Abstract
Bovine leukaemia virus (BLV) causes disease in cattle and it is related to human T lymphotrofic viruses HTLV-1 and HTLV-2. The objective of this study was to express and purify deleted and stable forms of the gp51 envelope glycoprotein of BLV using a baculovirus system. Two forms of the gp51 were synthesised: one comprised the gp51 N-terminal 174 amino acids and a single 6xHis tag (∆175-268gp51-His) and the second form contained the same amino acid sequence and a C-terminal Strep-tag II in addition to the 6xHis tag (∆175-268gp51-STH). The two proteins were expressed and purified by immobilized metal-affinity chromatography (IMAC) or by Strep-Tactin column. The Strep-Tactin technology was more efficient than IMAC method and achieved a high pure recombinant deleted gp51. In addition the ∆175-268gp51-STH protein was further concentrated by IMAC. This purified antigen could be used for the isolation of immunoreactive molecules and to develop a competitive ELISA test.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The BLV is an oncogenic retrovirus, a member of the Retroviridae, genus Deltaretrovirus. BLV has a world-wide distribution and it is the etiological agent of Enzootic Bovine Leucosis EBL [6, 25]. EBL is a lymphoproliferative disease of cattle and causes severe economic losses. National programmes for the eradication of BLV, based on detection and removal of infected animals by serological diagnosis, have been introduced in many countries. An early detection of antibodies, directed against the major envelope glycoprotein gp51 of BLV, is an efficient way to control the disease. Gp51 is directly involved in infectivity events and it is the major immunogenic protein [32]. Detection of BLV antibodies can be achieved by conventional ELISA tests that employ the gp51 protein as antigen [4, 5, 22]. The gp51 traditionally produced by foetal lamb kidney (FLK) cell line chronically infected with BLV (FLK-BLV), needs to be concentrated and partially purified before the use. Recently a gp51 recombinant protein (r-gp51) was expressed by baculovirus system in insect cells [10, 26]. The r-gp51 was efficiently utilized in the ELISA test (ELISAr-gp51), but the purification from the recombinant baculovirus was limited because of its tendency to form macromolecular aggregates.
Previous studies have shown that mouse monoclonal antibodies (Mabs), raised against BLV gp51, identify conformational and sequential epitopes (A through H) on the gp51 antigen [2]. Three of them (F, G, H) are of particular interest, since they are the target for Mabs that result in virus neutralization and syncytium inhibition, and they correspond to the epitopes recognized by antibodies present in the serum of naturally infected animals [3, 4]. Epitopes F, G and H are conformational and their recognition by antibodies requires the glycosylation of the gp51 [5]. Moreover, they are located within the weakly glycosylated N-terminal part of the protein. Epitopes A, B, B′, D, D′ and E are present on the denaturated gp51 and are thus termed sequential [23]. The E epitope is located between the N and C-teminal part of gp51, whereas epitopes A, B, D and the conformational epitope C, reside in the highly glycosylated C-terminal region of protein [18].
Currently, the 6xHis tag [21] is the most used affinity tag employed for purifying recombinant proteins by IMAC technology [31] using a Ni2+-nitrilotriacetic acid (Ni-NTA) matrix [12, 13]. Different intracellular and secreted recombinant proteins in fusion with an hexahistidyl sequence, expressed in the baculovirus expression system, have been purified by IMAC [8, 9, 24]. One-step metal affinity chromatography purification has been shown to be fairly effective for secreted antigen produced in mammalian cells grown in a serum-free medium [20].
Strep-tag II is a valid alternative to the 6xHis tag. The Strep-tag purification system is based on the high selective binding of engineered streptavidin (Strep-Tactin) to Strep-tag II fusion proteins [29, 30, 33]. Using this technology it is possible to purify recombinant proteins under physiological conditions, thus preserving the biological activity. The Strep-tag affinity chromatography has been used to purify proteins produced in Escherichia coli [11, 15], in yeast [1, 14], in plants [34] and in mammalian cell lines [7, 16].
The objective of this study was to develop an efficient and simple protocol for the production of a deleted form of gp51 protein, with high degree purity and yield rate. This recombinant protein may be applied for designing a direct ELISA to use in screening tests and for the production of diagnostic reagents, such as Mabs, carried out by traditional method or by screening of a phage display antibody library.
