Abstract
A soluble β-galactoside binding 14.5 kDa lectin was purified from the heart of Capra hircus. Its metal independent nature, preferential affinity for β-d-lactose and 90–94% homology with carbohydrate recognition domain of previously reported galectin-1 confirmed its inclusion in galectin-1 subfamily. The secondary structures of the deduced amino acid sequences were generally conserved with previously reported Gal-1. Exposure of the purified protein to varying temperature and pH, oxidant, thiol blocking reagents, denaturants and detergents resulted in significant changes in UV (ultraviolet), fluorescence, CD (circular dichroism) and FTIR (fourier transform infra red) spectra, thus strongly emphasizing the vitality of regular secondary structure of galectins for maintaining their active conformation. Bioinformatics studies corroborated the results obtained in wet lab. Our findings based on physico-chemical properties, oxidative inactivation and structural analysis of the goat heart galectin-1 suggests significant implications in potential biological and clinical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Galectins are the family of mammalian lectins which possess a conserved carbohydrate recognition domain (CRD) directed against β-galactoside structures; this binding requires the presence of thiol reducing agents and is independent of divalent cations [7]. Galectins’ CRD consists of 134 amino acids tightly bound into sandwich of a six stranded and a five stranded anti-parallel β-sheets that form extended β-sandwich with a typical jelly-roll topology [25]. Galectin-1 (Gal-1) is the most abundant member of galectin family and has been reportedly involved in wide range of significant biological functions [19]. Gal-1 has nearly ubiquitous distribution in animal tissues including heart of mammals [6]. In mammalian heart, Gal-1 is localized mainly in endocardial tissue, myocardial cell constituents, connective-tissue elements and vascular structures [6]. In the heart of healthy individuals, Gal-1 is involved in basic physiological functions like adhesion, transportation and apoptosis. However, the research work carried out in last decade have shown Gal-1 involvement in various cardiovascular disorders like Chagas’ disease [18], hypoxia-induced Pulmonary Hypertension [10], atherogenesis [11], atherosclerosis and restenosis [28]. Therefore, data regarding isolation, characterization, structural analysis and protein chemistry of heart galectins are important to gain a deeper understanding of the role of galectins in potentially life threatening cardiovascular diseases.
Amino acid sequences for different members of the galectin family have been isolated from several mammalian species in a wide range of taxonomic positions. However, with the exception of a few reports [38], the galectins from mammalian heart have not been extensively characterized. The selected organ i.e., Capra hircus heart is a prominent source of red meat, consumed extensively all around the world. The aims of the present study were to isolate and characterize a Gal-1 from Capra hircus heart, perform multiple sequence alignment of Gal-1 amino acid sequences deduced from heart of different species, and examine the effect of temperature, pH, oxidant, thiol blocking reagents, denaturants and detergents on the properties and regular secondary structure of native goat heart galectin-1 (GHG-1). In addition, response of GHG-1 activity in the presence of oxidizing agent was monitored to gain some information regarding its possible involvement in free radical induced oxidative stress mediated cardiovascular disorders [5, 16]. Bioinformatics studies were performed to further corroborate the results obtained in wet lab.
2 Materials and Methods
2.1 Reagents
Molecular weight markers (14.4–97.4 kDa), sugars (β-d-lactose, d-galactose, methyl-β-d-galactopyranoside, p-nitrophenyl-β-d-galactopyranoside, d-galactosamine, methyl-α-d-galactopyranoside, p-nitrophenyl-α-d-galactopyranoside, d-glucose, d-mannose, d-fucose, d-sucrose (Glc-α-1,2-Fuc), d-melibiose, d-glucosamine, d-cellobiose and d-fructose), Sephadex G100 and G50, coommassie brilliant blue (CBB) G-250 and R-250 were purchased from Sigma Aldrich (St Louis, MO, USA). All other chemicals used were of the analytical grade and were purchased from Merck India Ltd and Qualigens Fine Chemicals, India.
2.2 Preparation of Goat Heart Homogenate
Fresh goat heart was collected from local slaughter house. One hundred gram heart tissue was removed from its pericardium, minced into small pieces and suspended in 200 ml ice-chilled PBS ‘A’ (75 mM sodium phosphate pH 7.2, containing 0.15 M NaCl, 10 mM EDTA, 5 mM β-ME, 200 mM lactose and 0.02% (w/v) sodium azide). Homogenization was carried out at 4 °C in a stainless steel vessel using mixer table homogenizer. The homogenate was centrifuged in a Beckman C-24 BL cooling centrifuge (JA-20 rotor) (Frederick, Maryland, USA) for 30 min at 10,000 rpm at 4 °C and the supernatant collected was centrifuged twice for 30 min at 15,000 rpm at 4 °C.
2.3 Ammonium Sulphate Fractionation
The supernatant collected was subjected to 0–30% ammonium sulphate fractionation. The fractionated solution was centrifuged again for 30 min at 15,000 rpm at 4 °C, and the supernatant thus obtained was subjected to 30–70% ammonium sulphate fractionation. The precipitate obtained was dissolved in PBS ‘B’ (75 mM sodium phosphate pH 7.2, containing 0.15 M NaCl, 5 mM β-ME and 0.02% (w/v) sodium azide). After extensive dialysis against PBS ‘B’, the samples were centrifuged for 30 min at 15,000 rpm at 4 °C to remove any aggregate formed and the clear supernatant was applied to column chromatography.
