Abstract
Conversion of the renewable biopolymers to magnetic adsorbents is a sustainable approach for environmental concerns. Herein, the microwave-assisted micro-carboxymethylated cellulose (MCMC) with DS = 0.76, the in situ polymerized acrylamide, and nano g-Fe2O3-SO3H were fabricated in a three-dimensional hydrogel network by one-pot radical polymerization with an aromatic bis-amide cross-linker. This SO3H/phenyl implanted various methods characterized nano-composite hydrogel as a granular porous network with excellent swelling performance for the adsorption of heavy metal ions (HMIs). The results of the influences of pH, organic solvent, and salt onto the hydrogel swelling capacity (SC) showed an increase by raising the pH, a decrease in organic solvents, and a decrease in chloride salt solutions by order of Na+ < Ca2+ < Fe3+. The mechanism and kinetics of hydrogel swelling with a capacity of 60.7 g/g were consistent with the non-Fickian diffusion and Schott's second-order kinetic models. As an adsorbent, the hydrogel removed Pb2+ and Hg2+ with an adsorption capacity 91.12 mg/g and 79.64 mg/g at neutral pH 7. The adsorption isotherms of hydrogel for Pb2+ and Hg2+ were adequately fitted with the Langmuir model (R2 = 0.99). The chelation of carboxylate groups to HMIs, electrostatic interactions between the charged surface groups and HMIs, and hydrogen bonding are significant parameters of the adsorption mechanism. The successive adsorption/desorption cycles for HMIs removal offer high potential of this hydrogel for the treatment of industrial wastewaters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution by heavy metals ions (HMIs) has recently raised a global concern for the protection of the hydrosphere, ecosystems, and human health [1, 2]. Due to the continuous accumulation, high toxicity, strong permeability, and non-biodegradability, HMIs create big problems in living organisms at even trace concentrations [3,4,5]. Lead and mercury (Pb2+ and Hg2+) are of the most studied toxic HMIs, which damage to the brain, nerves, kidney, lipid membrane, and photosynthesis [6, 7]. The main pollution sources of Hg2+ are mercury batteries, contaminated foods, and dental amalgam, while Pb2+ leaches to the food pyramid and hydrosphere from the mining, lead processing, print industry, ceramics, batteries, pigments, plastics, cables, leaded gasoline, and battery recycling [8, 9]. While a fraction of discharged Pb2+ and Hg2+ into the industrial wastewaters can be removed during the ordinary water treatments, development of new methods for removal of toxic HMI contaminants is highly desirable for the protection of hydrosphere [10, 11]. Although waste water can be treated by various technologies (Table 1) [12,13,14], such as photocatalysis [15], microwave catalysis [16], advanced oxidation catalysis (AOP) [17], biological [18], ion exchange [19], and adsorption that is among the best methods developed to removal HMIs [20].
Adsorption is of the lasting methods developed to remove HMIs [21], due to the simple practical design of adsorbent, no by-product, easy operation, low cost, easy recycling, and eco-environmental advantages [22]. Adsorption of Pb2+ and Hg2+ by cellulose-based adsorbents, with advantages of high efficiency, biocompatibility, multi-functionality, and low-cost, are among the promising methods for removal of these HMIs from the wastewaters [22, 23]. However, difficult reusing, low thermal stability, and high hydrophilic properties are issues of the biocompatible cellulosic adsorbents, which magnetizing [24], combining with the other polymers, or targeted functionalizing are responses to these restrictions [25]. Carboxymethyl cellulose (CMC) is an adhesive, biodegradable [26], and pH-sensitive cellulose derivative prepared by carboxymethyl functionalization of micro- [27] or nano- celluloses [28] derived from various sources. Owing to these advantages, CMC is a promising component for task-specific biomaterials and hydrogels applied for adsorptive removal of either dyes or HMIs from the wastewaters [29, 30].
Hydrogels are three-dimensional network polymers with excellent water swelling and super-absorptivity to sorb various organic/inorganic contaminants onto their surface or swollen network [31]. Although hydrogel manipulation has a direct relationship to the adsorbed pollutant kind, copolymerization of monomers and cross-linkers with the selected carbohydrate polymers, peptides, or carbon materials is a general method for preparing of the hydrogels [32,33,34]. Representative examples are fabricated micro/nanoCMC with the native polyacrylamide (PAAmide), biopolymers, and cross-linkers in the non-magnetic/magnetic networks [29, 30, 35] as well as CMC-based hydrogels used for drug-delivery [36], HMIs removal [25], dye removal [37], and wound-dressing [38]. Typically, CaCl2 [39], citric acid [40], maleic group [41], or bisamides [42] are cross-linkers of these hydrogels, while Fe3O4 magnetic nanoparticles (MNPs) used as their magnetizing agents [28, 36]. Due to the high surface area, diffusion power, and active groups of MNPs that increase the adsorption efficiency, researchers have focused on the promising adsorbents magnetized by these magnetically separable nanoparticles to remove various pollutants from the wastewaters [43] and especially HMIs [44].
Microwave (MW) irradiation is an ecofriendly alternative to conventional heating for providing the necessary energy to promote the reactions in a very fast time [35]. MW-assisted water-based radical polymerization is among these accelerated reactions due to the fast absorbance of MW by polar media of water which in MW energy produces free radicals by the selective excitation of polar bonds and increases the monomer conversion ratio, purity, and yield of the polymers by reducing side reactions [45, 46].
Recently, we have prepared a hydrogel from the cotton-derived nanoCMC, γ-Fe2O3-SO3H, native polyacrylamide, and a chlorophenyl-bisamide linker for dye removal [47]. Due to the conceivable oxidation of the Fe3O4 MNPs to the more stable magnetic phase γ-Fe2O3 [48] and no report on the pH-responsive hydrogel based on the γ-Fe2O3-SO3H MNPs, aromatic cross-linkers, and CMC [47], design of a pH-intelligent composite hydrogel with efficient responses to HMIs removal is highly desirable. The objects of this work were simple preparation of the γ-Fe2O3-SO3H, microwave-assisted preparation of micro-CMC (MCMC), composition of the γ-Fe2O3-SO3H, MCMC, and the in situ polymerized acrylamide (AAm), and preparation of a novel magnetic composite hydrogel by cross-linking of the composite via the microwave-assisted radical polymerization with phenylated bisamide linker using the radical initiator ammonium persulfate (APS). After characterization of the composite hydrogel, the influences of pH, solvent, and salts on the hydrogel swelling together with the swelling kinetics were evaluated. The adsorption efficiency, mechanism, adsorption kinetics for Pb2+ and Hg2+ removal, and reusability of the hydrogel adsorbent were then investigated.
