2004). This ENM model showed restricted habitat suitability for this species in specific mountain regions of western (Sierra Madre Occidental) and central Mexico (TMVB) primarily because of its temperate climate due to altitude, as indicated by the preferential presence of S. niagarae in lakes located at around 2500 m asl in central Mexico.
The projected LGM scenario for S. niagarae was modeled with the CCSM4 projection which includes a mean temperature decrease of − 5.5 °C (Brady et al. 2013). This decrease in temperature is in agreement with pollen-based and diatom-based transfer functions from Lake Chalco, which estimated a temperature reduction during the LGM in central Mexico between − 4 and − 5 °C (Correa-Metrio et al. 2013; Caballero et al. 2019). The LGM scenario of S. niagarae identifies areas with highest potential in southern EUA as well as the western and central highlands in Mexico (Fig. 7b). This high probability distribution in the highlands of Mexico is confirmed by the distribution of lacustrine fossil records in north-western and central Mexico with a high abundance of S. niagarae (Fig. 7b).
Comparing the projected distributions under current and LGM conditions for S. niagarae, there are important latitudinal differences which imply an expansion of habitat suitability for this species in central Mexico during the LGM. The LGM scenario suggest that the highlands along the Sierra Madre Occidental could have acted as a migration corridor offering suitable habitats for this species to disperse into central Mexico, which is one of the migration corridors suggested by Ceballos et al. (2010).
Stewart et al. (2009) reported that temperate taxa could remain with a limited northern distribution (northern refugia), but based on the latitudinal displacements of S. niagarae during cold events, we speculate that its fundamental niche expands southwards, whereas during warm periods (e.g. present day) its maximum extension is in USA and Canada, while in Mexico is present with a scattered distribution at low densities. This southern refugia pattern is consistent with the responses of temperate taxa during glacial periods, as for example the boreal and cool-temperate conifers in eastern North America that had a southern displacement during the LGM (Jackson et al. 2000).
Our findings highlight the importance of Stephanodiscus niagarae as indicator of cold periods in the central and western mountains of Mexico. Potentially, S. niagarae could contribute in paleolimnologic and paleoclimatic reconstructions as a stratigraphic marker for previous glacial periods.
References
American Public Health Association (APHA) (1995) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
American Public Health Association (APHA) (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
American Public Health Association (APHA), American Water Works Association (AWWA), Water Pollution Control Federation (WPCF) (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
Armienta MA, Vilaclara G, De la Cruz-Reyna S et al (2008) Water chemistry of lakes related to active and inactive Mexican volcanoes. J Volcanol Geotherm Res 178:249–258. https://doi.org/10.1016/j.jvolgeores.2008.06.019
Avendaño D, Caballero M, Ortega-Guerrero B et al (2018) Environmental conditions at the end of the Isotopic Stage 6 (IS 6: > 130000 years) in the center of Mexico: Characterization of a section of laminated sediments from Lake Chalco. Rev Mex Ciencias Geol 35:168–178. https://doi.org/10.22201/cgeo.20072902e.2018.2.649
Bradbury JP (1971) Paleolimnology of Lake Texcoco, Mexico. Evidence from diatoms. Limnol Oceanogr 16:180–200. https://doi.org/10.4319/lo.1971.16.2.0180
Bradbury JP (2000) Limnologic history of Lago de Patzcuaro, Michoacan, Mexico for the past 48,000 years: impacts of climate and man. Palaeogeogr Palaeoclimatol Palaeoecol 163:69–95. https://doi.org/10.1016/S0031-0182(00)00146-2
