Abstract
Insulin-like growth factors (IGFs) play active role in mitogenic and metabolic processes. In the peripheral circulation, they are mostly bound to specific IGF-binding proteins (IGFBPs). Proteolysis of IGFBPs releases free, active IGFs. IGFBP-2 is the second most abundant of the six binding proteins and its concentration increases in catabolic states. The possible interaction between IGFBP-2 and other proteins in the circulation was investigated in this study. Our results showed that IGFBP-2 associates with α2-macroglobulin (α2M), a protease inhibitor. Formation of IGFBP-2/α2M complexes most likely contributes to the regulation of IGFBP-2 proteolysis and, thus, the activity of IGFs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The insulin-like growth factor (IGF) system plays crucial role in cellular growth, and metabolism, affecting all phases of cell development such as proliferation, differentiation, migration and survival [8, 12]. IGF-I and IGF-II, their receptors (IGF-1R and IGF-2R), the insulin (IR) and the hybrid receptor (IR/IGF-1R) and six IGF-binding proteins (IGFBPs) are members of the IGF system [8, 12].
Free IGFs exert their actions through binding to specific receptors. They are mostly inactive when attached to IGFBPs but can be released by proteolysis. Thus, IGFBPs increase the half-life of IGFs and regulate the intensity of their activity [12]. The affinities of IGFBPs and IGF receptors for IGFs are similar [13, 19]. IGFBP-3 is the major IGF-binding protein in the circulation of healthy people, followed by IGFBP-2, the physiological role of which still remains to be fully elucidated [2, 5].
IGFBP-2 is a 36 kDa protein, not post-translationally modified, and it is not known to form complexes with other proteins in the circulation [6, 27]. The concentration of IGFBP-2 in blood ranges from 370 to 550 μg/L but does not exhibit diurnal variation or changes related to daily food intake [8, 14]. While it is synthesized in almost every cell in the body, IGFBP-2 in the peripheral circulation is mostly derived from the liver [20]. Circulating IGFBP-2 levels increase several times in acute or chronic pathophysiological states, such as shock, extreme fasting [28], hypoxemia, malignancy [15] or after trauma. In these situations IGFBP-2 may become the major IGF-binding protein [1, 21]. Many hormones and growth factors regulate IGFBP-2 expression. The expression of IGFBP-2 is influenced by growth hormone, IGFs, follicle-stimulating hormone, transforming growth factor-β, estradiol, glucocorticoids and insulin [11].
In this study we have investigated whether IGFBP-2, up to now known to exist only as a separate molecular entity, forms complexes with other proteins in the circulation. Thus, IGFBP-3 is known to bind to transferrin (Tf) [25] and IGFBP-1 to α2-macroglobulin (α2M) [26]. In order to analyse possible interactions of IGFBP-2 with other blood proteins, immunoaffinity and lectin-affinity methods were used.
2 Materials and Methods
2.1 Serum Samples
Sera were obtained from venous blood of healthy adult volunteers (n = 20, 10 males and 10 females, 28–56 years old) after fasting for 12 h. Sera were stored frozen at −20 °C until used. This study was approved by the local ethical committee in the Institute for the Application of Nuclear Energy.
2.2 Immunoaffinity chromatography
In order to detect possible IGFBP-2 complexes with the eleven most abundant serum proteins, an immunoaffinity matrix (Beckman Coulter, Fullerton, USA) with immobilised antibodies against these proteins was used. These included antibodies against albumin, Tf, IgG, IgA, IgM, HDL apolipoproteins I and II, haptoglobin, α1-antitrypsin, α1-acid glycoprotein and α2M. The matrix (1.2 mL) was incubated with 0.5 mL of serum diluted 1:25 in 10 mM Tris-HCl/1.5 M NaCl buffer, pH 7.4, at room temperature for 15 min using a rotator. Unbound proteins were removed by centrifugation for 30 s at 2,000×g and the matrix was washed three times with the dilution buffer. Proteins bound to immobilised antibodies were eluted with 0.1 M Gly-HCl buffer, pH 2.5, in three 0.5 mL portions. Eluates were separated by centrifugation as described. Eluted fractions were neutralised with 50 μL of 1 M Tris–HCl buffer, pH 8.0. The matrix was then neutralised with 0.6 mL of 2 M Tris–HCl buffer, pH 8.0, and three times washed with the dilution buffer before application of the next sample.