Materials and Methods
Materials
Microcellulose (MC) was prepared following a previously method [49] and converted to the microCMC (MCMC) by carboxymethylation with the sodium chloroacetate under microwave irradiation. Cross-linker was synthesized by a new microwave-assisted method considering our previous method [50], while acrylamide, iron salts, PhCHO, ammonium persulfate (APS), HNO3 (65%), NaOH, Pb(NO3)2, and HgCl2 were obtained from the Merck Company (Germany). Pb2+ and Hg2+ solutions were prepared by dissolving salts in deionized water.
Material Characterization
Hydrogel and materials were characterized by the techniques given in Table 2. Fourier transform infrared spectrometer was used to obtain FT-IR spectra. Field emission scanning electron microscopy (FESEM) was applied for surface analysis of MCMC and hydrogel. The thermal stability of hydrogel or compositions were characterized by a thermo gravimetric analysis (TGA). Magnetization measurements were analyzed by Vibrating-sample magnetometer (VSM). Concentration of Pb2+ or Hg2+ was analyzed with an atomic absorption spectrophotometry (AAS) and atomic fluorescence spectrometry (AFS).
Etherification of the Cotton-Derived Microcellulose (MC) to MCMC
Aqueous NaOH (25 mL, 20% w/v) was added drop-wise during 60 min to a vigorously stirring suspension of MC (2 g) in 2-propanol (50 mL) at room-temperature, and the mixture stirred for 2 h. Sodium monochloroacetate (4.3 g) was then added and the mixture was kept under microwave irradiation for 8 min at 400 W power. The given solid was filtered and added to an aqueous alcohol solution (100 mL), neutralized with acetic acid, filtered, washed three times with aqueous ethanol, and dried at 40 °C for 24 h. The determined degree of substitution (DS) and viscosity for the given MCMC by the conductometric titration method and Ostwald Viscometer were 0.76 and 17.40 Pa s, respectively. The molecular weight of the as-prepared MCMC derived from viscosity was 211,787 Da.
Preparation of the γ-Fe2O3-SO3H MNPs [48] by a Modified Method
Chlorosulfonic acid (1.4 mL) was added drop-wise to the prepared γ-Fe2O3 MNPs [49] in a flask under solvent-free conditions and the mixture was magnetically stirred for 30 min. Then, EtOH (50 mL) was added, the brownish solid was collected by a magnet, and dried at 70 °C for 2 h.
Synthesis of Cross-Linker N,N'-(Phenylmethylene)Bisacrylamide (NNPDA)
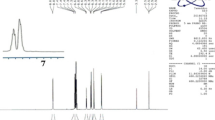
Molybdate sulfuric acid (MSA) (10 mol%) [50] was added to a stirring mixture of benzaldehyde (2 mmol) and acrylamide (4 mmol) under solvent-free conditions and the final mixture was irradiated by microwave at 400 W for 2 min. After reaction completion (TLC monitoring), hot acetone (10 mL) was added, the catalyst was separated by filtration, and the bisacrylamide product (NNPDA) was obtained after evaporation of the acetone under reduced pressure or filtration of the crystals after the addition of cold water. The chemical structure of NNPDA was confirmed by melting point, 1H NMR, and FT-IR (Figs. 1, 2A). With the melting point = 273–276 °C, characterization data from 1H NMR spectrum of NNPDA in DMSO-d6 showed δ = 5.65 (dd, J1 = 12 Hz, J2 = 4 Hz, 2H, =CH2), 6.15 (dd, J1 = 16 Hz, J2 = 4 Hz, 2H, =CH2), 6.36 (dd, J1 = 16 Hz, J2 = 12 Hz, 2H, =CH–), 6.65 (s, 1 H, CHbenzilic), 7.35 (d, J = 8.5 Hz, 3 H, Hortho/para), 7.45 (t, J = 8 Hz, 2 H, Hmeta), and 8.85 (s, J = 7.6 Hz, 2 H, 2 × NH) ppm.
Preparation of Hydrogel
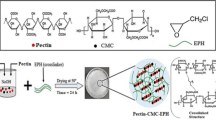
The APS solution (0.05 g in 5 mL H2O) was added to a stirred mixture of AAm (0.5 g), MCMC (1.0 g) and Fe2O3-SO3H (0.5 g) in water (50 mL) and the final mixture was stirred for 30 min. Then, cross-linker NNPDA (0.05 g) and extra APS solution (0.05 g in 5 mL H2O) were added drop-wise by syringe needle at room temperature and stirred vigorously for 2 min under microwave irradiation at 400 W. The hydrogel was collected on the vessel wall, washed with distilled water, and dried at 70 °C for 24 h. The preparation of hydrogel is summarized in Scheme 1.
Statistical Analysis
To give the accurate data, all swelling and adsorption experiments of this study are carried out in triplicates and the values are mean of three experiments ( ±) standard error (SE) by one-way ANOVA with Tukey–Kramer Multiple Comparisons Test.
Swelling Test for the As-Prepared Hydrogel
The finely powdered hydrogel (0.2 g) was immersed in excess deionized water (200 mL) at room temperature and allowed to swell by stirring for 1 h. After equilibrium, the swelling capacity (SC) was measured at room temperature based on the Eq. (1) [51]:
where Ws and Wdry are the weights of swelled and dry hydrogel after the t time.
Swelling Kinetics of the Hydrogel
The dried hydrogel (30 mg) was fully immersed in excess distilled water (DW) or 0.9% (w/v) NaCl solution at room temperature. After the 1 h contact time and equilibrium, the swollen hydrogel was taken from the medium at the time intervals, weighed after the rapid removal of the excess surface water or salt solution, and the SC (g/g) at different specified times (t) was calculated by Eq. (1).
Effects of Salt, pH, and Organic Solvents on Hydrogel Swelling
The salt and pH responses of the hydrogel were evaluated according to the earlier methods [51, 52], while the organic solvent influence on the hydrogel swelling followed with acetone and ethanol as the two corporate organic solvents. Typically, the dried hydrogel was immersed into the various volume ratios of acetone or ethanol in water (0–100%) at room temperature and the SC was calculated after the equilibrium by the Eq. (1).