Bradbury JP, Colman SM, Dean WE (2004) Limnological and climatic environments at Upper Klamath Lake, Oregon during the past 45 000 years. J Paleolimnol 31:167–188. https://doi.org/10.1023/B:JOPL.0000019232.74649.02
Brady EC, Otto-bliesner BL, Kay JE, Rosenbloom N (2013) Sensitivity to glacial forcing in the CCSM4. J Clim 26:1901–1925. https://doi.org/10.1175/JCLI-D-11-00416.1
Brugam RB (1983) The relationship between fossil diatom assemblages and limnological conditions. Hydrobiologia 98:223–235. https://doi.org/10.1007/BF00021023
Caballero M, Ortega Guerrero B (1998) Lake levels since about 40,000 years ago at Lake Chalco, near Mexico City. Quat Res 50:69–79. https://doi.org/10.1006/qres.1998.1969
Caballero M, Lozano S, Ortega B et al (1999) Environmental characteristics of Lake Tecocomulco, northern basin of Mexico, for the last 50,000 years. J Paleolimnol 22:399–411. https://doi.org/10.1023/A:1008012813412
Caballero M, Lozano-García S, Ortega-Guerrero B, Correa-Metrio A (2019) Quantitative estimates of orbital and millennial scale climatic variability in central Mexico during the last ∼40,000 years. Quat Sci Rev 205:62–75. https://doi.org/10.1016/j.quascirev.2018.12.002
Ceballos G, Arroyo-Cabrales J, Ponce E (2010) Effects of Pleistocene environmental changes on the distribution and community structure of the mammalian fauna of Mexico. Quat Res 73:464–473. https://doi.org/10.1016/j.yqres.2010.02.006
Cobos ME, Townsend Peterson A, Barve N, Osorio-Olvera L (2019) Kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 7:e6281. https://doi.org/10.7717/peerj.6281
Colman SM, Bradbury JP, Rosenbaum JG (2004) Paleolimnology and paleoclimate studies in Upper Klamath Lake, Oregon. J Paleolimnol 31:129–138. https://doi.org/10.1023/B:JOPL.0000019235.72107.92
Correa-Metrio A, Bush M, Lozano-García S, Sosa-Nájera S (2013) Millennial-scale temperature change velocity in the continental northern neotropics. PLoS ONE 8:e81958. https://doi.org/10.1371/journal.pone.0081958
Davies SJ, Metcalfe SE, Caballero ME, Juggins S (2002) Developing diatom-based transfer functions for Central Mexican lakes. Hydrobiologia 467:199–213. https://doi.org/10.1023/A:1014971016298
Edlund M, Kingston J, Heiskary S (2004) Expanding a sediment diatom reconstruction model to eutrophic Southern Minnesota Lakes. Environmental Outcomes Division Minnesotta Pollution Control Agency. CFMS Contract No. A45276, Minnesota
Edmondson WT, Abella SEB, Lehman JT (2003) Phytoplankton in Lake Washington: long-term changes 1950–1999. Arch Hydrobiol Suppl 139:275–326
Fritz S (2007) Salinity and climate reconstruction from diatoms in continental lake deposits. In: Elias SA (ed) Encyclopedia of quaternary science. Elsevier, Amsterdam, pp 514–522
Fritz SC, Juggins S, Battarbee RW (1993) Diatom assemblages and ionic characterization of lakes of the Northern Great Plains, North America: a tool for reconstructing past salinity and climate fluctuations. Can J Fish Aquat Sci 50:1844–1856. https://doi.org/10.1139/f93-207
Fritz SC, Cumming BF, Gasse F, Laird KR (2001) Diatoms as indicators of hydrologic and climatic change in saline lakes. In: Stoermer EF, Smol JP (eds) The diatoms: applications for the environmental and earth sciences. Cambridge University Press, Cambridge, pp 41–72
Gasse F (1986) East African diatoms: Taxonomy, Ecological Distribution. Bibliotheca Diatomologica, Stuttgart
Gent PR, Danabasoglu G, Donner LJ, Holland MM, Hunke EC, Jayne SR, Lawrence DM, Neale RB, Rasch PJ, Vertenstein M, Worley P, Yang ZL, Zhang M (2011) The community climate system model version 4. J Clim 24:4973–4991. https://doi.org/10.1175/2011JCLI4083.1
