Abstract
In this work, a chitosan (CHI) biopolymer was developed by loading different wt% of carbon-doped TiO2 (C–TiO2) with CHI to attain an efficient adsorbent of chitosan/carbon-doped TiO2 (CHI/C–TiO2). The fabricated materials were deployed for the removal of organic pollutants (methyl orange, MO; and reactive orange 16, RO16) and sulfur dioxide capture. The synthesized composites were characterized by BET, FTIR, XRD, TEM, SEM–EDX, pHpzc, and pH-potentiometric titrations. Statistical modeling represented by the Box–Behnken design (BBD) was utilized for optimization of the impacts of the various parameters; A: C–TiO2 particles loading (0–50%), B: dose (0.04–0.15 g), C: pH (4–10), and D: temperature (30–50 °C) on the adsorption of MO and RO16 dyes. The adsorption isotherms were obtained at equilibrium and under dynamic conditions, where the best fit to the isotherm results was shown by the Langmuir model and pseudo-first-order kinetic model, respectively. The maximum adsorption capacities of CHI/C–TiO2-50 (containing 50% of C–TiO2) was estimated at 196.6 mg/g and 270.5 mg/g for MO and RO16 dyes, respectively. This work revealed that the designed biomaterial (CHI/C–TiO2-50) could be realized as an effective adsorbent for environmental remediation that includes decontamination of wastewater and SO2 gas capture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air and water pollution is one of the fundamental problems that pose a great threat to the ecosystem and human health. In recent years, industrial development has undergone tremendous growth in various parts of the world, and consequently, increased water and air pollution can occur due to release of effluents containing chemicals into water bodies and release of toxic gases released into the atmosphere [1]. Organic dyes are one of the chemicals that are widely used in many industries such as textiles, pharmaceuticals, plastics, cosmetics, paper, and leather [2]. The release of these dyes into freshwater environments causes many problems for biota, such as reducing the effectiveness of photosynthesis, in addition to human health problems [3]. Methyl orange (MO) and reactive orange 16 (RO16) are among the azo dyes that are typical of anionic model organic pollutants [4, 5]. These dyes are non-degradable which makes them a great danger to aquatic environments, including their health hazards [6].
On the other hand, sulfur dioxide (SO2) is considered one of the most harmful gases, which is produced from several industries such as the combustion of fossil fuels (e.g., coal, gas, and oil) in the power plants, and the smelting of mineral ores [7]. This gas causes several environmental phenomena such as photochemical smog, acid rain, and haze [8]. These phenomena can negatively affect climatic systems (e.g., the atmosphere, rivers, snow cover, and sea ice), plant communities, human health, and animal growth [8, 9]. In light of the foregoing, the treatment of wastewater containing organic pollutants and the removal of toxic gases before release into the environment is an urgent matter to preserve the ecosystem and human health. Several methods have been reported in the treatment of dyeing wastewater including adsorption [10], nanofiltration [11], photocatalytic degradation [12], and electrocoagulation [13], while in air pollution with SO2 gas, the common methods applied for capturing SO2 employ liquid alkaline solutions (e.g., Ca(OH)2, and Mg(OH)2), and adsorbent materials (e.g., activated carbon) [7]. In general, adsorption technology is a preferred method for pollutant removal from water and air. Adsorption is characterized by unique and favourable features (e.g., simplicity of design, low investment cost, lack of secondary pollution, removal efficiency, and regeneration ability) that position it at the forefront of methods applied to air and water pollution control [9, 14]. Activated carbon (AC) is one of the most popular adsorbents in the field of adsorption of air and water pollutants due to its amazing properties such as porosity, high adsorption capacity, high surface area, and thermal stability [15, 16]. Despite these impressive properties of AC, it is economically expensive to produce. For this reason, research efforts have shifted to low-cost, renewable, and environmentally friendly adsorbents.