Adsorption Experiments for Hg2+ and Pb2+
The adsorption experiments for Hg2+ and Pb2+ were performed with an adjusted stirring shaker bath at room temperature and 100 rpm, where data are mean value of repeated experiments for three times. The concentrations of Pb2+ in the solutions were analyzed by atomic absorption spectrophotometry and all the Hg2+ concentrations analyzed by the atomic fluorescence spectrometry. The adsorption capacity (qe (mg/g)) and removal percent for each of the Hg2+ and Pb2+ were calculated from Eqs. (2) and (3) [53]:
where C0 and Ce (mg/L) are the initial and equilibrium concentrations of Hg2+ and Pb2+, V (L) is the volume of the sample solution, and m (g) is dried hydrogel weight. The influence of the initial pH on the Hg2+ and Pb2+ adsorption by hydrogel was examined, where the pH of the HMI solutions were adjusted at 2–7 using 0.01 M HCl and 0.01 M NaOH. Thus, the dry magnetic hydrogel (0.1 g) was immersed into the each of the HMIs solutions (100 mg/L (100 mL)) at the selected pH for 12 h to reach the adsorption equilibrium. Samples were then taken out with a syringe and the residual concentration of Pb2+ or Hg2+ in solutions were determined by atomic adsorption (AA) and atomic fluorescence analysis.
Results and Discussion
Hydrogel Preparation and Characterization
To prepare the designed hydrogel, firstly the γ-Fe2O3-SO3H MNPs were prepared by the reaction of the magnetic phase of γ-Fe2O3 with ClSO3H and used afterward as a stable and pH responsive magnetizing agent (Scheme 1). Secondly, the gemini bisamide N,N'-(phenylmethylene)diacrylamide (NNPDA) was synthesized by microwave-assisted reaction of acrylamide and benzaldehyde in 2:1 ratio [50], while microCMC (MCMC) with DS = 0.76 was prepared by etherification of the MC with sodium chloroacetate. The MCMC was then cross-linked to the in situ polymerized acrylamide and γ-Fe2O3-SO3H by the cross-linker NNPDA via the free-radical polymerization in the presence of the radical initiator APS under microwave irradiation at 400 W. To determine the optimum ratio of various components of composite hydrogel, the adsorption properties of the three hydrogels prepared from MCMC, the in situ polymerized acrylamide, γ-Fe2O3-SO3H, and cross-linker NNPDA were tested before characterization. Based on the best given adsorption results and our previous experience on the corresponding hydrogel [47] the weight ratio of 1:0.5:0.5:0.05 was selected for the preparation of this composite hydrogel (Scheme 1).
By cross-linking, the two end vinyl groups in aromatic cross-linker NNPDA were covalently bonded to the in situ polymerized acrylamide and MCMC. Additional hydrogen bonding of the –polar –OH and –CONH2 with the –COO− and SO3- groups in MCMC chains and γ-Fe2O3-SO3H reinforced the three-dimensional network of this implanted hydrogel by either the acid-sensitive -SO3H groups or the aromatic phenyl groups (Scheme 2).
To characterize the hydrogel, the FT-IR spectra of the MCMC, γ-Fe2O3-SO3H, cross-linker NNPDA, and hydrogel are compared in Fig. 2A. The similar vibrational bands observed at 3400–3100 cm−1, 3000–2080 cm−1, ~ 1600 cm−1, and 1100–1000 cm−1 in both spectra of MCMC and hydrogel are related to the stretching of O–H, C–H, C=O, and C-O groups. In the γ-Fe2O3-SO3H spectrum, the characteristic vibrational adsorption bands are acidic OH, asymmetric and symmetric stretches of O=S=O, and the lattice Fe/O stretch observed at 3000–3300 cm−1, 1350–1100 cm−1, and 625 cm−1, respectively. For the phenylbisamide cross-linker, the peaks observed at 3275 cm−1, 1669 cm−1, 1626 cm−1, 1559–1411 cm−1, and 1087 cm−1 are assigned to the vibrational modes of NH, C=O amide, aromatic ring, overlapped NH-bending with the vinyl C=C stretching, and C-N groups. While the absence of the peaks at 1715–1730 cm−1 and 1240–1250 cm−1 in the FT-IR spectrum of the MCMC revealed the anionic COO− from of carboxylic acid, major vibrational bands at 3500–3000 cm−1, 2920 cm−1, 1600 cm−1, 1422 cm−1, and 1063 cm−1 related to the OH, CH, asymmetric COO−, symmetric COO−, and C–O stretching confirms its structure. Appearance of the vibrational adsorption bands at 620 cm−1, 1326 cm−1, 1411 cm−1, 1667–1600 cm−1, and 3000–3400 cm−1 in the FT-IR spectrum of hydrogel approves insertion of the Fe/O, SO3, aromatic ring, carboxamide, carboxylate, acidic OH, and amide-NH groups in the hydrogel matrix. The comparative VSM curves of γ-Fe2O3, γ-Fe2O3-SO3H, and hydrogel showed no hysteresis loop and super-paramagnetic properties of this magnetic hydrogel at room temperature (Fig. 2B). The magnetization saturation (Ms) of the hydrogel is 14.30 emu/g and lower than either γ-Fe2O3-SO3H (28.58 emu/g) or γ-Fe2O3 (60.02 emu/g). The reason of this decrease in Ms is due to the dilution of the magnetic phase γ-Fe2O3-SO3H by non-magnetic components of PAAmide and MCMC in the hydrogel matrix, although super-paramagnetic properties of hydrogel is enough for magnetically separation during the water treatment.
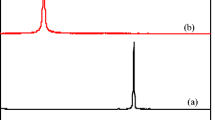
The comparative XRD patterns of MCMC, hydrogel, and γ-Fe2O3-SO3H show much similarity of hydrogel crystal planes to the γ-Fe2O3-SO3H phase than relatively amorphous MCMC (Fig. 2C). While, the XRD pattern of γ-Fe2O3-SO3H phase show peaks at 2θ = 29.36°, 31.48°, 45.37°, 54.14, 57.39° and 62.76° due to the crystal planes of (220), (311), (400), (422), (511), and (440), resemblance of these 2θs to crystal planes of hydrogel describe no significant change of it by entering to the three-dimensional network of hydrogel.
Comparing the TGA curves of MCMC, γ-Fe2O3-SO3H, and hydrogel shows ~ 45% higher thermal stability and lower weight loss for the cross-linked hydrogel than that MCMC (Fig. 2D), apparently due to the entering the stable inorganic phase γ-Fe2O3-SO3H and cross-linking of the hydrogel in a three-dimensional network. A comparative loss of mass analysis from 325 to 360 °C for sulfonic group degradation [54] in the TGA of γ-Fe2O3-SO3H and hydrogel showed ~ 10% higher loss of -SO3H groups by hydrogel than that γ-Fe2O3-SO3H at this temperature range.