2.3 Lectin-Affinity Chromatography
Agarose-immobilised lectins (Vector, Burlingame, USA) were used to isolate serum glycoproteins that might have interacted with IGFBP-2: SNA (Sambucus nigra agglutinin), LCA (Lens culinaris agglutinin), Con A (lectin from Canavalia ensiformis), PHA-E (Phaseolus vulgaris erythroagglutinin) and RCA-I (Ricinus communis agglutinin I). Equilibration and elution buffers for each matrix were prepared following procedures recommended by the producer. Matrices (2.0 mL) were packed into columns and 1.0 mL of sera diluted 1:10 in the appropriate buffer was circulated through each column at room temperature for 1 h. Unbound proteins were removed with 20 mL of dilution buffer. The bound glycoproteins were eluted with 7 mL of hapten sugar (0.2–0.5 M, as suggested by the producer) in 0.1 M acetic acid, pH 3.0. PHA-E-bound glycoproteins were eluted with 0.1 M acetic acid. Eluted fractions were neutralised with 2 M Tris–HCl buffer pH 8.9 and dialysed against distilled water overnight at 4 °C. Matrices were neutralised with 20 mL of the appropriate dilution buffer before application of the next sample.
2.4 Immunoprecipitation
A preactivated matrix, AminoLink®Plus Coupling Resin (Pierce Biotechnology, Rockford, USA) was used to immobilise separately antibodies against IGFBP-2 (Santa Cruz Bitechnology, Santa Cruz, USA; goat), α2M (AbD Serotec, Kidlington, Oxford, UK; rabbit) and Tf (INEP, Belgrade, Serbia; goat). The matrix (50 μL of 50 % slurry) was incubated with 0.2 mL of serum diluted 1:20 in the buffer supplied by the manufacturer, at 4 °C overnight. Unbound proteins were removed by centrifugation for 30 s at 2,000×g and the matrix was washed three times with the dilution buffer. Proteins bound to immobilised antibodies were stripped with the elution buffer (pH 2.5) in three 50 μL portions. Eluates were separated by centrifugation as described. Eluted fractions were neutralised with 10 μL of 1 M Tris–HCl buffer pH 8.0. The matrix was then neutralised with 0.1 mL of 2 M Tris–HCl buffer pH 8.0 and washed three times with the dilution buffer before application of the next sample.
2.5 Immunoblotting
The presence of a specific protein in fractions obtained after immuno- and lectin-affinity chromatography, and immunoprecipitation was detected by immunoblotting. SDS-PAGE (10 % gel) under reducing conditions was followed by electrotransfer to nitrocellulose membrane and immunoblotting [18]. The primary antibodies employed were the same as those used for immunoprecipitation. HRP-coupled secondary antibodies were anti-goat IgG (Biosource, Camarillo, USA; swine) and anti-rabbit IgG (AbD Serotec, Kidlington, Oxford, UK; sheep). Immunoreactive proteins were visualised with enhanced chemiluminescence (ECL) reagent (Pierce Biotechnology, Rockford, USA). Molecular markers were from Bio-Rad Laboratories (Hertfordshire, UK).