Chitosan (CHI) is one of the most environmentally friendly, renewable, and efficient adsorbents employed in adsorption technology [17]. In acidic media, CHI is a natural cationic polysaccharide that consists of glucosamine units [18]. CHI has several unique advantages over other common adsorbents, such as its ease of modification, biodegradability, super absorbency, hydrophilicity, and nontoxicity [19]. The chemical structure of CHI is characterized by abundant amino and hydroxyl groups, which are likely to be active adsorption sites for the removal of acid gases such as SO2 and acid dyes [20, 21]. Despite the foregoing, some of the properties of CHI require further development such as the effective surface area, chemical stability, and mechanical strength. Several strategies have been employed in developing the CHI properties such as composite formation with inorganic materials [22], grafting with organic groups [23], and crosslinking [24]. The development of CHI by composite formation with inorganic materials has attracted much attention due to its extraordinary properties like improved surface area, absorption capacity, chemical stability, and mechanical strength [22].
Carbon-doped TiO2 (known as Kronos) is one of the most promising materials that can be employed in modifying the properties of CHI due to its distinctive characteristics such as its surface area, chemical stability, mechanical strength, photo activity, and adsorption efficiency of inorganic/organic contaminants [25, 26]. Many studies have reported on the development of CHI by TiO2 and its utilization in various applications such as wastewater treatment [27], biosensor [28], and antimicrobial [29], but with carbon-doped TiO2 (C–TiO2), no studies were reported thus far. Furthermore, the CHI -based composites for capturing hazardous gases were recently explored, for example, CHI grafted Leca biocomposite for the capture of carbon dioxide (CO2) [30], adsorption of formaldehyde gas by CHI crosslinked with MOF-199@ aminated graphene oxide aerogel [31], CHI extracted from shrimp shells loaded with Cd ions for the capture of hydrogen sulfide (H2S) [32], CHI aerogel for the removal of ammonia (NH3) gas [33], and ferrocene- CHI for sorption of carbon monoxide (CO) gas [34].
Herein, this study aims to develop a CHI biopolymer by loading different wt% of C-doped TiO2 onto CHI to attain an efficient adsorbent, referred to as CHI/C-doped TiO2 (CHI/C–TiO2). The synthesized composites were fully characterized by BET, FTIR, XRD, TEM, SEM–EDX, pHpzc, and pH-potentiometric titrations. The fabricated materials were applied for the removal of organic pollutants (MO and RO16) and SO2 capture. Statistical modeling represented by Box–Behnken design (BBD) was utilized for optimization of the impacts of the C–TiO2 particles loading, dose, pH, and temperature on the adsorption of MO and RO16 dyes. In addition, several kinetic and isothermal models have been utilized to investigate the adsorption properties and mechanism of the CHI/C–TiO2 composite.
Materials and Methods
Materials
Chitosan (CHI, deacetylation ≥ 75%), and Carbon-doped TiO2 (C–TiO2, Kronos) were supplied by Sigma–Aldrich. The organic dyes including methyl orange (MO; C14H14N3NaO3S; MW: 327.32; λmax = 464 nm), and reactive orange 16 (RO16; C20H17N3Na2O11S3; MW: 617.54 g/mol; λmax = 493 nm) were obtained from ACROS, Organics. The chemicals including acetic acid (CH3COOH), sodium chloride (NaCl), sodium hydroxide (NaOH), and hydrochloric acid (HCl) were obtained from R&M Chemicals.