Graham RW, Lundelius EL Jr, Graham MA, Schroeder EK, Toomey RS III, Anderson E, Barnosky AD, Burns JA, Churcher CS, Grayson DK, Guthrie DR, Harington CR, Jefferson GT, Martin LD, McDonald GH, Morlan RE, Semken HA Jr, Webb DS, Werdelin L, Wilson MC (1996) Spatial response of mammals to late quaternary environmental fluctuations FAUNMAP working group. Science 272:1601–1606. https://doi.org/10.1126/science.272.5268.1601
Håkansson H (2002) A compilation and evaluation of species in the general Stephanodiscus, Cyclostephanos and Cyclotella with a new genus in the family Stephanodiscaceae. Diatom Res 17:1–139. https://doi.org/10.1080/0269249X.2002.9705534
Håkansson H, Locker S (1981) Stephanodiscus Ehrenberg 1846, a Revision of the species described by Ehrenberg. Nova Hedwigia 35:117–150
Håkansson H, Kling H (1989) A light and electron microscope study of previously described and new Stephanodiscus species (Bacillariophyceae) from Central and Northern Canadian lakes, with ecological notes on the species. Diatom Res 4:269–288. https://doi.org/10.1080/0269249X.1989.9705076
Hewitt G (2000) The genetic legacy of the Quaternary ice ages. Nature 405:907–913. https://doi.org/10.1038/35016000
Hijmans RJ, Cameron SE, Parra JL et al (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. https://doi.org/10.1002/joc.1276
Israde-Alcántara I, Garduño-Monroy VH, Ortega Murillo R (2002) Paleoambiente lacustre del cuaternario tardío en el centro del lago de Cuitzeo. Hidrobiologica 12:61–78
Israde-Alcántara I, Miller WE, Garduño-Monroy VH et al (2010a) Palaeoenvironmental significance of diatom and vertebrate fossils from Late Cenozoic tectonic basins in west-central México: a review. Quat Int 219:79–94. https://doi.org/10.1016/j.quaint.2010.01.012
Israde-Alcántara I, Velázquez-Durán R, Socorro Lozano García M et al (2010b) Evolución Paleolimnológica del Lago Cuitzeo, Michoacán durante el Pleistoceno-Holoceno. Bol Soc Geol Mex 62:345–357
Israde-Alcántara I, Domínguez-Vázquez G, Gonzalez S et al (2018) Five Younger Dryas black mats in Mexico and their stratigraphic and paleoenvironmental context. J Paleolimnol 59:59–79. https://doi.org/10.1007/s10933-017-9982-y
Jackson ST, Webb RS, Anderson KH et al (2000) Vegetation and environment in Eastern North America during the last glacial maximum. Quat Sci Rev 19:489–508
Julius M, Stoermer EF, Taylor CM, Schelske CL (1998) Local extirpation of Stephanodiscus niagarae (Bacillariophyceae) in the recent limnological record of Lake Ontario. J Phycol 34:766–771. https://doi.org/10.1046/j.1529-8817.1998.340766.x
Kilham SS, Theriot EC, Fritz SC (1996) Linking planktonic diatoms and climate change in the large lakes of the Yellowstone ecosystem using resource theory. Limnol Oceanogr 41:1052–1062. https://doi.org/10.4319/lo.1996.41.5.1052
Kolbe RW (1927) Zur ökologie, morphologie und systematik der brackwasser-diatomeen: Die kieselalgen des Sperenberger salzgebiets. G. Fischer Jena, Berlin-Dahlem
Krammer K, Lange-Bertalot H (1986) 2/1. Bacillariophyceae. 1. Teil: Naviculaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Sübwasserflora von Mitteleuropa. G. Fischer Verlag, Stuttgart
Krammer K, Lange-Bertalot H (1988) 2/2. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Sübwasserflora von Mitteleuropa. G. Fischer Verlag, Stuttgart
Krammer K, Lange-Bertalot H (1991) 2/4. Bacillariophyceae. 4. Teil: Achnanthaceae. Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Sübwasserflora von Mitteleuropa. G. Fischer Verlag, Stuttgart
Krammer K, Lange-Bertalot H, Håkansson H, Nörpel M (1991) 2/3 Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Sübwasserflora von Mitteleuropa. G. Fischer Verlag, Stuttgart
Lashaway AR, Carrick HJ (2010) Effects of light, temperature and habitat quality on meroplanktonic diatom rejuvenation in Lake Erie: Implications for seasonal hypoxia. J Plankton Res 32:479–490. https://doi.org/10.1093/plankt/fbp147