2 Materials and Methods
2.1 Plasmid and Construction of Recombinant Baculoviruses
Standard methods for molecular biology were used [28]. The gp51 coding gene was recovered from plasmid pB4gp51 [10] after being digested with Xho I and Hind III restriction enzymes and purified. Primers OplE2 forward and reverse (Invitrogen Corporation, Carlsbad, USA) were used to amplify the V5-6xHis double tag from the pIZT/V5His vector (Invitrogen). The amplified sequence was cloned in pCR 2.1 vector and sequenced. The Xho I-Hind III fragment containing the gp51 gene and the V5-6xHis tag insert recovered from pCR 2.1 by Hind III and Nsi I digestion, were cloned into the pFastBac 1 vector (Invitrogen) cut with Sal I and Pst I enzymes to generate the pFB1gp51/V5-H is plasmid. Two primers were designed in accordance with the nucleotide sequence of BLV [27] to amplify the inner region of gp51 protein. The forward primer, including the underlined Nde I restriction site, was 5′-AGGGCCATGGTCACATATG-3′ (nucleotide 5,127–5,145) and the reverse primer, containing an Age I site, was 5′-CAGTCTGGACCGGTCCGTGCTGTTTGATTTA-3′ (nucleotide 5,425–5,455). The insert obtained was purified by MiniElute PCR Purification KiT (Qiagen GmbH, Germany) and after digestion with Nde I-Age I enzymes, was cloned into the pFB1gp51/V5-H is plasmid digested with the same endonucleases. The resultant construct, coding the first 174 amino acids of the N-terminal part of the gp51 in frame with the 6xHis tag, was named as ∆175-268gp51-His/pFB1.
The ∆175-268gp51-His/pFB1 was modified to contain the Strep-tag II and 6xHis double tag at the C-terminus of the deleted r-gp51. The Strep-tag II nucleotide sequence was introduced downstream of position 5,441 of BLV sequence, using two-step PCR methods. First, the inner part of gp51 was amplified using the forward primer 5′-AGGGCCATGGTCACATATG-3′ and the reverse primer 5′-GAACTGCGGGTGGCTCCAGCTAGCCCGTGCTGTTTGATTT-3′ including the Nhe I spacer restriction site and the overlapping part of Strep-tag II coding sequence. Subsequently this fragment was purified and subjected to the second amplification step using the same forward primer and the reverse primer 5′-GGATCCACCGGTTTTTTCGAACTGCGGGTGGCTCCA-3′ including the Age I restriction site and the terminal part of Strep-tag II. The resulting fragment was purified, digested with Age I and Nde I and cloned in the ∆175-268gp51-His/pFB1 plasmid cut with the same enzymes. The resultant construct (∆175-268gp51-STH/pFB1) was verified by sequencing.
Recombinant baculoviruses expressing both recombinant ∆175-268gp51-His and ∆175-268gp51-STH proteins, were obtained according to the instruction manual of the Bac-to-Bac Baculovirus Expression System (Invitrogen).
2.2 SDS–PAGE and Western Blotting
Samples of culture medium containing ∆175-268gp51-His and ∆175-268gp51-STH and r-gp51 proteins, were subjected to immunoblot analysis. Samples were mixed with 4× NuPAGE sample buffer (Invitrogen) containing 100 mM DDT and heated to 95 °C for 5 min. ∆175-268gp51-His and ∆175-268gp51-STH were mixed with 2× Native Tris-Gly Sample buffer, in non-reduced conditions, and loaded. Proteins and the prestained BenchMark protein marker (Invitrogen) were subjected to electrophoresis using 12% Bis–Tris precasting NuPAGE gels in MOPS buffer and transferred to a PVDF membrane as described in the manufacturer’s instructions (Invitrogen). The membranes were treated with phosphate buffer saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) containing 5% skimmed milk and incubated for 2 h with an anti-BLV gp51 Mab that recognized a linear epitope. Detection was performed by SuperSignal West Pico Chemiluminescence Substrate using an anti mouse antibody conjugated with horseradish peroxidase (Pierce Biotechnology, Rockford, USA).
Protein purity was determined by staining the gel with GelCode Blue Stain Reagent (Pierce) in accordance with the instructions protocol.