To show changes in hydrogel surface by cross-linking, the FESEM images of MCMC and hydrogel at 200 nm and 1 µm resolution were compared in respect to the surface particle’s shape, size, and arrangement (Fig. 3). Based on the images, the surface morphology of MCMC gel (Fig. 3A) is crumpled and different from the hydrogel surface bearing the compactly dispersed semi-spherical granules with the mean particle size 20–35 nm (Fig. 3B). Such changes in surface morphology of hydrogel (Fig. 3B–D) are due to the hydrogen bonding network between the polar groups of MCMC, γ-Fe2O3-SO3H, and the in situ formed PAAamide as well as phenyl implantation of the hydrogel by cross-linker NNPDA.
BET analyses for the γ-Fe2O3-SO3H, MCMC, and hydrogel compared in Fig. 4 show a decrease in surface area, pore volume, and pore diameter of the hydrogel versus its components, probably due to the blocking of the pores in γ-Fe2O3-SO3H by NCMCC and in situ formed PAAmide chains.
Kinetics and Mechanism of Hydrogel Swelling
To investigate the kinetically parameters of hydrogel swelling, the recorded swelling capacity (SC) of hydrogel in deionized water and 0.9% NaCl solution at times between 10 and 100 min were compared in Fig. 5A and B, while data are mean value of three repeated experiments. With a higher amount for water than NaCl, SC of hydrogel increased sharply at the initial 40 min swelling time in both media and then became slower until reaching equilibrium. Mechanistically, the hydrogel swelling divides into two molecular steps containing the diffusion of the water molecules into the hydrogel network and secondly relaxation of the polymeric chains in accordance with the Fickian diffusion model and Schott's second-order-kinetic models [52]. To describe the dispersion mechanism of solvent molecules in the first step, the Fickian diffusion model was applied according to the Eq. (4):
where F is the fractional adsorption at t time (min), Mt (g) and Me (g) are the weights of absorbed water at t and equilibrium times, K is the characteristic constant, and n is the diffusional supporter of the solvent [51]. Moreover, the n < 0.5 indicates Fickian diffusion and water molecules transport by the concentration gradient, the 0.5 < n < 1 indicates non-Fickian diffusion and water adsorption by dual diffusion and polymer chains relaxation mechanism, and the n > 1 indicates abnormal diffusion by the polymer chains relaxation [51]. By scheming the ln (F) versus the ln (t), the corresponding model parameters including n, K, and R2 for swelling of this hydrogel were calculated as 0.84, 0.031, and 0.99 in water and 0.59, 0.075, and 0.98 in 0.9% NaCl, respectively (Fig. 5C, D). While the Figs. exhibit straight lines with the linear correlation coefficient (R2 ≥ 0.98), the 0.5–1 value of n for this hydrogel in both media reveals dominance of the non-Fickian diffusion at the initial step of swelling followed by the mix diffusion models.
Next, Schott's second-order kinetic model applied to express the hydrogel swelling kinetics [55] by following Eq. (5):
where Qt (g/g) and Qe (g/g) are the SC at t time (min) and theoretic equilibrium time and k (g/g min) are the initial swelling rate constant. By plotting t/Qt versus t (Fig. 5E, F), parameters including the correlation coefficients (R2), calculated swelling capacity (Qe, g/g), and k were obtained by linear regression as 0.99, 76.33 g/g, and 8.82 × 104 g/g.min and 0.98, 35.09 g/g, and 6.78 × 104 g/g.min for swelling of hydrogel in water and NaCl solution, respectively. According to the Fig. 5E and F, straight lines with the linear correlation coefficient (R2 > 0.98–0.99) confirm the excellent fitting of the experimental data with the Schott's second-order kinetic model, while matching the calculated Qe (g/g) to its experimental value supports this perfect fitting.
Consequently, the swelling process of hydrogel is consistent with the non-Fickian diffusion mechanism and Schott's second-order kinetic model.
The pH Effect on Hydrogel Swelling
Due to the strong relationship between pH and hydrogel swelling in adsorptive removal of pollutants, the pH sensitivity and swelling behaviors of the hydrogel at pH between 2 to 12 were studied (Fig. 6A). According to data, the swelling tendency of hydrogel increased significantly by pH increasing, so the SC reached to 42 g/g at pHs between 2.0 to 4.0, gently increased to ~ 60 g/g at pH ranged ~ 4.0–7.0, and raised to the maximum 65.39 g/g at pH 12. The pH-dependency of hydrogel during the swelling is due to the existence of the strong pH responsive –SO3H and –COOH groups to proton concentration. So, the strong hydrogen bonding of the polar protonated surface –SO3H and –COOH groups at acidic media helps to the hydrogel collapsing in solution. By increasing the pH, the acidic –SO3H and –COOH groups were sequentially deprotonated, hydrogen-bonding interactions were weakened, and the electrostatic repulsion between negatively-charged –SO3ˉ and –COOˉ groups enhances the SC by expanding the hydrogel network. At alkaline media, the SC was relatively constant due to the deprotonating saturation of the pH-sensitive groups of –SO3− and –COO− formed at initial pH increasing.
The Salt Effect on Hydrogel Swelling
To evaluate the salt effect on the hydrogel swelling, the calculated SC in different concentrations (0.02–0.1 mol/L) of the NaCl, CaCl2, and FeCl3, which had similar anion but dissimilar cations, were compared. Figure 6B clearly shows the strong dependency of SC on the salt type and concentration, although increase in the concentration of all three salts results in the decrease in SC. Such decrease in SC is related to the higher water-affinity of the salt ions versus the hydrogel matrix to adsorb water. By increasing the salt concentration from 0.02 to 0.1 mol/L, the decrease in SC for these salts showed the order FeCl3 > CaCl2 > > NaCl, so the SC decreased from 57.23 to 35.96 g/g for NaCl, from 9.08 to 5.12 g/g for CaCl2, and from 7.77 to 3.12 g/g for FeCl3. Generally, by increasing in the total concentration of ions, the osmotic pressure difference between the interior and outer solutions of the hydrogel decreases by reducing the ions ratio and leads to shrinkage of the hydrogel network. Besides, by raising the electrolyte concentrations, the increased chelation and electrostatic interactions between the metal cations and the anionic –COO− groups of hydrogel led to the decrease in electrostatic repulsion and hence SC. However this order of SC decrease is compatible with the order of cation charge as Fe3+ > Ca2+ > Na+, due to the exclusive abilities of the Fe3+ and Ca2+ than Na+ to chelate with the carboxylate groups of MCMC in the hydrogel matrix that led to the additional ionic cross-linking and lower SC [51, 52, 56]. To certify the chelating of the Ca2+ to the hydrogel, the FT-IR spectra of the hydrogel and Ca2+/hydrogel complex were compared in Fig. 7. Declining the stretching mode of CO amide at ~ 1667 cm−1 and shifting/broadening for the symmetrical/asymmetrical –NH2 stretching (~ 3414/ ~ 3279 cm−1), symmetrical/asymmetrical COO− stretching (~ 1561/1414 cm−1), and also C–O stretching (1115 cm−1) in the spectrum of Ca2+/hydrogel complex approve involvement of these groups in chelation of hydrogel to Ca2+ (Fig. 7).