2.4 Sephadex G50 Gel Filtration Chromatography
Sephadex G50 gel filtration column was packed according to the method of [23]. Sephadex G50 powder (10 g) was allowed to swell in sufficient amount of distilled water and left overnight. The gel formed was boiled for 6 h in boiling water bath, suspended in PBS ‘B’ and allowed to settle down. The superfine particles in supernatant were removed by decanting. A previously cleaned glass column (2 × 60 cm) mounted on a vertical support was filled with distilled water; glass wool was fitted at the outlet of the column and checked for leaks and air bubbles. The de-aerated gel slurry was gently poured with the help of a glass rod into the column with care to avoid generation of air bubbles. The column was left standing overnight. Flow rate was increased gradually and after accomplishing a constant flow rate higher than that required for final elution the column was adjusted to the required flow rate (10 ml/h). The packed column was thoroughly washed with two bed volumes of PBS ‘B’. In order to check the uniform packing and to determine the void volume of the column, 0.2% (w/v) solution of blue dextran in PBS ‘B’ was passed through the column. The volume of blue dextran or galectin solution applied on the column was not more than 2–3% of the total bed volume. The column was thoroughly washed and equilibrated with PBS ‘B’ prior to application of the sample. The dialyzed sample obtained after 30–70% ammonium sulphate fractionation was applied at room temperature to the gel filtration column. The column was then eluted with PBS ‘B’ at a flow rate of 10 ml/h, 3 ml fractions were collected and protein concentration was determined in each fraction by the method of Lowry [27]. The fractions of protein peak were dialyzed against PBS ‘B’ to remove lactose, tested for hemagglutination activity [26] and subjected to a lactosyl-Sepharose 4B affinity column.
2.5 Lactosyl-Sepharose 4B Affinity Chromatography
Lactosyl-Sepharose 4B affinity column was packed according to the method of [17]. One hundred ml of sedimented Sepharose 4B beads were suspended in 100 ml of 0.5 M Na2CO3 and washed several times until pH 11.0 was achieved. The gel was filtered over a Buchner funnel. The drained gel was collected and re-suspended in 100 ml of 0.5 M Na2CO3 and 10 ml of divinyl sulfone was slowly added. The mixture was stirred gently for 70 min at 23 °C, filtered on Buchner funnel and washed several times with 0.5 M Na2CO3. The drained gel was suspended in 100 ml of a 10% lactose (w/v) solution in 0.5 M Na2CO3. The reaction was allowed to proceed for 24 h in cold with gentle mixing. The mixture was filtered and washed with water and then with PBS ‘B’. The column was packed and stored in PBS containing 0.02% sodium azide in cold. The column was generally repacked after 5 uses. The fractions pooled together after gel filtration was subjected to the affinity column. The unbound material was eliminated by washing with PBS ‘B’ until no protein was detected in the effluent. The bound material was then eluted at 4 °C with 200 mM lactose in PBS ‘B’ at a flow rate of 10 ml/h, 3 ml fractions were collected and protein concentration was determined in each fraction by the method of Lowry [27]. The fractions of protein peak were again dialyzed against PBS ‘B’ to remove lactose, tested for hemagglutination activity and used for further studies.
2.6 Polyacrylamide Gel Electrophoresis (PAGE)
To test homogeneity of the purified galectin, native PAGE and sodium dodecyl sulphate (SDS)–PAGE were performed by Tris–Glycine buffer system [21]. The galectin samples for native PAGE were diluted with 10 mM Tris–HCl buffer pH 8.0, containing 1 mM EDTA, 10% glycerol and 2.5% (v/v) 2-mercaptoethanol and a trace of bromophenol blue as a tracking dye and loaded on the gel. The galectin samples for SDS–PAGE were diluted with 10 mM Tris-HCl buffer pH 8.0, containing 1 mM EDTA, 2% SDS, 10% glycerol and 2.5% (v/v) β-mercaptoethanol, boiled for 5 min and loaded on the gel. The electrophoresis gels were stained with CBB R-250 dye.
2.7 Molecular Weight Determination
Molecular weight of the purified galectin was determined using Sephadex G100 gel filtration column under native (without β-ME) as well as reduced (with β-ME) conditions. The purified galectin along with standard molecular weight markers were loaded on the column. The column then was eluted with PBS ‘B’ at a flow rate of 10 ml/h, 3 ml fractions were collected and galectin concentration was determined in each fraction by the method of Lowry [27]. The data were analyzed according to the theoretical treatment of Andrews [4]. The linear plot between elution volume (Ve)/void volume (Vo) and log M was used for calculating molecular weight of the purified galectin.
The subunit molecular weight of the purified galectin was calculated by the procedure of Weber and Osborn [39] using its mobility on SDS–PAGE. The mobility of marker proteins determined under identical conditions was plotted against the logarithm of molecular weight. The analysis of data indicated a linear relationship between log M and relative mobility (Rm) and the plot was used for calculating molecular weight of the purified galectin.
2.8 Determination of Stokes Radii and Diffusion Coefficients
Stokes radii of the purified galectin was determined using the gel filtration data. The partition coefficient (Kav) value of each marker protein was calculated from formula [Kav = (V e − V o)/(V t − V o)], where V e stands for elution volume, V o stands for void volume and V t designates total volume of the column. A linear plot between the square root of the negative logarithm of Kav and Stokes radii of marker proteins was used for calculating Stokes radii of the purified galectin.
Diffusion coefficient (D) values of the purified galectin was computed using the equation, D = KT/6πηr, where, ‘K’ is the Boltzman constant (1.386 × 10−16 erg/deg), ‘T’ is the absolute temperature (303 k), ‘η’ is the coefficient of viscosity of the medium (0.0100 P for water and dilute aqueous salt solutions at 20 °C) and ‘r’ is Stokes radius.
2.9 Carbohydrate Binding and Human Blood Group Specificity
Saccharide specificity of the purified galectin was determined by sugar inhibition tests using two fold serial dilutions of sugar solutions. To assess these inhibition tests, standard solutions (100 mM) of following saccharides were prepared in 0.15 M NaCl: β-d-lactose, d-galactose, methyl-β-d-galactopyranoside, p-nitrophenyl-β-d-galactopyranoside, d-galactosamine, methyl-α-d-galactopyranoside, p-nitrophenyl-α-d-galactopyranoside, d-glucose, d-mannose, d-fucose, d-sucrose (Glc-α-1,2-Fuc), d-melibiose, d-glucosamine, d-cellobiose and d-fructose. To determine the minimum concentration required for inhibition of hemagglutination by saccharides, 25 μl serial diluted test saccharides were added to each well containing 25 μl purified galectin containing four agglutinating units. Fifty microlitres of trypsinized erythrocytes was added into each well and contents were mixed by gentle shaking and incubated for an hour at room temperature. Highest dilution of the test saccharides required for complete inhibition was noted. Hemagglutination ability of the purified galectin was also scanned using trypsinized as well as untrypsinized preparations of human erythrocytes (blood groups A, B, AB and O) by two fold serial dilution method.