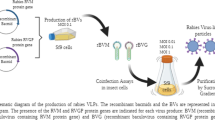
2.3 Infection and Proteins Production
Sf21 cells (Invitrogen) were cultured in roller bottles (Corning Life Sciences, NY) in HYQ SFX-Insect serum-free medium (Hyclone, Loan Road, USA) containing 100 μg/mL of streptomycine, 0.25 μg/mL amphotericin B, 100 UI/mL penicillin and 10 μg/mL of gentamycin. Cells were seeded at 2 × 106 cells/mL in 100 mL of fresh medium and infected with 0.05 of Plaque Forming Unit of recombinant baculoviruses for cell. Three days post infection the culture medium containing the secreted proteins was harvested, centrifuged at 5,000×g for 10 min and stored at −20 °C.
2.4 Dialysis and Sequential Ultrafiltration
Insect cell surnatant (100 mL) containing ∆175-268gp51-His was dialyzed at 4 °C against buffer WA (50 mM NaCl, 10 mM NaH2PO4, 4 mM NaHCO3, 10 mM MgSO4, 15 mM MgCl2, 50 mM KCl, 7 mM CaCl2, pH 7.5) using a 10.000 MWCO SnakeSkin Pleated Dialysis Tubing (Pierce). The recovered antigen was stored at −20 °C.
The pH of 100 mL of culture medium containing ∆175-268gp51-His was increased from 6.2 to 7.5 by an addition of 500 mM NaOH and centrifuged at 2,000×g for 10 min. The supernatant was diluted with 100 mL of buffer WA and concentrated again to 100 mL by sequential ultrafiltration with VivaFlow 50 PES 30.000 MWCO (VivaScience AG, Goettinger, Germany). The washes by ultrafiltration were repeated for other three times and on each step 25 mL fraction of the medium was collected. For scale-up purification, 500 mL of the medium containing protein was ultrafiltrated as described above.
2.5 IMAC
The ∆175-268gp51-His protein was purified by IMAC using HIS-Select Nickel Affinity Gel suspension (SIGMA, Saint Louis, Missouri USA). The ultrafiltrate fractions were mixed with IMAC matrix equilibrated with buffer EB (300 mM NaCl, 50 mM Tris–HCl and 10 mM imidazole, pH 8). The suspensions were incubated on an orbital shaker (180 rpm) for 20 min, centrifuged and washed with 20 matrix volumes (MVs) of buffer WB (300 mM NaCl, 50 mM Tris–HCl, 5 mM CaCl2 and 10 mM imidazole, pH 8). Two elution steps were performed with 2 MVs of buffer WB containing 250 mM of imidazole (WI). The eluted fractions were combined as single pool and stored at −80 °C. Scale-up purification of ultrafiltrate antigen was performed with 1 mL of Ni2+ matrix and the elution was carried out with buffer WI.
Concentration of ∆175-268gp51-STH was carried out using 100 mL of pooled eluted Strep-Tactin fractions with 0.5 mL metal-chelate affinity matrix as described above.
2.6 Strep-Tactin Affinity Chromatography
A 5 mL Strep-Tactin MacroPep column (IBA BioTAGnology, Göttingen, Germany) was equilibrated with two column bed volumes (CVs) of buffer W (100 mM Tris–HCl pH 8, 150 mM NaCl and 5 mM CaCl2). For the purification of ∆175-268gp51-STH, NaCl, Tris–HCl pH 8 and Tween 20 were added to the culture medium at the final concentration of 800 mM, 100 mM and 0.05%, respectively. Batches of 50, 100, 200 mL were filtered and loaded by gravity or using a peristaltic pump. The purification of ∆175-268gp51-STH and the regeneration of the Strep-Tactin matrix was carried out according to the IBA protocol.
The purified protein was quantified by the microassay procedure using the Bio-Rad Protein Assay reagent (BIO-RAD Laboratories, Hercules, USA).
2.7 Capture ELISA
The previously described ELISAr-gp51 [10] was used to determine the antigen characteristics of ∆175-268gp51-His and ∆175-268gp51-STH proteins by replacing the entire r-gp51 with deleted proteins.
The ∆175-268gp51-STH protein was analyzed by a modified ELISAr-gp51, where the usual anti-BLV gp51 Mab catcher was replaced by an anti-Strep tag II Mab (IBA BioTAGnology).