The Organic Solvent Effect on Hydrogel Swelling
Due to the plentiful application of acetone and ethanol in practical experiments, the swelling capacity of the prepared hydrogel was evaluated in these two organic solvents at various volume ratios from 0 to 100 vol% (Fig. 8). According to the swelling curves the SC of the hydrogel decreased by increasing the ratio of organic solvents in two stages. The first stage of SC decrease appeared at ~ < 20% volume ratio in 6.60% and 18.90% for EtOH:H2O and acetone:H2O, respectively. The sharper SC reduction in EtOH:H2O versus acetone:H2O at this stage is due to the higher hydrogen-bonding of EtOH with hydrogel components than acetone. The second stage of SC decrease occurred at the over than 20 vol% of organic solvents with a higher slope in acetone:H2O versus EtOH:H2O. This SC trend is well-matched with the dielectric constant order of solvents as acetone < ethanol < water [56] for the inhibition of the –SO3H and –COOH groups to interact with the hydrogel network when swelled [57]. The second reason for such swelling trend is better interaction of the acetone, as a less hydrophilic solvent, than EtOH with the present hydrophobic phenyl implants in cross-linker of hydrogel (Scheme 2).
Adsorption Studies
In order to show the efficiency of the as-prepared SO3H/phenyl implanted hydrogel in the adsorption of Pb2+ and Hg2+ at optimized conditions, influence of pH on the adsorption, adsorption performance, adsorption isotherm, adsorption mechanism, and reusability of the hydrogel adsorbent were studied.
Effect of pH on the Adsorptive Removal of Hg2+ and Pb2+ by Hydrogel
The pH is an influential factor on the adsorption of HMIs because of the either change in solubility of metal cations or the ionization of the pH-sensitive groups exist on the adsorbent. At pH higher than 7 the metal ions of Pb2+ and Hg2+ are precipitated as hydroxide or hydro-complexes [6] in the aqueous medium, thus, the pH effects on the capacity of hydrogel for adsorption of Pb2+ and Hg2+ was evaluated at pHs 2.0–7.0 and calculated as removal% by following the Eq. (3) (Fig. 9A, B). As data show, removal% of Pb2+ and Hg2+ increased significantly by increase the pH, so the removal efficiency for Pb2+ increased rapidly from 35.68% at pH 2 to 91.12% at pH 7, while a similar removal% trend was observed at the same pH interval for Hg2+ from 15.29 to 79.64%. The existence of the protonated active groups on the hydrogel surface and the competition between H+ and Pb2+ or Hg2+ cations to adsorb on the active sites of the hydrogel surface are reasons proposed for these trends. By increasing the pH, the –SO3H and –COOH groups of hydrogel deprotonate to the anionic –SO3− and –COO− as the powerful chelating groups and the chelating and electrostatic interactions between the adsorbent and Hg2+ or Pb2+ ions increased the higher removal percent. Moreover, the higher removal of Pb2+ than Hg2+ by hydrogel can be attributed to the lesser atomic radius of Pb2+ for easier entering to the hydrogel pores during the adsorption removal [6].
Comparison of the Hydrogel Performance with the Other Adsorbents
Among the various adsorbents used for removal of Pb2+ and Hg2+, the cellulose/CMC-based adsorbents are highlighted to improve the Pb2+ and Hg2+ removal efficiencies by chelation [29, 58]. To demonstrate the superiority of the prepared three-dimensional hydrogel in this work, its performance for Pb2+ and Hg2+adsorption in respect to the removal% was compared with the previously corresponding adsorbents (Fig. 9C, D). According to Figs, the higher values of removal% for the as-prepared hydrogel confirmed its higher performance than those of the previously reported adsorbents [29, 58,59,60,61,62,63]. The reasons for the excellent performance of this composite hydrogel is due to numerous active COO−, CONH2, SO3−, and OH groups in hydrogel, which leads to extra chelation power and absorption capacity of the hydrogel.
Adsorption Isotherm
To describe the association between the concentrations of Pb2+ and Hg2+ with the hydrogel, and their adsorbed amounts at equilibrium the adsorption isotherms were obtained using the Langmuir and Freundlich model equations. At first, Langmuir model was studied by following the Eq. (6) [6]:
where qe is the adsorbed Pb2+ and Hg2+ at equilibrium (mg/g), Ce is the concentration of metal ion in the supernatant at equilibrium (mg/L), qm (mg/g) and b (L/mg) are Langmuir constants for the respective adsorption capacity and the adsorption energy. Figure 10A indicates the Langmuir isotherm plots regarding the Pb2+ and Hg2+ adsorption by the hydrogel. By regression analysis of the plots the R2 value was found to be 0.99 for the either Pb2+ or Hg2+ adsorption. Alternatively, Freundlich model was studied by following the Eq. (7) [6]:
where k (mg/g) and n are the Freundlich constants related to the multilayer adsorption capacity and intensity, respectively. The values of the correlation coefficient R2 for the adsorption of Pb2+ and Hg2+ by hydrogel in Freundlich isotherm model were 0.95 and 0.92 (Fig. 10B). The higher R2 value in the Langmuir isotherm model (R2 = 0.99) for adsorption of both of Pb2+ and Hg2+ by hydrogel revealed better-fitting of the experimental data with the Langmuir adsorption isotherm that offers monolayer adsorption of the HMIs by homogeneous sites of the adsorbent. The fine analysis of the isotherm constants and variables reveals more operative of homogeneous surfaces than the heterogeneous surfaces in the adsorption process (Table 3).