Synthesis of CHI/C–TiO2
The synthesis of CHI/C–TiO2 was based on the approach described in previous work [35]. Initially, 1 g of CHI flakes was poured into 60 ml of CH3COOH acid solution (5% v/v) and stirred at 500 rpm on a magnetic stirrer for 24 h at 25° C to dissolve the CHI flakes. Then, the CHI solution was added drop-wise into 1000 ml NaOH (2 M) solution to obtain CHI beads. The resulting CHI hydrogel beads were washed with distilled water until the pH became neutral. For comparison, CHI/C–TiO2 composites were fabricated by loading different wt. % of C–TiO2 with CHI before adding to the solution of CH3COOH, i.e. 25:75 wt% (C–TiO2:CHI), which was termed as CHI/C–TiO2-25 and 50:50. wt% (C–TiO2:CHI), which was termed as CHI/C–TiO2-50. Finally, the fabricated composites were crushed and then sieved to particle size (250 μm) for the dye removal tests, along with SO2 capture.
Characterization
The textile properties (e.g., specific surface area, and average pore width) of the CHI/C–TiO2-50 were obtained through Brunauer–Emmett–Teller (BET) method (Micromeritics ASAP 2060). The morphological characteristics of CHI/C–TiO2-50 before and after adsorption of MO and RO16 dyes were achieved by recording scanning electron microscope (SEM, Zeiss Supra 40 VP) images. The energy dispersive X-Ray analysis (EDX) was performed to the chemical qualitative analysis of the CHI/C–TiO2-50 before and after adsorption of MO and RO16 dyes. X-ray powder diffraction spectra (XRD, X’Pert PRO, PANalytical) were used to evaluate the phase determination of the CHI/C–TiO2-50 composite. Fourier Transform Infrared (FTIR) spectra (Perkin-Elmer, Spectrum RX I) was used to determine the surface chemical characteristics of the CHI/C–TiO2-50 composite before and after MO and RO16 dyes adsorption. The free amino (-NH2) groups in the CHI/C–TiO2-50 was computed by pH-potentiometric titrations [36]. Zero-point of charge (pHpzc) analysis was employed to determine the surface charge of the CHI/C–TiO2-50 [37].
Experimental modeling
BBD was used in this research work as an efficient and reliable statistical method to provide MO removal (%) and RO16 removal (%) as responses based on several independent factors that affect the adsorption properties: C–TiO2 loading, pH, adsorbent dosage, and temperature. The statistical analysis and numerical demonstration of the experimental results related to MO and RO16 removal were acquired through the software program of Design-Expert (Stat-Ease, version 13). Table 1 shows the parameters evaluated inside the BBD model, along with their levels and symbols. A polynomial equation (Eq. 1) was applied to correlate the experimental results and estimate the MO removal (%) and RO16 removal (%).
where Y, and \({(X}_{i}\) and \({X}_{j})\) symbol the forecasted response (MO removal (%) or RO16 removal (%)); and the evaluated factors. \({\beta }_{0}\) and \({\beta }_{i}\), denote the regression coefficients of intercept, and the linear; whereas, \({\beta }_{ii}\) and \({\beta }_{ij}\) signify quadratic and interaction effects. The BBD model produced 29 runs to examine the influence of the following factors: A: C–TiO2 loading (0–50%), B: adsorbent dose (0.04–0.15 g), C: pH (4–10), and D: temperature (30–50 °C) on MO and RO16 dyes adsorption process. The actual values of responses (MO and RO16 removal) along with their experimental conditions are recorded in Table 2. In 250 ml flasks that contain 100 ml of the dye solution, a constant quantity of the adsorbent was added. Then, these sealed flasks were transferred into a shaker water bath (WNB7-45, Memmert) at a speed of up to 90 strokes/min. The next step after the adsorption process was to remove the adsorbents from solutions with a syringe filter (0.45 μm). Then, the residual concentrations of MO and RO16 in solutions was estimated using spectrophotometry with a UV–Vis spectrometer (HACH DR 2800) at λmax 464 nm and 493 nm, respectively. The removal efficiency (R, %) of MO and RO16 dyes were calculated using Eq. 2 shown below.
where \({C}_{o}\) (mg/L) and \({C}_{e}\) (mg/L) represent the dye concentrations (MO or RO16) before and after the adsorption process, respectively.