3 Results
An immunoaffinity matrix with immobilised antibodies against eleven abundant serum proteins was used to assess possible interactions with IGFBP-2. Unbound proteins and those bound to the matrix were analysed by immunoblotting with anti-IGFBP-2 antibody. In Fig. 1 it can be seen that IGFBP-2 was detected among proteins bound to the matrix. Three immunoreactive species were distinguished: a band of mass between the 31 and 45 kDa markers, corresponding to the IGFBP-2 monomer, and two other bands at masses ≥100 kDa. The same proteins were detected in the unbound fraction, together with an IGFBP-2 fragment (mass between the 21 and 31 kDa markers) and an immunoreactive species close to the 66 kDa marker. These data suggested the existence of a complex between IGFBP-2 and at least one other serum protein.
Immunoblot of the immunoaffinity matrix unbound (U) and bound (B) IGFBP-2 species. Serum samples were allowed to interact with a matrix with immobilised antibodies against eleven most abundant serum proteins in 10 mM Tris–HCl/1.5 M NaCl buffer, pH 7.4, at room temperature for 15 min; specifically bound proteins were eluted with 0.1 M Gly-HCl buffer, pH 2.5 and subjected to SDS-PAGE (10 % gel) under reducing conditions (200 V, 45 min) followed by electrotransfer to nitrocellulose membrane (100 V, 1 h) and immunoblotting with anti-IGFBP-2 antibody (1: 1,000 dilution)
Some serum proteins are simple polypeptides, while others are glycoproteins. IGFBP-2 is a simple protein, without carbohydrate moiety and alone it does not bind to lectins. However, after interacting with some other serum (glyco)proteins, an IGFBP-2 complex may or may not bind to lectins depending on the nature of its partner in complex formation—whether it is a glycoprotein or simple protein. In the second experiment, lectin-affinity chromatography followed by immunoblotting was employed to investigate whether IGFBP-2 interacted with glycoproteins. Five lectin-bound matrices were used and they all interacted with glycoprotein(s) that formed complexes with IGFBP-2 (Fig. 2). The SNA- and Con A-bound fractions contained monomer IGFBP-2, three immunoreactive species of higher masses and traces of IGFBP-2 fragments. These protein profiles resembled the IGFBP-2 immunoreactive profile exhibited by the serum itself (i.e. the unbound fraction in Fig. 1). RCA-I-, LCA- and PHA-E-bound fractions contained IGFBP-2 monomer and traces of IGFBP-2 complexes and fragments.
IGFBP-2 immunoblot of protein fractions specifically eluted from immobilised lectins: SNA (1), Con A (2), RCA-I (3), LCA (4) and PHA-E (5). Immobilised lectins were incubated with serum samples in buffers recommended by the producer for each lectin, at room temperature for 1 h; specifically bound glycoproteins were eluted with hapten sugar (0.2–0.5 M, as suggested by the producer) in 0.1 M acetic acid, pH 3.0 or, in the case of PHA-E, bound glycoproteins were eluted with 0.1 M acetic acid; eluted fractions were subjected to SDS-PAGE (10 % gel) under reducing conditions (200 V, 45 min) followed by electrotransfer to nitrocellulose membrane (100 V, 1 h) and immunoblotting with anti-IGFBP-2 antibody (1: 1,000 dilution)
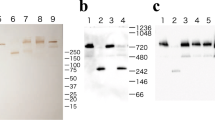
Since it is known that IGFBP-3 forms complexes with Tf and IGFBP-1 with α2M, and that both Tf and α2M are glycoproteins, the next experiment was directed towards detecting possible interactions between IGFBP-2 and Tf or α2M. Serum samples were subjected to immunoprecipitation using matrix-immobilised anti-IGFBP-2, anti-Tf or anti-α2M antibodies. Proteins specifically bound to these matrices were eluted and analysed by immunoblotting. Immunoblotting with anti-IGFBP-2 antibody (Fig. 3, samples 1–4) detected the presence of immunoreactive species in eluates obtained from matrices with immobilised anti-IGFBP-2 or anti-α2M antibody. IGFBP-2 was not detected in the eluate from anti-Tf resin. According to these results, IGFBP-2 formed complexes with α2M but not with Tf. In order to confirm this finding, immunoblotting with anti-α2M antibody was performed (Fig. 3, samples 5–7). α2M immunoreactive protein of high molecular mass was detected in the eluate obtained from the matrix with immobilised anti-IGFBP-2 antibody, indicating that this antibody could bind IGFBP-2/α2M complexes besides its natural ligand. α2M immunoblotting performed after elution of proteins precipitated with anti-α2M antibodies revealed a presence of α2M fragments. According to literature data, α2M may cleave into several fragments. The fragment at approximately 60 kDa is the major one [10]. α2M fragments were not precipitated with anti-IGFBP-2 antibody, implicating that only intact α2M is capable of binding IGFBP-2 in a complex.