Adsorption Mechanism
To characterize the mechanism of HMI adsorption by hydrogel, the FT-IR spectrum and XPS analysis of the hydrogel before and after the Pb2+adsorption and the FT-IR spectra of the hydrogel before and after the Hg2+adsorption were compared in Figs. 11 and 12. Disappearing the peak related to stretching mode of CO amide (~ 1667 cm−1) in the spectrum of hydrogel after HMI adsorption together with the peak shifting/broadening for the symmetrical/asymmetrical -NH2 stretching (~ 3414/ ~ 3279 cm−1), symmetrical/asymmetrical COO− stretching (~ 1563/1419 cm−1), and also C–O stretching (1115 cm−1) approve the involvement of the OH, COO−, and CONH2 groups of the hydrogel in the adsorption process (Fig. 11).
For fine analysis, the XPS wide scan spectra of the hydrogel before and after the Pb2+ adsorption were compared in Fig. 12. The peak at 142.4 eV in the XPS of the hydrogel after Pb2+ adsorption is credited to the Pb 4f orbital that is an evidence its adsorption by the hydrogel. There are three peaks in the Pb 4f spectrum (Fig. 12B), due to the chelation of Pb2+ to the COO−, CONH2, and the O–H groups of hydrogel by chemisorption as the main mechanism for adsorption of Pb2+.
Desorption and Reusability
Due to the importance of the reusability of adsorbents in practical uses and higher removal percent of Pb2+, the reusability effectiveness of this hydrogel for the four sequence adsorption–desorption runs Pb2+ removal were performed So, after each adsorption step, the hydrogel was separated from the solution by an external magnet, washed with 0.01 M HNO3 for desorption, dried, weighted, and recycled hydrogel was used in further run (Table 4).
According to the results, the adsorption capacity of hydrogel preserved after the fourth adsorption–desorption cycle with a less than 2% decrease from the 91.12%, although without the significant change in hydrogel mass. Correspondingly, comparison of the FT-IR spectrum, FESEM image, and XRD of the reused hydrogel after the fourth reusing run for Pb2+ removal with the fresh hydrogel revealed no change in the characteristic adsorption bands and surface morphology of the adsorbent (Fig. 13).
Consequently, all results supported this hydrogel as an effective reusable adsorbent for the adsorptive removal of Pb2+ and Hg2+ as the most toxic HMIs.
Effect of Ions and Humic Acid on Co-adsorption of HMIs
Due to the complexity in practical wastewater treatment as well as the presence of inorganic anions, cations, and humic acids (HA) in wastewater matrix, the adsorption removal of Pb2+ and Hg2+ were studied in the presence of the HA and Na+, Ca2+, SO42−, and PO43− ions (Fig. 14). When experiments were performed at various initial HA concentrations, adsorption of Pb2+ and Hg2+ increased by increasing the HA amounts, owing to the role of HA as a good complexion agent that binds to the Pb2+ and Hg2+ ions via the COOH and OH phenolic groups in HA. Reversely, adsorption decreased in the presence of cations due to their competition with Pb2+ and Hg2+ to chelate with numerous active groups of hydrogel including COO−, CONH2, SO3−, and OH groups. The results of the adsorption removal of Pb2+ and Hg2+ by hydrogel in the presence of the SO42− and PO43− anions revealed no significant changes in removal percent of these HIMs (Fig. 14).
Conclusions
In this study, a magnetic hydrogel implanted with phenyl and SO3H groups was prepared from the tabloid-derived carboxymethyl cellulose, in situ polymerized acrylamide, and Fe2O3-SO3H that cross-linked to a new aromatic grafting linker. The FT-IR, FESEM, TGA, XRD, and VSM analyses revealed structure of the as-prepared magnetic hydrogel. The swelling capacity of this hydrogel increased by increasing the pH, decreased in organic solvents, and decreased also in salt solutions with the order Na+ < Ca2+ < Fe3+. The mechanism of hydrogel swelling is approved with non-Fickian diffusion and Schott's pseudo-second-order models. This magnetic hydrogel has intelligent responses to salt, pH, and organic solvents. The adsorption capacity of hydrogel for the removal of Pb2+ and Hg2+ from the aqueous solutions were studied at pH ranged in 2–7, while maximum adsorption of these heavy metal ions was at neutral pH and for the Pb2+ removal. The kinetic of adsorption of Pb2+ and Hg2+ by hydrogel ions well fitted with the Langmuir adsorption model with R2 = 0.99. Therefore the magnetic hydrogel prepared in this work could become an environmentally friendly and recyclable adsorbent for HMIs from the aqueous solutions.
References
Qasem NA, Mohammed RH, Lawal DU (2021) Removal of heavy metal ions from wastewater: A comprehensive and critical review. NPJ Clean Water 4(1):1–15
Malik LA, Bashir A, Qureashi A, Pandith AH (2019) Detection and removal of heavy metal ions: a review. Environ Chem Lett 17(4):1495–1521
Wu Y, Pang H, Liu Y, Wang X, Yu S, Fu D, Chen J, Wang X (2019) Environmental remediation of heavy metal ions by novel-nanomaterials: a review. Environ Pollut 246:608–620
Krabbenhoft DP, Sunderland EM (2013) Global change and mercury. Science 341(6153):1457–1458
Sonmez B, Celikkol AN (2021) Pullulan based hydrogels for the removal of various metal ions from aqueous solutions. J Environ Chem Eng 9(5):106188–106196
Khozemy EE, Nasef SM, Mohamed TM (2020) Radiation synthesis of superabsorbent hydrogel (wheat flour/acrylamide) for removal of mercury and lead ions from waste solutions. J Inorg Organomet Polym Mater 30(5):1669–1685
Setoodehkhah M, Momeni S (2018) Water soluble schiff base functinalized Fe3O4 magnetic nano-particles as a novel adsorbent for the removal of Pb(II) and Cu(II) metal ions from aqueous solutions. J Inorg Organomet Polym Mater 28(3):1098–1106
Brodkin E, Copes R, Mattman A, Kennedy J, Kling R, Yassi A (2007) Lead and mercury exposures: interpretation and action. CMAJ 176(1):59–63