2.10 Amino Acid Sequencing of the Purified Goat Heart Galectin
Purified galectin (2 mg in 3 ml of PBS containing 5 mM β-ME and 0.5 M NaCl) was dialyzed against 0.01 M ammonium hydrocarbonate and lyophilized. The lyophilized galectin was dissolved in 400 μl of 8 M deionized urea/0.05 M Tris-HCl pH 8.3, reduced with 0.045 M dithiothreitol (40 μl) at 50 °C for 30 min, and then carboxamidomethylated with 0.1 M iodoacetamide (80 μl) under nitrogen atmosphere at room temperature for 2 h. The carboxamidomethylated galectin was diluted to 2 ml with distilled water and digested with trypsin and high specificity chymotrypsin at 37 °C for 20 h using 1:20 enzyme/substrate ratio. The tryptic and chymotryptic peptides were separated by reverse phase HPLC (Pharmacia LKB, Sweden) on a Vydac C18 column (4.7 × 250 mm) equilibrated with 0.1% trifluoroacetic acid in water (v/v). The column was eluted at a flow rate of 1.0 ml/min with 0–60% acetonitrile linear gradient in 0.1% trifluoroacetic acid (v/v) during 10 min. The major peptides selected were applied to a polybrene coated glass filter and sequenced in an Applied Biosystems model 477A automatic sequencer run according to the manufacturer’s instructions. Searches for the similarities to the determined sequences were performed with the aid of the Swiss-Prot protein sequence data bank. The peptides were aligned by similarity.
2.11 Equilibrium Dialysis
The binding of lactose to the purified galectin was quantitatively studied in PBS ‘B’ by equilibrium dialysis in a dialysis bag (3.0 ml capacity) made from Sigma cellulose membrane. The dialysis bag containing 100 μg/ml of the galectin solution was placed in plastic vials containing 1 ml lactose solution in the range of 40–400 μM. After equilibrating for 24 h at 37 °C, a portion was taken from the protein free compartment and its carbohydrate content was estimated [14]. The amount of lactose bound per mole galectin was calculated according to Scatchard analysis.
2.12 Effect of Divalent Metal Cations
To examine the effect of divalent metal cations on galectin activity, demetallization of purified galectin (100 μg/ml) was carried out using 0.1 M EDTA followed by remetallization of the sample with 0.1 M CaCl2 and MnCl2, [Sr(CH3COO)2], MgCl2, and NiCl2 in PBS ‘B’. Hemagglutination activity of each sample was tested by microtitre plate assay.
2.13 Determination of Sulfhydryl Groups
The exposed thiol (-SH) groups were determined according to the procedure of Ellman [15]. For the determination of exposed -SH groups, appropriate amount of galectin was added in 100 mM Tris–EDTA buffer (pH 8.0) to give a total volume of 3.0 ml of the solution, followed by addition of 0.1 ml DTNB solution (40 mg DTNB in 10 ml of 0.1 M Tris HCl, pH 8.0). After 15 min, the color developed was read at 412 nm. The number of free thiol groups was calculated in ‘milli-moles’ using cysteine (0.125 M) as standard.
2.14 Thermal and pH Stability
Thermal stability of the purified galectin (100 μg/ml) was determined by incubating the samples in PBS ‘B’ at various temperatures (30–80 °C) for 30 min. The samples were then cooled on ice and hemagglutination activity of each sample was tested by microtitre plate assay.
For determining the pH stability, the purified galectin (100 μg/ml) samples in 50 μl of normal saline containing 5 mM β-ME was incubated with 50 ml of the following buffers, 0.1 M sodium acetate buffer (pH 3.5–5.5), 0.1 M sodium phosphate buffer (pH 6.5–7.5), 0.1 M Tris–HCl buffer (pH 8.5–9.5) and 0.1 M glycine–NaOH buffer (pH 10.5–11.5) for 24 h at 4 °C. Hemagglutination activity of each sample was tested by microtitre plate assay.
2.15 Effect of Thiol Blocking Agents, Denaturants and Detergents
The rate of reaction of the purified galectin (100 μg/ml) with 70 mM each of iodoacetamide, iodoacetate, p-hydroxymercuricbenzoate (pHMB) and N-ethylmaleimide (NEM) in PBS ‘B’ was determined at room temperature. The effect of denaturants on the purified galectin (100 μg/ml) was determined by incubating protein samples with increasing concentrations (0–8 M) of urea, guanidine HCl (GdnHCl) and thiourea in PBS ‘B’37 °C for 1 h. The effect of different detergents was monitored by incubating the purified galectin (100 μg/ml) with increasing concentrations (1–5 M) of SDS, Tween-20 and Triton X-100 in PBS ‘B’ at 37 °C for 1 h. After designated time, hemagglutination activity of each sample was tested by microtitre plate assay.
2.16 UV–VIS Spectroscopy
The requirement of reducing agent for maintenance of the galectin activity was further investigated by measuring the time-course effect of an oxidizing agent on native protein by adding 5 mM H2O2 in PBS ‘B’ without β-ME. The UV spectra of the purified galectin (100 μg/ml in PBS ‘B’ containing β-ME), and that of oxidized galectin (100 μg/ml in PBS ‘B’ containing 5 mM H2O2 in the absence of β-ME) were measured on UV-1700 Pharmaspec UV–VIS double beam spectrophotometer (Shimadzu Corp., Kyoto, Japan) in the wavelength region of 220–320 nm.