2.8 Indirect ELISA
The quantification of ∆175-268gp51-STH protein contained in the conditioned insect cell medium and the unbound antigen of Strep-Tactin column flowthrough was calculated by an indirect ELISA test. Briefly, purified ∆175-268gp51-STH protein was diluted in carbonate/bicarbonate buffer in a predetermined optimal range (0.5–100 ng/mL), while conditioned culture medium and Strep-Tactin column flowthrough were diluted 1:800 and 1:100 in the same buffer, respectively. One-hundred microliter of each dilution was loaded on six wells of an ELISA plate (MaxiSorp; Nalgene Nunc International, Rosckiede, Denmark) and incubated at 4 °C ON. After washing with PBS containing 0.05% Tween 20 (PBST), three wells for each dilution were incubated with anti-BLV gp51 Mab, while the other three wells with a Mab anti-DD′ epitope (VMDR Inc., Wa, USA) used as a negative control. Then the plate was incubated with anti-mouse Mab conjugated to horseradish peroxidise. Substrate solution containing 3,3′,5,5′-tetramethylbenzidine (TMB) was added and the optical density at 450 nm (OD450) was measured. The results were expressed as ΔOD450, obtained by subtracting the mean of negative OD450 values from the mean of positive OD450 values. The ΔOD450 values obtained from the absorbed purified ∆175-268gp51-STH, were plotted against the respective concentration range (0.5–100 ng/mL), to generate the calibration curve. The amount of protein in the insect cell medium and Strep-Tactin column flowthrough dilutions was calculated by intrapolation of the respective ΔOD450 values on the linear regression equation derived from standard curve.
3 Results
3.1 Expression and Characterization of ∆175-268gp51-His and ∆175-268gp51-STH Proteins
The sequential C-terminal epitopes A, B, B′, D and D′ of gp51 were removed by polymerase chain reaction (PCR). Two different recombinant baculoviruses were constructed, both expressing the first 174 amino acids of the N-terminal of gp51, with a C-terminal single 6xHis tag and a double Strep-II/6xHis tag, respectively.
These proteins were analysed by Western blotting using anti-BLV gp51 Mab. Under denatured and reduced conditions the Mab revealed the presence of two identical immunoreactive bands, with a molecular weight around 35 kDa for both deleted proteins and 48 kDa for r-gp51 (Fig. 1).
Western blot analysis of the expressed recombinant proteins. Proteins were resolved on a 12% SDS–PAGE, transferred on PVDF membrane and incubated with the anti-BLV gp51 Mab. Lane 1 and 2: supernatant of Sf21 cells containing ∆175-268gp51-His and ∆175-268gp51-STH proteins; Lane 3: culture medium of uninfected Sf21 (negative control); Lane 4: supernatant of Sf21 cells containing r-gp51 protein (positive control). The positions of the molecular weight markers are indicated on the right
The ELISAr-gp51 test was used to determine the reactivity of the two deleted proteins against BLV-infected cattle serum (positive serum) that recognize conformational epitopes of the glycoprotein [3, 4]. The OD450 values obtained analysing the new recombinant proteins and the r-gp51 showed a substantial overlap (Fig. 2a). No alterations of the conformational structure of protein were observed in order to the deletion of the majority of C-terminus and the addition of the tags. Moreover, our studies demonstrated that ∆175-268gp51-His and ∆175-268gp51-STH proteins were more stable than the entire gp51 (data not shown).
a Comparison of OD450 values between ∆175-268gp51-His, ∆175-268gp51-STH and the entire r-gp51. Culture medium of each recombinant protein was diluted from 1:2 to 1:256 in PBST plus 5% skimmed milk and loaded on ELISA plate. b Analysis of the deleted recombinant proteins with the anti-Strep tag II Mab
The ELISAr-gp51 performed with the anti-Strep tag II Mab showed that the OD450 obtained with the medium containing the ∆175-268gp51-STH (dilution 1:2), was approximately four times higher than that observed for the ∆175-268gp51-His infected Sf21 medium used as a negative control (Fig. 2b). These results indicated that the Strep-tag II was efficiently exposed on the molecules of recombinant protein.