Meeks CJ (1974) Chlorophylls. In: Stewart PDW (ed) Algal physiology and biochemistry. Blackwell Scientific Publications, Oxford, pp 161–175
Metcalfe S (1995) Holocene environmental change in the Zacapu Basin, Mexico: a diatom-based record. The Holocene 5:196–208. https://doi.org/10.1177/095968369500500207
Metcalfe S, Say A, Black S et al (2002) Wet conditions during the last glaciation in the Chihuahuan Desert, Alta Babicora Basin, Mexico. Quat Res 57:91–101. https://doi.org/10.1006/qres.2001.2292
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) Vegan: community ecology package. The R Project for Statistical Computing. http://CRAN.R
Oliva-Martínez MG, Ramírez-Martínez JG, Garduño-Solórzano G, Cañetas Ortega J, Ortega MM (2005) Diatoms of three bodies of water from wetlands Jilotepec-Ixtlahuaca, Estado de Mexico. Hidrobiologica 15:1–26
Organization for Economic Cooperation and Development (OECD) (1982) Eutrophication of waters. Organization for Economic Cooperation and Development, Paris
Osorio-Olvera L, Lira-Noriega A, Soberón J, Townsend PA, Falconi M, Contreras-Díaz RG, Martínez-Meyer E, Barve V, Barve N (2020) ntbox: an R package with graphical user interface for modeling and evaluating multidimensional ecological niches. Methods Ecol Evol 11:1199–1206. https://doi.org/10.1111/2041-210X.13452
Pardi MI, Graham RW (2019) Changes in small mammal communities throughout the late Quaternary across eastern environmental gradients of the United States. Quat Int 530–531:80–87. https://doi.org/10.1016/j.quaint.2018.05.041
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Modell 190:231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026
Pla S, Paterson AM, Smol JP et al (2005) Spatial Variability in Water Quality and Surface Sediment Diatom Assemblages in a Complex Lake Basin: Lake of the Woods, Ontario, Canada. Int J Gt Lake Res 31:253–266. https://doi.org/10.1016/S0380-1330(05)70257-4
R Development Core Team (2009) R: a language and environment for statistical computing, 3.1. http://www.R-project.org
Rawson DS (1956) Algal indicators of trophic lake types. Limnol Oceanogr 1:18–25
Reavie ED, Barbiero RP, Allinger LE, Warren GJ (2014a) Phytoplankton trends in the Great Lakes 2001–2011. J Gt Lake Res 40:618–639. https://doi.org/10.1016/j.jglr.2014.04.013
Reavie ED, Heathcote AJ, Chraïbi S (2014b) Laurentian Great Lakes phytoplankton and their water quality characteristics, including a diatom-based model for paleoreconstruction of phosphorus. PLoS ONE 9:e104705. https://doi.org/10.1371/journal.pone.0104705
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 64:205–221
Reynolds CS (1999) Non-determinism to probability, or N: P in the community ecology of phytoplankton nutrient ratios: Arch. Fur Hydrobiol 146:23–35
Servicio Meteorológico Nacional (SMN) (2019) Información Estadística Climatológica. https://smn.conagua.gob.mx/es/climatologia/informacion-climatologica/informacion-estadistica-climatologica. Accessed 1 May 2019
Sigala I, Caballero M, Correa-Metrio A et al (2017) Basic limnology of 30 continental waterbodies of the Transmexican Volcanic Belt across climatic and environmental gradients. Bol Soc Geol Mex 69:313–370. https://doi.org/10.18268/bsgm2017v69n2a3
Spaulding SA, Kociolek JP, Wong D, Kociolek IP (1999) A taxonomic and systematic revision of the genus Muelleria (Bacillariophyta). Phycologia 38:314–341. https://doi.org/10.2216/i0031-8884-38-4-314.1
Spaulding SA, Van de Vijver B, Hodgson DA, McKnigth DM, Verleyen E, Stanish L (2010) Diatoms as indicators of environmental change in antarctic and subantarctic freshwaters. In: Smol J, Stoermer EF (eds) The diatoms: applications for the environmental and earth sciences. Cambridge University Press, New York, pp 267–284