2.17 Fluorescence Spectroscopy
Intrinsic fluorescence of the purified galectin (100 μg/ml) was measured at 25 °C in a Hitachi F-200 spectrofluorometer (Hitachi, Tokyo, Japan) equipped with a DR3 recorder. The solutions were selectively irradiated using an excitation wavelength of 280 nm with 10 nm band pass. Emission spectra were measured in the range of 300–400 nm. Changes in the fluorescence spectra in the presence of 5 mM H2O2, 0.1 M lactose, 8 M urea and 5 M SDS solutions were also recorded. Appropriate controls containing the substances used for treatment were also taken. Each spectrum was the average of five scans.
2.18 Circular Dichroism (CD) Spectroscopy
All CD measurements were carried out on Jasco spectrophotometer model J-810 using a SEKONIC XY plotter (model SPL-4301A) with a thermostatically controlled (25 °C) cell holder attached to a NESLAB model RTE-210 water bath with an accuracy of ±0.10 °C. The instrument was equipped with a microcomputer and pre-calibrated with (+)-10-camphorsulfonic acid. CD measurements were taken in the far UV region (200–250 nm) as well as the near UV region (250–350 nm) using purified galectin concentration of 250 μg/ml and 1 mg/ml, respectively. Original spectrum of the purified galectin was recorded with a scan speed of 100 nm/min with a response time of 1 s. Changes in the CD spectra in the presence of 5 mM H2O2, 0.1 M lactose, 8 M urea and 5 M SDS solutions were also recorded. Appropriate controls containing the substances used for treatment were also taken. Each spectrum was the average of five scans.
2.19 Fourier Transform Infra Red (FTIR) Spectroscopy
All FTIR measurements were carried out on NICOLET (ESP) 560 spectrophotometer (USA) equipped with a transmission OMNIC ESP 5.1 software and a DTGS detector. Original spectrum of the purified galectin (150 μg/ml) at 37 °C was recorded with a resolution of 4 cm−1 and 128 scans. Changes in the FTIR spectra in the presence of 5 mM H2O2, 0.1 M lactose, 8 M urea and 5 M SDS solutions were also recorded. The changes in the peak frequency and intensity were then assigned to conformational change within the protein. Appropriate controls containing the substances used for treatment were also taken. Each spectrum was the average of five scans.
2.20 Bioinformatics Studies
The amino acid sequence of the purified galectin was formatted using DNA tools (http://biology.semo.edu/cgi-bin/dnatools.pl). BLAST-P (http://www.ncbi.nlm.nih.gov/BLAST [3]) and CLUSTALW (www.ebi.ac.uk/clustalw) were used to further corroborate the amino acid sequence. Peptide cutter program of ExPASy tools (http://ca.expasy.org/’) was used to predict the purified galectin digestion by trypsin and high specificity chymotrypsin digestion in reverse phase HPLC. The theoretical average mass and monoisotopic mass of the purified galectin were predicted using ExPASy tools and compared with the findings of wet lab. Probable secondary structure of the purified galectin was explored using 3D-JIGSAW Comparative Modelling Server program of ExPASy tools.
3 Results and Discussion
3.1 Isolation and Purification of Goat Heart Galectin
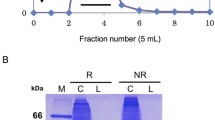
About 70% of total active galectin was precipitated after ammonium sulphate fractionation, resulting in 3.5 fold purification. After extensive dialysis against PBS ‘B’, the salt fractionated galectin was chromatographed first on Sephadex G50 gel filtration column followed by lactosyl-Sepharose 4B affinity column, both equilibrated with PBS ‘B’. The results of purification from 100 g goat heart tissue are shown in Table 1. The purified galectin yield (1.09 mg) represented 66% yield with a fold purification of 1618.1. Figure 1 shows the elution profile of the purified goat heart galectin. The purified galectin moved as a single band on native PAGE (Fig. 1 inset) as well as SDS–PAGE (Fig. 2 inset), suggesting homogeneity of the purified galectin with respect to charge and mass.
Molecular weight determination using SDS–PAGE: electrophoresis of the purified protein was performed on 12.5% acrylamide gel in reducing condition. The relative mobilities (Rm) were plotted against their molecular weight using least square analysis of the data. The molecular weight markers used were: Phosphorylase b (97.4 kDa), Bovine Serum Albumin (68 kDa), Ovalbumin (45 kDa), Carbonic Anhydrase (29 kDa), Soyabean Trypsin Inhibitor (20.1 kDa), Lysozyme (14.4 kDa). The elution position of dimer and monomer GHG-1 are indicated by arrows A and B, respectively. Inset shows the SDS–PAGE of GHG-1: Lane ‘a’ contains molecular weight markers (35 μg) in descending order of their molecular weight and lane ‘b’ contains 35 μg of purified protein, Gel was stained using CBB R 250 dye
3.2 Molecular Weight Determination
On Sephadex G100 gel filtration column purified goat heart galectin eluted as a monomer of 14.5 kDa under reducing conditions, whereas they eluted as a dimer of 29 kDa under non-reducing conditions (Fig. 2), thus, revealing their homo-dimeric nature. SDS–PAGE also demonstrated the molecular weight of purified galectin as 14.5 kDa (Fig. 2 inset).
3.3 Determination of Stokes Radii and Diffusion Coefficients
Stokes radii of dimer and monomer GHG-1 was found to be 26 and 17.5 Å, respectively. The diffusion coefficient of GHG-1 corresponding to the value of stokes radius was computed to be 8.57 × 10−15 and 12.7 × 10−15 cm2/s, respectively. These findings further confirmed the homo-dimeric nature of purified galectin. The dimeric nature of β-galactoside binding galectin s provides them the potential to bind to glycoconjugates. The positive and negative effects of Gal-1 on cell growth might be influenced by both dose and the relative distribution of monomeric versus dimeric form [36].