3.2 Purification of ∆175-268gp51-His
Insect cell supernatant containing ∆175-268gp51-His recombinant protein was dialyzed against buffer WA in order to remove chelant agents that bind and strip Ni2+ ions from the Ni-NTA matrix. This antigen was analyzed by the ELISA test prior to purification. The low OD450 values of positive serum in the ELISA showed that the antigenic characteristics of this recombinant protein were lost over time (data not shown). Previously studies demonstrated that the complete r-gp51 tend to form macromolecular aggregate revealed by SDS–PAGE performed without reducing agents (data not shown). To ensure that the protein was not aggregate or degraded by proteases, a Western Blot in reduced and non-reduced conditions was carried out. The results depicted in Fig. 3 show that the anti-BLV gp51 Mab revealed the same protein bands before and after the dialysis. In order to decrease the processing time, the sample containing the ∆175-268gp51-His was washed by ultrafiltration. Under these conditions the ∆175-268gp51-His protein remained stable even after four washes as showed by ELISA assay (data not shown).
Western blot analysis of the culture medium containing ∆175-268gp51-His protein. Proteins were resolved on a 12% SDS–PAGE, transferred on PVDF membrane and incubated with the anti-BLV gp51 Mab. Lanes 1 and 2: immunoreactive bands before and after dialysis under reduced conditions; Lane 3: uninfected Sf21 culture medium; Lanes 4 and 5: immunoreactive bands of non-reduced protein before and after dialysis. The positions of the molecular weight markers are indicated on the right
IMAC method was employed to purify recombinant protein from the crude culture medium and ultrafiltrated washed fractions. Figure 4 shows that the binding capacity of matrix was the same for all samples, excluding the crude culture medium where the Ni2+ ions were stripped. This method removed most of the proteins but significant impurities remained in the eluted fractions, and they seemed to increase with the number of the ultrafiltration washes. These contaminant proteins apparently could be reduced adding NaCl and Tween 20 at a concentration, respectively, of 0.8 M and 0.05% into the ultrafiltrate medium (Fig. 4f, lane 3). The purified antigen remained stable after IMAC purification as observed by the high values of the OD450 of the ELISAr-gp51 (data not shown).
a–f Coomassie blue-stained of 12% SDS–PAGE of IMAC purified samples containing the ∆175-268gp51-His protein. a Crude culture medium; b culture medium after the first wash; c after the second wash; d after the third and e after the fourth wash by tangential ultrafiltration; f culture medium containing 800 mM of NaCl and 0.05% of Tween 20 after fourth wash by tangential ultrafiltration. Lane 1: culture medium before purification; Lane 2: IMAC column flowthrough; Lane 3: IMAC eluted fractions. Purified r-protein is indicated by an arrow. The positions of the molecular weight markers are indicated on the left
Unexpectedly the results were not reproducible working at the same conditions in a scaled-up purification. Ni2+ ions were stripped even using an antigen washed for four times by ultrafiltration.
The stability of purified ∆175-268gp51-His protein was tested after freezing and thawing by the ELISAr-gp51 and a loss of functionality was noted after two of these cycles.
3.3 Purification and Quantification of ∆175-268gp51-STH
Crude culture medium containing the double tagged ∆175-268gp51-STH protein was purified by Strep-Tactin matrix. The deleted recombinant protein was present both in all elution fractions and in the column regeneration buffer containing HABA. Furthermore, the gel stained with GelCode Blue reagent showed that some contaminant proteins were present in the first eluted fraction (data not shown). To overcome this problem, NaCl and Tween 20 were added to the crude culture medium prior the purification and in addition the amount of elution buffer was increased. In this case it was possible to obtain more ∆175-268gp51-STH protein with a high degree of purity (Figs. 5, 6). Nevertheless, a relative discrete amount of purified ∆175-268gp51-STH was lost with the regeneration buffer. The ELISAr-gp51 test showed that the purified protein maintained the same antigenic characteristic of crude antigen. Protein quantification was carried out using the Bio-Rad Protein microassay on pooled protein fractions of two different batches of production. The concentration of eluted protein was estimated as 6.2 and 8.1 μg/mL for the first and second 50 mL batches of produced antigen. Recombinant protein recovery was estimated by the ELISA method using a standard curve that was constructed with different dilutions of the purified yield. The dilutions of protein that showed linear responses of ΔOD450 variation (R 2 value > 0.995) occurred between 0.15 and 1.25 ng. The estimated recovery was 59 and 64.6% for the first and second batches. The production of r-protein in the insect cell system was calculated to be approximately 4.4 and 5.2 mg/L for the two batches (Table 1). These data were confirmed by the ELISAr-gp51 where, at the same dilutions, the ΔOD450 values of the second batch were higher than the first (data not shown).