Stewart JR, Lister AM, Barnes I, Dalén L (2009) Refugia revisited: Individualistic responses of species in space and time. Proc R Soc B Biol Sci 277:661–671. https://doi.org/10.1098/rspb.2009.1272
Stoermer EF, Jang JJ (1970) Distribution and relative abundance of dominant plankton diatoms in Lake Michigan. Gt Lakes Res Div, Michigan
Stoermer EF, Ladewski TB (1976) Apparent optimal temperatures for the occurrence of some common phytoplankton species in southern Lake Michigan. Gt Lakes Res Div, Michigan
Stoermer EF, Emmert G, Schelske CL (1989) Morphological variation of Stephanodiscus niagarae Ehrenb. (Bacillariophyta) in a Lake Ontario sediment core. J Paleolimnol 2:227–236. https://doi.org/10.1007/BF00202048
Theriot EC, Stoermer EF (1981) Some aspects of morphological variation in S. niagarae (Bacillariophyceae). J Phycol 17:64–72. https://doi.org/10.1111/j.1529-8817.1981.tb00820.x
Theriot EC, Stoermer EF (1984a) Principal component analysis of character variation in Stephanodiscus niagarae Ehrenb.: Morphological variation related to Lake Trophic status. In: Mann DG (ed) 7th international diatom symposium. Otto Koeltz Science Publishers, Koenigstein, pp 97–111
Theriot EC, Stoermer EF (1984b) Principal component analysis of variation in Stephanodiscus rotula and S. niagarae (Bacillariophyceae). Syst Bot 9:53–59. https://doi.org/10.2307/2418407
Theriot EC, Fritz SC, Whitlock C, Conley DJ (2006) Late Quaternary rapid morphological evolution of an endemic diatom in Yellowstone Lake, Wyoming. Paleobiology 32:38–54. https://doi.org/10.1666/02075.1
Valadez F, Oliva G, Vilaclara G et al (2005) On the presence of Stephanodiscus niagarae Ehrenberg in central Mexico. J Paleolimnol 34:147–157. https://doi.org/10.1007/s10933-005-0810-4
Waltari E, Hijmans RJ, Peterson AT et al (2007) Locating pleistocene refugia: comparing phylogeographic and ecological niche model predictions. PLoS ONE 2:e563. https://doi.org/10.1371/journal.pone.0000563
Xu J, Ho AYT, Yin K et al (2008) Temporal and spatial variations in nutrient stoichiometry and regulation of phytoplankton biomass in Hong Kong waters: Influence of the Pearl River outflow and sewage inputs. Mar Pollut Bull 57:335–348. https://doi.org/10.1016/j.marpolbul.2008.01.020
Yu A (2011) Stephanodiscus niagarae. In: Diatoms North Am. https://diatoms.org/species/stephanodiscus_niagarae. Accessed 6 Sep 2019
Acknowledgements
This research was funded by DGAPA-IV-100215 “Cambio Climático y Medio Ambiente en la historia del lago de Chalco” and DGAPA-PAPIIT-IN103819 “Variabilidad climática y paleoambientes durante la terminación II (130 ka): el paso del penúltimo glacial (MIS 6) al penúltimo interglaciar (MIS 5)”. Diana Avendaño thanks the Posgrado de Ciencias de la Tierra, UNAM and CONACyT (CVU 854736) for finnancial support. We also thank: Dr. Ma. Aurora Armienta and the staff in the Laboratorio de Quimica Análitica, Instituto de Geofisica, UNAM for major anions and SiO2 analysis; Ariadna Martinez and Daniela Cela from the “Red de Ecología Funcional” laboratory at the Instituto de Ecología, A.C. (INECOL), Xalapa, Mexico, for the nutrients analysis; Laura Gómez Lizárraga for excellent technical assistance with the different stages of sample preparation with the scanning electron microscope and Alejandra Ubaldo Guerra provided assistance during the field work. We also thank Dr. Whitmore, Dr. Reavie and two anonymous reviewers for their valuable comments that greatly improved our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Avendaño, D., Caballero, M. & Vázquez, G. Ecological distribution of Stephanodiscus niagarae Ehrenberg in central Mexico and niche modeling for its last glacial maximum habitat suitability in the Nearctic realm. J Paleolimnol 66, 1–14 (2021). https://doi.org/10.1007/s10933-021-00178-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-021-00178-w