3.4 Carbohydrate Binding and Human Blood Group Specificity
Most β-galactoside binding lectins display affinity towards lactose and N-acetyllactosamine with subtle differences in their carbohydrate specificities [32]. Therefore, a number of simple sugars and complex saccharides were screened for sugar specificity of the purified galectin by hemagglutination inhibition assay. Structural variations in the CRD topology of galectins has been reported to contribute to their extraordinary sensitivity towards corresponding sugar residues [33], and formed the basis of our studies regarding their specificity towards various saccharides. Hemagglutination inhibition studies using a number of saccharides (Table 2) led to the conclusion that the purified goat heart galectin is specific for saccharides bearing non-reducing terminal d-galactose linked in a β-configuration. This is further supported by the observation that methyl-α-d-galactopyranoside and p-nitrophenyl-α-d-galactopyranoside are weak inhibitors as compared to methyl-β-d-galactopyranoside and p-nitrophenyl-β-d-galactopyranoside [2]. The affinity for β-galactosides satisfied the first criterion for inclusion of the purified goat heart galectin in Gal-1 subfamily.
The inhibition of galectin activity by d-galactose and d-galactosamine indicates that a free hydroxyl or a free amino group at C2 is required for a monosaccharide to cause inhibition of galectin binding to erythrocytes [34]. Moreover, the configuration at C4 is also important, since neither d-glucose nor d-glucosamine caused inhibition. The fact that lactose is far more potent inhibitor for hemagglutination activity of the purified galectin suggests that carbohydrate binding site of the purified galectin could have extended geometry, which is only partially occupied by the galactose molecule [31]. This finding is quite consistent with the X-ray crystallographic studies using small disaccharides which predicted the 4-OH and 6-OH groups of galactose and 3-OH group of glucose comprising lactose structure are critically important for high affinity binding [25]. An ammonium group on C2 (d-galactosamine, d-glucosamine) decreases the inhibitory properties, likely because of its positive charge. Unlike human brain Gal-1, which required low concentrations of β-d-galactose, p-nitrophenyl-β-d-galactoside and d-fucose (1.4, 0.4, and 25 mM, respectively) for complete hemagglutination inhibition [9], the purified goat heart galectin required higher concentrations of these saccharides, suggesting its own unique specificity.
The concentration of the purified galectin required for hemagglutination varied markedly with the type of human erythrocytes. The purified protein agglutinated native human erythrocytes with marked preference for the blood group A, probably mediated by the polylactosaminoglycans found on the surface of human erythrocytes [30]. The overall order of preference was A>O>AB>B.
3.5 Amino Acid Sequencing
For determining the amino acid sequence the tryptic and chymotryptic fragments of purified protein were separated by reversed phase HPLC (Fig. 3). The peaks T1–T9 representing pure peptides were subjected to amino acid analysis and automated sequencing (Fig. 4) except for T6. The presence of an acetyl group was deduced from its blocked N-terminus as reported for other Gal-1 [34].
HPLC profile of peptides obtained by tryptic (a) and chymotryptic (b) digestion of the purified lectins: Peptides were separated by reversedphase HPLC on a Vydac C18 column equilibrated with 0.1% (v/v) trifluoroacetic acid in water. Elution was performed at a flow rate of 1.0 ml/min with 0–60% acetonitrile linear gradient in 0.1% (v/v) trifluoroacetic acid for 10 min, and the elution was monitored at 220 nm. Peaks T1–T9 represent tryptic peptides and C1–C3 represent chymotryptic peptides, whose sequence was determined by Edman degradation and established from amino acid sequence
Comparison of GHG-1 amino acid sequence of with other Gal-1. GHG-1 sequence was compared with other Gal-1: CMG-1, cattle myelin Gal-1; BHG-1, bovine heart Gal-1; SBG-1, sheep brain galectin; HBG-1, human brain Gal-1; RLG-1, rat lung Gal-1. Identities to GHG-1 sequence are shown at the bottom right. Amino acids conserved in all Gal-1 are designated with asterisks (*). Amino acids from other Gal-1, which differ from those present at the same position in GHG-1, are indicated in bold (•)
The T6 sequence not established by tryptic digestion was subjected to Edman degradation and the resulting fragments were designated as C1–C3. The sequence alignment of the purified galectin with other Gal-1 characterized from cattle myelin sheath [1], bovine heart [35], human brain [8], rat lung [13] and sheep brain [34] showed a significant degree of identity (90–94%), in agreement with its high conservation throughout evolution. These results suggest that the 14.5 kDa Gal-1 is species specific rather than organ specific protein [8].
The analysis of amino acid sequence of the purified galectin indicates that the aromatic side chain of the conserved Trp68 stacks adjacent to the galactose ring. Such van Der Waal interactions between sugar and aromatic side chains are quite common in protein-carbohydrate complexes [20]. The axial 4-OH of galactose, a main determinant of the galectin specificity, forms two key electrostatic interactions; one with the N-atom of Arg48 and the other with the N-atom of His44 [34]. Both are invariant residues in the sequences of various galectins (Fig. 4). In addition, the conservation of amino acid residues that interact with carbohydrate ligands (His44, Asn46, Arg48, His52, Asp54, Asn61, Trp68, Glu71, and Arg73) allowed us to classify them as ‘prototype’ according to its molecular architecture as it neither has a link peptide joining two CRDs nor has the CRD joined to different types of domain as found in ‘tandem repeat’ and ‘chimera’ topologies, respectively. Besides, they show the characteristics of cytoplasmic proteins, such as an acetylated N-terminal amino group and the lack of hydrophobic trans-membrane segment and secretion signal peptide similar to other Gal-1 [7]. The conserved 134 amino acid CRD satisfied the second criterion for inclusion of the purified goat heart galectin in Gal-1 subfamily, and thus we designated it as goat heart galectin-1 (GHG-1).