Immunoblot of the IGFBP-2 (1–4) and α2M (5–7) species obtained by immunoprecipitation with anti-IGFBP-2 antibody (4, 7), anti-Tf antibody (2) or anti-α2M antibody (3, 6). Samples 1 and 5 are untreated sera. Immunoprecipitation was performed with matrices with separately immobilised antibodies against IGFBP-2, α2M and Tf. Matrices were incubated with serum samples in a buffer supplied by the manufacturer, at 4 °C overnight; specifically bound proteins were eluted with a supplied buffer pH 2.5 and subjected to SDS-PAGE (10 % gel) under reducing conditions (200 V, 45 min) followed by electrotransfer to nitrocellulose membrane (100 V, 1 h) and immunoblotting with anti-IGFBP-2 antibodies (1: 1,000 dilution) or anti-α2M antibody (1: 5,000)
All serum samples exhibited similar immunoblotting profiles. There were differences between samples in the relative abundance of immunoreactive proteins, but without any specific pattern related to age or gender.
4 Discussion
Our results have demonstrated that IGFBP-2 in the peripheral circulation forms complexes with α2M but not with Tf. One prerequisite for complex formation is the existence of appropriate structural elements in each subunit allowing interaction to occur. It is not known which protein sequences in IGFBP-1 and IGFBP-3 are directly involved in their respective binding to α2M and Tf. However, a unique feature of IGFBP-1 and IGFBP-2 is the presence of an RGD sequence that enables them to interact with integrins [9, 22], while IGFBP-3 was found to possess a metal-binding domain [23].
The formation of a specific protein association is often connected with performance of a unique physiological role. α2M is a large multi-domain protein (a tetramer of approximately 190 kDa subunits) known to inhibit many proteases, but also able to bind smaller proteins in a non-covalent, reversible manner, thereby regulating their rate of degradation [7, 24]. Proteolysis of binding proteins liberates ligands, which can then exert their functions. Circulating IGFBP-2 inhibits IGF actions [27], so IGFBP-2 proteolysis is a regulatory step in the balance between bound (inactive) and free (active) IGFs.
A number of pathophysiological states are characterised by elevated concentrations of IGFBP-2 [1, 15, 21, 28]. Increased proteolytic activity most often accompanies these situations [3]. A study on IGFBP-1/α2M complexes demonstrated that α2M protects IGFBP-1 from chymotrypsin in a dose-dependent manner [26]. Plasmin [16], calpain [4] and matrix metalloproteases [17] are known to degrade IGFBP-2, especially in severe catabolic illness. A complex relationship between the relative abundance of IGFBP-2, its proteases and protease inhibitors, including α2M, and the degree of their interaction may be expected to regulate the activity of IGFs.