Adsorption study of MO and RO16 dyes on CHI/C–TiO 2 -50
Determining the adsorption ability of CHI/C–TiO2-50 toward MO and RO16 dyes was accomplished by use of batch experiments. As determined in Table 2, the maximum removal of MO (81.6%) and RO16 (90.4%) dyes was accomplished in run eighteen under the following conditions: C–TiO2 loading = 50%, dosage = 0.095 g (adjusted to 0.1 g in adsorption tests), pH 4, and temperature = 40 °C. In this way, batch tests were carried out to study the uptake the MO and RO16 dyes at these previously mentioned ideal conditions over a range of initial concentrations (20–200 mg/l). The calculation of the adsorption capacity (qe, mg/g), of MO and RO16 dyes on the surface of the CHI/C–TiO2-50 at equilibrium employed Eq. 3.
where W (g) and V (L) are the amount of CHI/C–TiO2-50 and the volume of the dye solution (MO and RO16), respectively.
SO2 adsorption tests
The SO2 adsorption tests were carried out by Ultramat 23 Gas Analyzer integrated with computer dynamic simulation to adjust the gas flow rate of SO2 for the tests. 1 g of the synthesized adsorbent was placed in the reaction vessel and the temperature was maintained at 87 °C. Before SO2 capture, N2 (99.99%) was passed through the prepared adsorbent to exclude any undesired compounds. After that, a stream of SO2 (1000 ppm) gas was fed to the column containing adsorbent meanwhile keeping on the feed flow at 500 ml/min. Equation 4 was utilized to calculate the breakthrough adsorption capacity (S, mmol/g) of SO2 gas.
where q, tm, and G signify the gas flow rate (ml/min), is the breakthrough time (min), and the amount of the adsorbent (g). Co and Ci represent the initial concentration (ppm), and the outlet concentration (ppm) of SO2 gas.
Results and discussion
Characterization of the CHI/C–TiO2-50
Table 3 lists the physicochemical properties of the CHI/C–TiO2, specifically the amine content (%) and surface area characteristics. pH-potentiometric titration is a vital method used to estimate the proportion of primary amino groups (-NH2) existing in the backbone of the CHI/C–TiO2-50, which symbolizes adsorptive sites for anionic dyes such as MO and RO16 dyes. The –NH2 content in CHI/C–TiO2-50 determined by potentiometric titration reached 38.41%. This finding suggests that the CHI/C–TiO2-50 still has plenty of –NH2 to bind with MO and RO16 dyes. The results of the BET analysis showed that the surface area of the CHI/C–TiO2-50 composite is significantly higher than the cross-linked CHI composites containing 50% of TiO2 nanoparticles. For example, cross-linked CHI-glyoxal/TiO2 (17.74 m2/g) [38] and cross-linked CHI-tripolyphosphate/TiO2 (2.75 m2/g) [35] indicate that C–TiO2 possesses a high surface area that plays a significant role in improving the surface properties of CHI/C–TiO2-50 through supporting onto the CHI biopolymer matrix. As per IUPAC, CHI/C–TiO2-50 is a mesoporous material due to its average pore diameter of 7.31 nm.
The X-ray diffraction (XRD) profile of the CHI/C–TiO2-50 composite is illustrated in Fig. 1. The broad peak located at 20.3° in Fig. 1 relates to the semi-crystalline nature of CHI biopolymer [39]. In Fig. 1, several peaks at 2θ of 25.28°, 37.8°, 48.05°, 53.89°, 55.1°, 62.6°, 68.7°, 70.3° and 75.01° correspond to the [101], [004], [200], [105], [211], [204], [116], [220], and [215] crystalline phases of C–TiO2, respectively with the Miller indices (JCPDS file No. 00-021-1272) [40].