Coomassie blue-stained of 12% SDS–PAGE of the ∆175-268gp51-STH purified fractions by Strep-Tactin chromatography. Lane 1: crude culture medium; Lane 2: Strep-Tactin column flowthrough (initial fraction); Lane 3: Strep-Tactin column flowthrough (final fraction); Lane 4: flowthrough of the first wash; Lane 5: flowthrough of the fifth wash; Lanes 6–12: Strep-Tactin column eluted fractions; Lane 13: prestained BenchMark protein marker
Coomassie blue-stained of 12% SDS–PAGE of expression and purification of recombinant ∆175-268gp51-STH from 200, 100 and 50 mL of culture medium. Lanes 1, 4 and 8: crude culture medium; Lanes 2, 5 and 9: Strep-Tactin column flowthrough; Lanes 3, 6 and 10: Strep-Tactin column eluted fractions of 200, 100 and 50 mL of insect medium; Lane 7: prestained BenchMark protein marker with the values of molecular weight reported on the right
3.4 Concentration of ∆175-268gp51-STH
The ∆175-268gp51-STH was purified to >99% homogeneity after the Strep-Tactin purification, but its concentration was very low. IMAC method was employed to concentrate ∆175-268gp51-STH protein. The elution fractions obtained from different Strep-Tactin purifications were pooled and incubated with 0.5 mL of HIS-Select Nickel Affinity Gel. The recovery was >99% with a final concentration of 600 μg/mL and any contaminant proteins were observed as shown in Fig. 7. The high percentage was confirmed by the ELISAr-gp51 test, where a low OD450 value of IMAC flowthrough was observed (data not shown).
Concentration of ∆175-268gp51-STH protein obtained by IMAC. The eluted fractions were resolved on 12% SDS–PAGE and stained with Coomassie blue. Lane 1: pooled Strep-Tactin eluted fractions; Lane 2: IMAC matrix flowthrough; Lanes 3, 4 and 5: IMAC eluted fractions; Lane 6: prestained BenchMark protein marker
The ELISAr-gp51 showed that the concentrated antigen lost its antigenic characteristic after the second cycle of freezing and thawing.
4 Discussion
The methods employed for the purification of the gp51 produced in a baculovirus expression system failed to purify this antigen because of its characteristic to form aggregates. These macromolecular forms were probably due to the partial glycosilation of the gp51 protein synthesized in heterologous expression systems [10, 17, 26]. In order to circumvent this problem, a deleted form of gp51 was expressed in baculovirus. Two constructs were made using PCR method, where a 6xHis and Strep-II/6xHis tags were placed downstream of the gene encoding the first 174 amino acids of N-terminus of gp51 protein. These tags were chosen because mild elution conditions were needed to preserve the native structure of deleted proteins.
The main problem to purify ∆175-268gp51-His using IMAC technology was that the crude culture medium stripped the Ni2+ ions from the matrix. Therefore, a pre-treatment by tangential flow filtration was required with time consuming. Despite the presence of some contaminant proteins, the yield and the quality of the purified antigen was acceptable but limited to the small-scale purification. Large-scale purification of ∆175-268gp51-His protein was compromised because most of Ni2+ ions were stripped even though the ultrafiltration was carried out.
To overcome these problems, a second recombinant protein ∆175-268gp51-STH was expressed in insect cells and purified by Strep-Tactin procedure. Generally, Strep-Tactin purification method requires the addition of avidin to eliminate biotin or biotinilated proteins ([19]; and IBA data sheet) that irreversibly block the binding sites of Strep-tag II sequence [29]. However, some authors reported that the avidin binds to the Strep tag II and causes the precipitation of recombinant proteins [35]. According to these results, our data demonstrated that the addition of the avidin at concentration of 40 μg/mL removed, after centrifugation, part of ∆175-268gp51-STH (data not shown). Since the ∆175-268gp51-STH protein was expressed at a low level, the purification was performed in the absence of avidin. A total of 1.2 L of the baculovirus conditioned medium containing recombinant protein was purified with the same column without loss of binding capacity.