Abbreviations
- α2M:
-
Alpha 2-macroglobulin
- ConA:
-
Canavalia ensiformis lectin
- IGF:
-
Insulin-like growth factor
- IGFBP:
-
IGF-binding protein
- LCA:
-
Lens culinaris agglutinin
- PHA-E:
-
Phaseolus vulgaris erythroagglutinin
- IGF-R:
-
IGF receptor
- RCA-I:
-
Ricinus communis agglutinin I
- SNA:
-
Sambucus nigra agglutinin
- Tf:
-
Transferrin
References
Baričević I, Jones DR, Malenković V, Nedić O (2006) Clin Endocrinol 65:373–379
Baxter RC (1997) In: Le Roith D (ed) Advances in molecular and cellular endocrinology. Jai Press Inc., Greenwich
Bentham J, Rodriguez-Arnao J, Ross RJM (1993) Horm Res 40:87–91
Berg U, Bang P, Carlsson-Skwirut C (2007) Biol Chem 388:859–863
Boney M, Moats-Staats BM, Stiles AD, D’Ercole AJ (1994) Endocrinology 135:1863–1868
Collett-Solberg Paulo F, Pinchas C (1996) Endocrinol Metab Clin Exp 25:591–609
Doan N, Gettins PG (2007) J Biochem 407:23–30
Federici M, Porzio O, Zucaro L, Fusco A, Borboni P, Lauro D, Sesti G (1997) Mol Cell Endocrinol 129:121–126
Firth SM, Baxter RC (2002) Endocr Rev 23:824–854
Hergenhahn HG, Hall M, Soderhall K (1988) Biochem J 255(3):801–806
Hoeflich A, Reisinger R, Lahm H (2001) Cancer Res 61:8601–8610
Le Roith D (2003) Exp Diabesity Res 4:205–212
Le Roith D, Bondy C, Yakar S, Liu JL, Butler A (2001) Endocr Rev 22:53–74
Lin Y, Jiang T, Zhou K, Xu L, Chen B, Li G, Qui X, Jiang T, Zhang W, Song SW (2009) Neuro-Oncology 11:468–476
Liou JM, Shun CT, Liang JT, Chiu HM, Chen MJ, Chen CC, Wang HP, Wu MS, Lin JT (2010) J Clin Endocrinol Metab 95:1717–1725
Marcinkiewicz M, Gordon PV (2008) Growth Horm IGF Res 18:325–334
Miyamoto S, Nakamura M, Yano K, Ishii G, Hasebe T, Endoh Y, Sangai T, Maeda H, Schi-Chuang Z, Chiba T, Ochiai A (2007) Jpn J Cancer Res 98:685–691
Nedić O, Masnikosa R (2008) Metab, Clin Exp 57:658–661
Rapp R, Deger A, Blum W, Koch R, Weber U (1988) Eur J Biochem 172:421–425
Ross RJM, Chew SL, D’ Souza Li L, Yateman M, Rodriguez-Arnao J, Gimson A, Holly J, Camacho-Hubner C (1996) J Endocrinol 149:209–216
Sandberg-Nordqvist AC, Von Holst H, Holmin S, Sara VR, Bellander BM, Schalling M (1996) Mol Brain Res 38:285–293
Schütt BS, Langkamp M, Rauschnabel U, Ranke MB, Elmlinger MW (2004) J Mol Endocrinol 32:859–868
Singh B, Charkowicz D, Mascarenhas D (2004) J Biol Chem 279:477–487
Sottrup-Jensen L (1989) J Biol Chem 264:11539–11542
Weinzimer SA, Gibson TB, Collet-Solberg PF, Khare A, Liu B, Cohen P (2001) J Clin Endocrinol Metab 86:1806–1813
Westwood M, Aplin JD (2001) J Biol Chem 276:41668–41674
Wheatcroft SB, Kearney MT (2009) Trends Endocrinol Metab 20:153–162
Wolf E, Lahm H, Wu M, Wanke R, Hoeflich A (2000) Pediatr Nephrol 14:572–578
Acknowledgments
This work was supported by the Ministry of Education, Science and Technological Development of Serbia, Grant number 173042.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Šunderić, M., Miljuš, G. & Nedić, O. Interaction of Insulin-Like Growth Factor-Binding Protein 2 with α2-Macroglobulin in the Circulation. Protein J 32, 138–142 (2013). https://doi.org/10.1007/s10930-013-9471-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-013-9471-8