3.6 Equilibrium Dialysis
GHG-1 readily reacted with lactose, according to the following reaction:
where ‘n’ is the number of carbohydrate binding sites in the GHG-1 dimer, ‘Lac’ stands for lactose, ‘υ’ stands for the amount of lactose bound per mole galectin, and ‘K a’ is the association constant for interaction of Lac with GHG-1.
Equation 3 can be arranged to Eq. 4.
‘υ’ was determined in triplicate and plotted according to Eq. (4).
The linear Scatchard plot of equilibrium dialysis data of GHG-1 in PBS ‘B’ with lactose at 37 °C was drawn. The slope of the plot gave binding constant value (K a) for GHG-1 as 6.97 × 103 M−1. The number of binding sites calculated from the X intercept was found to be 1.9 per GHG-1 dimer.
3.7 Effect of Divalent Metal Cations and Determination of Sulfhydryl Groups
The divalent metal cations did not GHG-1 activity. The metal independent nature of GHG-1 further confirmed its inclusion in Gal-1 family, as reported for other Gal-1 [30]. Exposed thiol group analysis of GHG-1 indicated a molar ratio of 3.1, suggesting the presence of approx. three moles of sulfhydryl group per mole of protein.
3.8 Thermal and pH Stability
GHG-1 retained full hemagglutination activity for temperatures up to 42 °C. Beyond this temperature, a gradual drop of activity was observed, which was lost completely at 63 °C. The purified galectin was found to be quite stable in the pH range 5.5–9.5; however, it showed maximum hemagglutination activity near physiological pH 7.4. These findings suggest GHG-1 as the most stable mammalian heart galectin to have been purified till date, thus recommending the purified goat galectin for preferential use in various recognition studies.
3.9 Effect of Thiol Blocking Agents, Denaturants and Detergents
The structural and functional integrity of GHG-1 was assayed in the presence of various thiol blocking reagents, chaotropic denaturants and detergents in order to highlight the susceptibility of heart galectins towards different denaturing media. The fact that the reduced form of cysteine is indeed required for the maintenance of GHG-1 in its active form [30, 34] was confirmed by the finding that GHG-1 activity was differentially inhibited in the presence of various thiol blocking reagents. The loss of galectin activity thus could be due to possible conformational changes that occurred during modification of cysteine residues [25]. However, relatively slower rate of inactivation points towards the possibility of cysteine molecules being partially buried inside the galectin, and possibly not involved directly in carbohydrate binding but rather being present at a relatively distant site [2]. This easily explains the effectiveness of pHMB, the most hydrophobic blocking reagent, in most readily inhibiting the hemagglutination activity (50% inhibition in 17 min) followed by lesser hydrophobic NEM (50% inhibition in 25 min), iodoacetate (50% inhibition in 45 min) and iodoacetamide (50% inhibition in 55 min) (Fig. 5a).
a Effect of thiol blocking reagents on GHG-1 activity: the purified lectin (100 μg/ml) was incubated with 70 mM each of iodoacetamide, iodoacetate, p-hydroxymercuricbenzoate (pHMB) and N-ethylmaleimide (NEM) in PBS ‘B’ was determined at room temperature. b Effect of denaturants on GHG-1 activity: the purified lectin (100 μg/ml) was incubated with increasing concentrations (0–8 M) of urea, guanidine HCl (GdnHCl) and thiourea in PBS ‘B’37 °C for 1 h. c Effect of detergents on GHG-1 activity: The purified lectins (100 μg/ml) were incubated with increasing concentrations (1–5 M) of SDS, Tween-20 and Triton X-100 in PBS ‘B’ at 37 °C for 1 h. After designated time, hemagglutination activity of each sample was tested by microtitre plate assay
The inhibitory effect of various chaotropic denaturants on GHG-1 activity (Fig. 5b) showed that urea caused maximum loss in the galectin activity (50% decrease at 2.0 M), followed by GdnHCl (50% decrease at 4.0 M) and thiourea (50% decrease at 5.0 M). These findings indicate that GHG-1 globular nature is stabilized mainly by hydrogen bonding and hydrophobic interactions [29], suggesting a denaturant induced modification in the environment of the Trp residues.
SDS, a well known ionic detergent denatured GHG-1 even at a very low concentration (Fig. 5c), possibly by ordering water molecules and also by intercalating into hydrophobic galectin clefts with its long hydrocarbon chain [22]. Tween-20 and Triton X-100 being neutral detergents were less inhibitory on GHG-1, as they do not bind with galectins in detectable amount and are not very efficient in breaking non-covalent bonds which are responsible for the quaternary structure of multimeric proteins.
3.10 UV–VIS Spectroscopy
Like other β-galactoside binding galectins [32, 35], GHG-1 also shows hemagglutination activity only in the presence of reducing agents such as β-mercaptoethanol. Purified galectin showed no detectable loss of activity when kept for up to 4 months in PBS containing 0.3 M lactose at 4 °C, thus suggesting that lactose maintains the purified galectin in the active form even in the absence of a reducing agent, possibly by preventing oxidative inactivation due to formation of intra-sulphide linkages [12]. Since galectins remain in active conformation in the presence of reducing agents, we speculate that they contain an oxidizable residue whose integrity is crucial for their activity. GHG-1 presents a UV spectrum maximum at 280 nm (Fig. 6a), corresponding to the presence of single Trp residue and a large number of other aromatic residues. However, oxidation of the purified galectin in the presence of 5 mM H2O2 (without β-ME) resulted in the shift of absorption peak to 250 nm suggesting oxidation of Trp residue to an oxindole moiety, which absorbs maximally at 250 nm [24]. This result suggested that reducing agents not only prevent oxidation of Cys residues in galectins but also protect Trp from oxidation.