The Strep-Tactin purification method requires a high concentration of recombinant protein in crude materials, because the dissociation constant for the Strep-tag II/Strep-Tactin matrix interaction is in the micromolar range. The Strep-Tactin matrix showed very poor binding with a low percentage of recovery for concentrations of recombinant proteins below 1 μM [7, 33, 35]. For example, recovery of the SEAP protein expressed in mammalian cells at a concentration of 0.7–1.0 μM, was only 19% [7]. Our results showed that the recovery of ∆175-268gp51-STH was 59 and 64.6% for two different batches of antigen production (Table 1). The calculated concentration of expressed protein, with a molecular weight of about 35 kDa, was estimated to be approximately 0.15 and 0.18 μM, respectively. Although the concentration of ∆175-268gp51-STH protein in the insect medium was under 1 μM, we obtained a high percentage of recovery. The efficient binding of the recombinant protein may be explained by an optimal C-terminal tail conformation dictating the accessibility of the Strep-tag II and 6xHis double tag to the immobilized streptavidin. The only disadvantage of using Strep-Tactin purification is that the recombinant protein obtained was poorly concentrated. This antigen can be used in the ELISA method even prior to dilution, but other applications, such as crystallization, immunization studies or screening of phage display antibody library, required a concentration of the material. For this purpose, the recombinant protein was concentrated by IMAC method with a high recovery in a short period of time (Fig. 7).
The antigen eluted in buffer containing imidazole by IMAC showed a good concentration but a low stability. Protein purified by Strep-Tactin method was poorly concentrated (from 2 to about 5 times), but the antigen maintained its functionality even after many freeze–thaw cycles. It is probable that the presence of high concentration of imidazole [7] and/or the increase of the concentration of recombinant protein over a threshold value, could induce its precipitation.
In conclusion, we have developed a protocol for a rapid and efficient purification of a deleted recombinant BLV gp51, produced in a secreted form in insect cell medium. Previous purification technologies failed to purify the entire or deleted form of the antigen. In the immunopurification method, acid or basic elution denatured the protein. In other chromatographic procedures, such as anionic and cationic exchange, or molecular-weight exclusion chromatography, the loss of protein was very high. Using IMAC it is possible to purify ∆175-268gp51-His protein, but this method requires a pre-treatment of serum-free medium. The recovery of recombinant antigen was acceptable, but some contaminant proteins were observed in the eluates. The protein tagged with Strep-tag II and polyhistidine at its C-terminus can be purified by Strep-Tactin technology, in a single-step to >99% homogeneity with a good recovery. Finally, the IMAC can be employed as a rapid method to concentrate the ∆175-268gp51-STH protein.
Abbreviations
- BLV:
-
Bovine leukaemia virus
- HTLV:
-
Human T-cell leukaemia virus
- FLK:
-
Foetal lamb kidney
- EBL:
-
Enzootic bovine leucosis
- IMAC:
-
Metal-affinity chromatography
- PBS:
-
Phosphate buffer saline
References
Boettner M, Prinz B, Holz C, Stahl U, Lang C (2002) J Biotechnol 99:51–62
Bruck C, Mathot S, Portetelle D, Berte C, Franssen JD, Herion P, Burny A (1982) Virology 122:342–352
Bruck C, Portetelle D, Burny A, Zavada J (1982) Virology 122:353–362