Zahir F, Rizwi SJ, Haq SK, Khan RH (2005) Low dose mercury toxicity and human health. Environ Toxicol Pharmacol 20(2):351–360
Gil A, Amiri MJ, Abedi-Koupai J, Eslamian S (2018) Adsorption/reduction of Hg (II) and Pb (II) from aqueous solutions by using bone ash/nZVI composite: effects of aging time, Fe loading quantity and co-existing ions. Environ Sci Pollut Res 25(3):2814–2829
Gong Y, Wang Y, Lin N, Wang R, Wang M, Zhang X (2022) Iron-based materials for simultaneous removal of heavy metal (loid) s and emerging organic contaminants from the aquatic environment: recent advances and perspectives. Environ Pollut 299:118871–118885
Shrestha R, Ban S, Devkota S, Sharma S, Joshi R, Tiwari AP, Kim HY, Joshi MK (2021) Technological trends in heavy metals removal from industrial wastewater: a review. J Environ Chem Eng 9(4):105688–105706
Mansouri F, Chouchene K, Roche N, Ksibi M (2021) Removal of Pharmaceuticals from water by adsorption and advanced oxidation processes: state of the art and trends. Appl Sci 11(14):6659–6694
Liu N, Dai W, Fei F, Xu H, Lei J, Quan G, Zheng Y, Zhang X, Tang L (2022) Insights into the photocatalytic activation persulfate by visible light over ReS2/MIL-88B (Fe) for highly efficient degradation of ibuprofen: combination of experimental and theoretical study. Sep Purif Technol 297:121545–121555
Tamaddon F, Nasiri A, Yazdanpanah G (2020) Photocatalytic degradation of ciprofloxacin using CuFe2O4@methyl cellulose based magnetic nanobiocomposite. MethodsX 7:100764–100772
Lin N, Gong Y, Wang R, Wang Y, Zhang X (2022) Critical review of perovskite-based materials in advanced oxidation system for wastewater treatment: design, applications and mechanisms. J Hazard Mater 424:127637–127661
Yang Y, Li X, Jie B, Zheng Z, Li J, Zhu C, Wang S, Xu J, Zhang X (2022) Electron structure modulation and bicarbonate surrounding enhance Fenton-like reactions performance of Co-Co PBA. J Hazard Mater 437:129372–129385
Langbehn RK, Michels C, Soares HM (2021) Antibiotics in wastewater: from its occurrence to the biological removal by environmentally conscious technologies. Environ Pollut 275:116603–116619
Fu Z-J, Jiang S-K, Chao X-Y, Zhang C-X, Shi Q, Wang Z-Y, Liu M-L, Sun S-P (2022) Removing miscellaneous heavy metals by all-in-one ion exchange-nanofiltration membrane. Water Res 222:118888–118897
Chakraborty R, Asthana A, Singh AK, Jain B, Susan ABH (2022) Adsorption of heavy metal ions by various low-cost adsorbents: a review. Int J Environ Anal Chem 102(2):342–379
Ma L, Zhang X, Ikram M, Ullah M, Wu H, Shi K (2020) Controllable synthesis of an intercalated ZIF-67/EG structure for the detection of ultratrace Cd2+, Cu2+, Hg2+ and Pb2+ ions. Chem Eng J 395:125216–125228
Li W, Zhang L, Hu D, Yang R, Zhang J, Guan Y, Lv F, Gao H (2021) A mesoporous nanocellulose/sodium alginate/carboxymethyl-chitosan gel beads for efficient adsorption of Cu2+ and Pb2+. Int J Biol Macromol 187:922–930
Zhang K, Luo X, Yang L, Chang Z, Luo S (2021) Progress toward hydrogels in removing heavy metals from water: problems and solutions—A review. ACS ES&T Water 1(5):1098–1116
Tamaddon F, Mosslemin MH, Asadipour A, Gharaghani MA, Nasiri A (2020) Microwave-assisted preparation of ZnFe2O4@ methyl cellulose as a new nano-biomagnetic photocatalyst for photodegradation of metronidazole. Int J Biol Macromol 154:1036–1049
Wu S, Guo J, Wang Y, Huang C, Hu Y (2021) Facile preparation of magnetic sodium alginate/carboxymethyl cellulose composite hydrogel for removal of heavy metal ions from aqueous solution. J Mater Sci 56(23):13096–13107
Ma Q, Wang W, Ge W, Xia L, Li H, Song S (2021) Preparation of carboxymethyl cellulose-based hydrogel supported by two-dimensional montmorillonite nanosheets for methylene blue removal. J Polym Environ 29(12):3918–3931
dos Santos DM, de Lacerda BA, Ascheri DPR, Signini R, de Aquino GLB (2015) Microwave-assisted carboxymethylation of cellulose extracted from brewer’s spent grain. Carbohydr Polym 131:125–133
Karzar Jeddi M, Mahkam M (2019) Magnetic nano carboxymethyl cellulose-alginate/chitosan hydrogel beads as biodegradable devices for controlled drug delivery. Int J Biol Macromol 135:829–838
Godiya CB, Cheng X, Li D, Chen Z, Lu X (2019) Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J Hazard Mater 364:28–38
Varaprasad K, Jayaramudu T, Sadiku ER (2017) Removal of dye by carboxymethyl cellulose, acrylamide and graphene oxide via a free radical polymerization process. Carbohydr Polym 164:186–194
Kaith BS, Singh A, Sharma AK, Sud D (2021) Hydrogels: synthesis, classification, properties and potential applications—A brief review. J Polym Environ 29(12):3827–3841
Zainal SH, Mohd NH, Suhaili N, Anuar FH, Lazim AM, Othaman R (2021) Preparation of cellulose-based hydrogel: a review. J Mater Res Technol 10:935–952
Mondal S, Das S, Nandi AK (2020) A review on recent advances in polymer and peptide hydrogels. Soft Matter 16(6):1404–1454
Anjali J, Jose VK, Lee J-M (2019) Carbon-based hydrogels: synthesis and their recent energy applications. J Mater Chem A 7(26):15491–15518
Dacrory S, Kamal KH, Kamel S (2021) EDTA-functionalized magnetic graphene oxide/polyacrylamide grafted carboxymethyl cellulose hydrogel for removal of Pb2+ from aqueous solution. J Polym Environ 2021:1–14
Mohammadi R, Saboury A, Javanbakht S, Foroutan R, Shaabani A (2021) Carboxymethylcellulose/polyacrylic acid/starch-modified Fe3O4 interpenetrating magnetic nanocomposite hydrogel beads as pH-sensitive carrier for oral anticancer drug delivery system. Eur Polym J 153:110500–110509
Lin Q, Gao M, Chang J, Ma H (2016) Adsorption properties of crosslinking carboxymethyl cellulose grafting dimethyldiallylammonium chloride for cationic and anionic dyes. Carbohydr Polym 151:283–294
Javanbakht S, Nabi M, Shadi M, Amini MM, Shaabani A (2021) Carboxymethyl cellulose/tetracycline@UiO-66 nanocomposite hydrogel films as a potential antibacterial wound dressing. Int J Biol Macromol 188:811–819