a UV–VIS spectroscopy of GHG-1: the UV spectra of the purified lectin (100 μg/ml in PBS ‘B’ containing β-ME), and that of oxidized lectin (100 μg/ml in PBS ‘B’ containing 5 mM H2O2 in the absence of β-ME) were measured on UV-1700 Pharmaspec UV–VIS double beam spectrophotometer (Shimadzu Corp., Kyoto, Japan) in the wavelength region of 220–320 nm. b Fluorescence spectra of GHG-1: fluorescence spectrum of GHG-1 (100 μg/ml) was measured in the range of 300–400 nm. Changes in the fluorescence spectra in the presence of 5 mM H2O2, 0.1 M lactose, 8 M urea and 5 M SDS solutions were also recorded. Appropriate controls containing the substances used for treatment were also taken. Each spectrum was the average of five scans
3.11 Fluorescence Spectroscopy
When excited at 280 nm, GHG-1 exhibited maximum fluorescence emission 330 nm (Fig. 6b), typical of Trp residue in a hydrophobic environment. The fluorescence profile of the oxidized galectin showed a remarkable quenching in the fluorescence intensity accompanied with a blue shift from 318 to 338 nm. Oxidized galectin also exhibited a decreased activity, thus implicating intra-molecular disulphide bonding as the basis of active conformation of GHG-1 [40]. However, the presence of lactose prevented the oxidation of Trp which is supported by a slight decrease in the fluorescence of protein-carbohydrate complex. These findings stress upon the significance of reducing environment which is probably needed to reduce molecular oxygen normally present in the solutions to prevent it from oxidizing Trp residues [24]. It also indicates that the fluorophore is present within or in the vicinity of lactose binding site and may be readily accessible to lactose [24]. The involvement of Trp68 aromatic ring (a highly conserved amino acid) in interaction with hydrophobic part of the side chain of the conserved Lys63 assures its optimal orientation for interaction with lactose residues [34]. Although, there is no direct evidence that Trp is crucial for hemagglutination activity of heart galectins, but it may play some possible role in maintenance of the carbohydrate binding site of the galectin molecules as several research groups, based on X ray studies on bovine spleen galectin have shown that Trp 68, a highly conserved amino acid in β-galactoside binding galectins is found to make stacking interaction with galactose moiety [37]. The fluorescence emission spectra of GHG-1 were also recorded after treatment with urea, pHMB and SDS. The resulting decrease in the fluorescence intensity indicates that the Trp residue in the native galectins is located near an intra-molecular quenching group, and galectin denaturation increases this interaction. The effect of oxidant was also monitored by CD and FTIR analysis. A shift in far UV-CD (Fig. 7a), near UV-CD (Fig. 7b) and FTIR (Fig. 7c) spectra showed a major transition of native secondary structure of GHG-1 from β-pleated form to a more open and enriched α-helix conformation, thus, clarifying the reason for the loss of activity upon oxidation.
a Far UV-CD spectra of GHG-1: CD measurements were taken in the far UV region (200–250 nm) using GHG-1 concentration of 250 μg/ml. Original spectrum of GHG-1 was recorded with a scan speed of 100 nm/min with a response time of 1 s. b Near UV-CD spectra of GHG-1: CD measurements were taken in the near UV region (250–350 nm) using GHG-1 concentration of 1 mg/ml. Original spectrum of GHG-1 was recorded with a scan speed of 100 nm/min with a response time of 1 s. c FTIR spectra of GHG-1: original spectrum of GHG-1 (150 μg/ml) was recorded at 37 °C with a resolution of 4 cm−1 and 128 scans. Changes in the CD and FTIR spectra in the presence of 5 mM H2O2, 0.1 M lactose, 8 M urea and 5 M SDS solutions were also recorded. Appropriate controls containing the substances used for treatment were also taken. Each spectrum was the average of five scans
3.12 CD and FTIR Spectroscopy
The far UV-CD spectrum of native GHG-1 showed a low intensity spectrum with minimum around 220 nm (Fig. 7a), consistent with a predominantly β-sheet structure profile as reported for other Gal-1 [34]. The FTIR spectrum also correlates with CD analysis, consistent with the presence of large percentage of β-sheet structure in native GHG-1 as suggested by maximum absorbance peak at 1,632 cm−1 (Fig. 7c). The FTIR spectrum of native GHG-1 was found to be truncated between 1,650 and 1,573 cm−1. Presence of lactose with native GHG-1 did not result in any significant change in secondary and tertiary topology. However, exposure of GHG-1 to H2O2, urea, pHMB and SDS caused very marked changes in both far UV-CD (Fig. 7a) and near UV-CD (Fig. 7b) spectra as well as FTIR spectra (Fig. 7c), concomitant with the loss of β-sheet structure. The spectral change is consistent with disruption of regular secondary structures (25–50% α-helix and 35–60% β-sheet) to a random coil structure as well as remarkable changes in the tertiary structure. It is possible that the formation of disulfide bonds locks the galectin into a new, inactive conformation that neither can form the usual secondary and tertiary structure and nor can bind saccharides [13]. This strongly emphasizes the requirement of the regular secondary and tertiary structure and of the presence of complete polypeptide chain for maintaining the active galectin conformation.
3.13 Bioinformatics Studies
The prediction of bioinformatics studies were found to be strongly in agreement with the results obtained in wet lab. BLAST-P and CLUSTALW established GHG-1 amino acid sequence similarity with other Gal-1. The predictions for trypsin and high specificity chymotrypsin digestion of the purified galectin using peptide cutter program of ExPASy tools were in accordance with the result obtained for reverse phase HPLC (Table 3). ExPASy tools predicted average mass as well as monoisotopic mass of the purified galectin as 14.5 kDa, thus corroborating our findings. Secondary structure prediction of GHG-1 using 3D-JIGSAW Comparative Modelling Server of ExPASy tools (Fig. 8) was in accordance with known secondary structures of other Gal-1.
4 Conclusion
The present study for the first time reports the purification and characterization of a metal independent Gal-1 from Capra hircus heart. The appreciably high degree of thermal and pH stability suggests it to be one of the most stable mammalian heart galectin purified till date, proclaiming its preferred usage in various recognition studies and other potential biological and clinical applications. The sensitivity of GHG-1 carbohydrate binding activity to oxidative inactivation might serve to limit the duration and physical range of its influence. Moreover, marked reduction in the activity of oxidized protein and associated relevance from available literature suggest its potential role in free radical induced oxidative stress mediated cardiovascular disorders.