Bruck C, Portetelle D, Mammerickx M, Mathot S, Burny A (1984) Leuk Res 8:315–321
Bruck C, Rensonnet N, Portetelle D, Cleuter Y, Mammerickx M, Burny A, Mamoun R, Guillemain B, Van der Maaten M, Ghysdael J (1984) Virology 136:20–31
Burny A, Cleuter Y, Kettman R, Mammerickx M, Marbaix G, Portetelle D, Van der Broeke A, Willems L, Thomas R (1990) In: Gallo RC, Wong-Staal F (eds) Retrovirus biology and human disease. Marcel Dekker Inc, NY, pp 9–32
Cass B, Pham PL, Kamen A, Durocher Y (2005) Protein Expr Purif 40:77–85
Chazenbalk GD, Rapoport B (1995) J Biol Chem 270:1543–1549
Chen XS, Brash AR, Funk CD (1993) Eur J Biochem 214:845–852
De Giuseppe A, Feliziani F, Rutili D, De Mia GM (2004) Clin Diagn Lab Immunol 11:147–151
Han R, Zwiefka A, Caswell CC, Xu Y, Keene DR, Lukomska E, Zhao Z, Höök M, Lukomski S (2006) Appl Microbiol Biotechnol 72:109–115
Hochuli E, Dobeli H, Schacer A (1987) J Chromatogr 411:177–184
Hochuli E, Bannwarth W, Dobeli H, Gentz R, Stuber D (1988) Biotecnology 6:1321–1325
Holz C, Hesse O, Bolontina N, Stahl U, Lang C (2002) Protein Expr Purif 25:372–378
Humtsoe JO, Kim JK, Xu Y, Keene DR, Höök M, Lukomski S, Wary KK (2005) JBC 280:13848–13857
Junttila MR, Saarinen S, Schmidt T, Kast J, Westermarck J (2005) Proteomics 5:1199–1203
Legrein M, Portetelle D, Dumont J, Burny A, Hilger F (1989) Gene 79:227–237
Mamoun RZ, Morisson M, Rebeyrotte N, Busetta B, Couez D, Kettmann R, Hospital M, Guillemain B (1990) J Virol 64:4180–4188
O’Reilly DR, Miller LK, Luckow VA (1994) Baculovirus expression vector, a laboratory manual. Oxford University Press, New York
Pham PL, Perret S, Doan HC, Cass B, St-Laurent G, Kamen A, Durocher Y (2003) Biotechnol Bioeng 84:332–342
Porath J, Carlsson J, Olsson I, Belfrage G (1975) Nature 258:598–599
Portetelle D, Bruck C, Mammerickx M, Burny A (1983) J Virol Methods 6:19–29
Portetelle D, Couez D, Bruck C, Kettmann R, Mammerickx M, Van Der Maaten M, Brasseur R, Burny A (1989) Virology 169:34–41
Reddy RG, Yoshimoto T, Yamamoto S, Funk CD, Marnett LJ (1994) Expression of porcine leukocyte 12-lipoxygenase in a baculovirus/insect cell system, its characterization. Arch Biochem Biophys 312:219–226
Rice NR, Stephens RM, Gilden RV (1987) In: Burny A, Mammerickx M (eds) Enzootic leukosis and bovine leukemia virus. Nyhoff, Boston, pp 115–144
Russo S, Contermini L, Berkoviz-Siman-Tov R, Ponti W, Poli G (1998) FEBS Lett 436:11–16
Sagata N, Yasunaga T, Tsuzuku-Kawamura J, Ohishi K, Ogawa Y, Ikawa Y (1985) Prot Natl Acad Sci 82:677–681
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Skerra A, Schmidt TGM (1999) Biomol Eng 16:79–86
Skerra A, Schmidt TGM (2000) Methods Enzymol 326:271–304
Terpe K (2003) Appl Microbiol Biotechnol 60:523–533
Van der Maaten M, Miller JM (1990) In: Dinter Z, Morein B (eds) Virus infections of ruminants, vol 39. Elsevier Science Publishers B. V, Amsterdam, pp 419–429
Voss S, Skerra A (1997) Protein Eng 10:975–982
Witte CP, Noël LD, Gielbert J, Parker JE, Romeis T (2004) Plant Mol Biol 55:135–147
Wojczyk BS, Czerwinski M, Stwora-Wojczyk MM, Siegel DL, Abrams WR, Wunner WH, Spitalnik S (1996) Protein Expr Purif 7:183–193
Acknowledgments
The authors thank Ms Tamsin Anne Hickson for the English language revisions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Giuseppe, A., Forti, K., Feliziani, F. et al. Purification by Strep-Tactin Affinity Chromatography of a Delete Envelope gp51 Protein of Bovine Leukaemia Virus Expressed in Sf21 Insect Cells. Protein J 29, 153–160 (2010). https://doi.org/10.1007/s10930-010-9228-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-010-9228-6