Mohamood T, Fattima’Al-Zahara N, Abdul Halim AH, Zainuddin N (2021) Carboxymethyl cellulose hydrogel from biomass waste of oil palm empty fruit bunch using calcium chloride as crosslinking agent. Polymers 13(23):4056–4072
Capanema NSV, Mansur AAP, de Jesus AC, Carvalho SM, de Oliveira LC, Mansur HS (2018) Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int J Biol Macromol 106:1218–1234
Hashem M, Sharaf S, Abd El-Hady MM, Hebeish A (2013) Synthesis and characterization of novel carboxymethylcellulose hydrogels and carboxymethylcellulolse-hydrogel-ZnO-nanocomposites. Carbohydr Polym 95(1):421–427
Hosseinzadeh H, Javadi A (2016) Fabrication and characterization of CMC-based magnetic superabsorbent hydrogel nanocomposites for crystal violet removal. Polym Adv Technol 27(12):1609–1616
Zeng X, Zhang G, Junfeng Zhu WZ (2022) Adsorption of heavy metal ions in water by surface functionalized magnetic composites: a review. Environ Sci: Water Res Technol 5(5):1–32
Ren J, Zhu Z, Qiu Y, Yu F, Ma J, Zhao J (2021) Magnetic field assisted adsorption of pollutants from an aqueous solution: a review. J Hazard Mater 408:124846–124857
Kumar A, Kuang Y, Liang Z, Sun X (2020) Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: a review. Mater Today Nano 11:100076–100096
Karmakar M, Mondal H, Ghosh NN, Chattopadhyay PK, Singha NR (2021) Synthesis of gum tragacanth-grafted pentapolymer hydrogels for As (III) exclusion: roles of microwaves, RSM optimization, and DFT studies. Int J Biol Macromol 184:909–925
Tamaddon F, Ahmadi-AhmadAbadi E, Khoje-neamah E (2022) Nano-carboxymethylcellulose, polyacrylamide, and γ-Fe2O3-SO3H cross-linked to a hydrophobic linker: an organic-inorganic hydrogel for adsorptive removal of dyes. J Mol Struct 1270:133872–133886
Koukabi N, Kolvari E, Zolfigol MA, Khazaei A, Shaghasemi BS, Fasahati B (2012) A magnetic particle-supported sulfonic acid catalyst: tuning catalytic activity between homogeneous and heterogeneous catalysis. Adv Synth Catal 354(10):2001–2008
Tamaddon F, Arab D, Ahmadi-AhmadAbadi E (2020) Urease immobilization on magnetic micro/nano-cellulose dialdehydes: urease inhibitory of Biginelli product in Hantzsch reaction by urea. Carbohydr Polym 229:115471–115481
Tamaddon F, Kargar-Shooroki H, Jafari AA (2013) Molybdate and silica sulfuric acids as heterogeneous alternatives for synthesis of gem-bisamides and bisurides from aldehydes and amides, carbamates, nitriles or urea. J Mol Catal A: Chem 368–369:66–71
Dai H, Zhang Y, Ma L, Zhang H, Huang H (2019) Synthesis and response of pineapple peel carboxymethyl cellulose-g-poly (acrylic acid-co-acrylamide)/graphene oxide hydrogels. Carbohydr Polym 215:366–376
Dai H, Huang H (2017) Enhanced swelling and responsive properties of pineapple peel carboxymethyl cellulose-g-poly(acrylic acid-co-acrylamide) superabsorbent hydrogel by the introduction of carclazyte. J Agric Food Chem 65(3):565–574
Sun X-F, Xie Y, Shan S, Li W, Sun L (2022) Chemically-crosslinked xylan/graphene oxide composite hydrogel for copper ions removal. J Polym Environ 30:1–15
Singha NR, Dutta A, Mahapatra M, Roy JSD, Mitra M, Deb M, Chattopadhyay PK (2019) In situ attachment of acrylamido sulfonic acid-based monomer in terpolymer hydrogel optimized by response surface methodology for individual and/or simultaneous removal (s) of M (III) and cationic dyes. ACS Omega 4(1):1763–1780
Schott H (1992) Swelling kinetics of polymers. J Macromol Sci Part B Phys 31(1):1–9
Wang Y, Wang W, Shi X, Wang A (2013) Enhanced swelling and responsive properties of an alginate-based superabsorbent hydrogel by sodium p-styrenesulfonate and attapulgite nanorods. Polym Bull 70(4):1181–1193
Gao T, Wang W, Wang A (2011) A pH-sensitive composite hydrogel based on sodium alginate and medical stone: synthesis, swelling, and heavy metal ions adsorption properties. Macromol Res 19(7):739–748
Akl M, Ismail MA, Hashem MA, Ali DA (2021) Novel NS modified cellulose: synthesis, spectroscopic characterization and adsorption studies of Cu2+, Hg2+ and Pb2+ from environmental water samples. Res square 1:1–34
Yang S, Fu S, Liu H, Zhou Y, Li X (2011) Hydrogel beads based on carboxymethyl cellulose for removal heavy metal ions. J Appl Polym Sci 119(2):1204–1210
Özkahraman B, Acar I, Emik S (2011) Removal of Cu2+ and Pb2+ ions using CMC based thermoresponsive nanocomposite hydrogel. Clean: Soil, Air, Water 39(7):658–664
Hu Z-H, Omer AM, Ouyang Xk YuD (2018) Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb (II) from aqueous solution. Int J Biol Macromol 108:149–157
Mohammed N, Baidya A, Murugesan V, Kumar AA, Ganayee MA, Mohanty JS, Tam KC, Pradeep T (2016) Diffusion-controlled simultaneous sensing and scavenging of heavy metal ions in water using atomically precise cluster–cellulose nanocrystal composites. ACS Sustain Chem Eng 4(11):6167–6176
Rong L, Zhu Z, Wang B, Mao Z, Xu H, Zhang L, Zhong Y, Sui X (2018) Facile fabrication of thiol-modified cellulose sponges for adsorption of Hg2+ from aqueous solutions. Cellulose 25(5):3025–3035
Acknowledgements
The authors gratefully admit partial support of this work by the Yazd University Research Council.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
FT Conceptualization, Supervision, Project administration, Methodology, Formal analysis, Data curation, Writing-review and editing. EA-A Methodology, Original draft, Conceptualization, Formal analysis, Investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tamaddon, F., Ahmadi-AhmadAbadi, E. Microwave-Assisted Fabrication of a pH/Salt Responsive Hydrogel from the Micro-CMC, In Situ Polymerized Acrylamide, and Nanoγ-Fe2O3-SO3H Cross-Linked by a Phenyl Bisamide Linker for Pb2+ and Hg2+ Removal. J Polym Environ 31, 406–422 (2023). https://doi.org/10.1007/s10924-022-02628-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02628-6