Abbreviations
- Gal-1:
-
Galectin-1
- CD:
-
Circular dichroism
- CBB:
-
Coomassie brilliant blue
- FTIR:
-
Fourier transform infra red
- pHMB:
-
para-Hydroxymercuricbenzoate
- HPLC:
-
High performance liquid chromatography
- SDS–PAGE:
-
Sodium dodecyl sulphate polyacrylamide gel electrophoresis
- UV–VIS:
-
Ultraviolet visible
- GHG-1:
-
Goat heart galectin-1
- PBS:
-
Phosphate buffered saline
- CRD:
-
Carbohydrate recognition domain
References
Abbott WM, Mellor A, Edwards Y, Feizi T (1989) Biochem J 259(1):283–290
Ali N, Salahuddin A (1989) Biochim Biophys Acta 992:30–34
Altschul SF, Warren G, Webb M, Eugen WM, David JL (1990) J Mol Biol 215:403–410
Andrews P (1965) Biochem J 96:595–606
Aragno M, Mastrocola R, Alloatti G, Vercellinatto I, Bardini P, Geuna S, Catalano MG, Danni O, Boccuzzi G (2008) Endocrinology 149(1):380–388
Bardosi A, Bardosi L, Hendrys M, Wosgien B, Gabius HJ (1990) Am J Anat 188(4):409–418
Barondes SH, Cooper DNW, Gitt MA, Leffler H (1994) J Biol Chem 269:20807–20810
Bladier D, Caer JPLF, Joubert R, Caron M, Rossier J (1991) Neurochem Int 18:275–281
Bladier D, Joubert R, Avellana-Adalid V, Kemeny JL, Doinel C, Amourous J, Caron M (1989) Arch Biochem Biophys 269:433–439
Case D, Irwin D, Ivester C, Harral J, Morris K, Imamura M, Roedersheimer M, Patterson A, Carr M, Hagen M, Saavedra M, Crossno J Jr, Young KA, Dempsey EC, Poirier F, West J, Majka S (2007) Am J Physiol Lung Cell Mol Physiol 292:L154–L164
Chellan B, Narayani J, Appukuttan PS (2007) Exp Mol Path 83:399–404
Cho M, Cummings RD (1995) J Biol Chem 270:5198–5206
Clerch LB, Whitney P, Hass M, Brew K, Miller T, Werner R, Massaro D (1988) Biochemistry 27:692–699
Dubois M, Gilles KA, Hamilton JK (1956) Anal Chem 28:350–356
Ellman GL (1959) Arch Biochem Biophys 82:70–77
Elola MT, Chiesa ME, Fink NE (2005) Comp Biochem Phys 141:23–31
Ersson B, Asberg K, Porath J (1973) Biochim Biophys Acta 310(2):446–452
Giordanengo L, Gea S, Barbieri G, Rabinovich GA (2001) Clin Exp Immunol 124(2):266–273
Hasan SS, Ashraf GM, Banu N (2007) Cancer Lett 253:25–33
Kasai K, Hirabayashi J (1996) J Biochem (Tokyo) 119:1–8
Laemmli UK (1970) Nature 227:680–685
Lanio LE, Alfarez C, Pazos S, Martinez D, Martinez Y, Casallanovo F, Abuin E, Schreier S, Lissi E (2003) Toxicon 41:65–70
Laurent TC, Killander J (1964) J Chromat 14:317–330
Levi G, Teichberg VI (1981) J Biol Chem 256:5735–5740
Liao DI, Kapadia G, Ahmed H, Vasta GR, Herzberg O (1994) Proc Natl Acad Sci USA 91:1428–1432
Lis H, Belenky D, Rabinkov A, Sharon N (1994) Cell biology: a laboratory handbook. Academic Press Inc., New York
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) J Biol Chem 193(1):265–275
Moiseeva EP, Williams B, Goodall AH, Samani NJ (2003) Biochem Biophys Res Comm 310:1010–1016
Nelson L, Cox MN (2001) Macmillon worth publishers, New York, USA, pp 159–202
Ola MS, Tabish M, Khan FH, Banu N (2007) Cell Biol Int 31:578–585
Rabinovich GA, Toscano MA, Ilarregui JM, Rubinstein N (2004) Glycoconj J 19:565–573
Rabinovich GA, Toscano MA, Jackson SS, Vasta GR (2007) Curr Opin Struc Biol 17:513–520
Reuter G, Gabius HJ (1999) Cell Mol Life Sci 55:368–422
Shahwan M, Alqirim MT, Zaidi SMKR, Banu N (2004) Biochemistry (Moscow) 69:506–512
Southan C, Aitken A, Childs RA, Abbott WM, Feizi T (1987) FEBS Lett 214:301–304
Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG (2006) J Immunol 176:778–789
Vasta GR, Ahmed H, Odom EW (2004) Curr Opin Struc Biol 14:617–630
Waard AD, Hickman S, Kornfeld S (1976) J Biol Chem 251:7581–7587
Weber K, Osborn M (1969) J Biol Chem 244:4406–4412
Whitney PL, Powell JT, Sanford GL (1986) Biochem J 238:683–689
Acknowledgments
The financial assistance in the form of scholarships to the first author (GMA) from University Grants Commission (UGC), New Delhi is thankfully acknowledged. The authors are grateful to Aligarh Muslim University, UGC-SAP and DST-FIST for the facilities. Thanks are due to all friends and fellows for their critical suggestions during experimental set up and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashraf, G.M., Banu, N., Ahmad, A. et al. Purification, Characterization, Sequencing and Biological Chemistry of Galectin-1 Purified from Capra hircus (goat) Heart. Protein J 30, 39–51 (2011). https://doi.org/10.1007/s10930-010-9300-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-010-9300-2