TEM analysis was performed to accurately determine the particle size of C–TiO2. The TEM images of C–TiO2 particles at several wavelengths (500 nm, 200 nm, 100 nm, and 50 nm) are illustrated in Fig. 2a–d, respectively. According to the TEM results in Fig. 2, the size of C–TiO2 particles is 8.60 nm (Fig. 2d). These results concur with the results of the BET analysis, which support that the surface area of the CHI/C–TiO2-50 was 89.34 m2/g. The presence of nano-sized particles (C–TiO2) in the formula designed (CHI/C–TiO2-50) for adsorption of anionic dyes and SO2 gas impart a high adsorption efficiency to remove organic and inorganic pollutants.
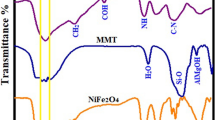
The surface functional groups of the CHI/C–TiO2-50, and CHI/C–TiO2-50 after MO and RO16 dyes adsorption were determined by FTIR analysis. The FTIR spectra of CHI/C–TiO2-50, and CHI/C–TiO2-50 after the adsorption of MO and RO16 dyes are shown in Fig. 3a–c. The characterized bands of CHI/C–TiO2-50 composite (Fig. 3a) at 3698 cm−1, 3500–3650 cm−1, 2270 cm−1, 1660 cm−1, 1450 cm−1, 1380 cm−1, 1075 cm−1, 770 cm−1, and 540 cm−1 are attributed to the hydroxyl groups of TiO2, –OH and –NH2 stretching, stretching of C≡C, N–H bending vibration, deformations of C–O–H, C–N stretching, C–O–C stretching, stretching vibration of Ti–O–Ti and Ti–O bond, respectively [41, 42]. The FTIR spectra of the CHI/C–TiO2-50 composite after MO (Fig. 3b) and RO16 (Fig. 3c) adsorption showed a remarkable change in the bands of –NH2 and –OH groups, which is notably shifted, and broadened, confirming that the –OH and NH2 groups participate in the MO and RO16 adsorption process. Besides, the peak at 1500 cm−1 can be attributed to the aromatic rings (C=C) of the MO and RO16 dyes further confirm the loading or adsorption of MO and RO16 dyes onto the CHI/C–TiO2-50 surface.
Exploration of the surface morphology of the CHI/C–TiO2-50 before and after adsorption of MO and RO16 dyes as well as estimates of its chemical composition was achieved by SEM–EDX. Figure 4a–c provides SEM–EDX images of (a) CHI/C–TiO2-50, and CHI/C–TiO2-50 following adsorption of (b) MO and (c) RO16 dyes. As seen in Fig. 4a, the surface features for the 50% C–TiO2 particles loaded onto CHI reveal that the CHI/C–TiO2-50 had a granular surface containing tiny pores, which illustrates the embedding of C–TiO2 particles onto the polymer matrix of CHI. The EDX spectrum of the CHI/C–TiO2-50 reveals the presence of Ti, O, C, and N. The appearance of a Ti signature in the EDX spectrum is further evidence that the C–TiO2 particles were effectively incorporated onto the CHI matrix. The CHI/C–TiO2-50 surface was altered into a smooth, packed, and uniform surface after adsorption of MO (Fig. 4b) and RO16 (Fig. 4c), consistent with the uniform dye surface coverage. This result was confirmed by EDX, which showed that the content of C (%) was increased because of loading the dye (MO or RO16) molecules.
Model validation
A statistical analysis of variance (ANOVA), including the statistical evaluation of the significant impacts of the factors and their interactions related to the MO and RO16 dyes removal was carried out. The ANOVA findings for the removal of the MO and RO16 dyes are listed in Table 4. The MO and RO16 removal models have F-values of 23.63 and 18.12, respectively, implying that the models are critical [43]. The correlation coefficients (R2) of 0.96 (MO removal) and 0.95 (RO16 removal) indicate that the models are well fitted and that there is a favourable agreement between theoretical and actual removal values [44]. Statistically, the BBD model terms have statistical significance when the p-value ≤ 0.05. Accordingly, A, B, C, AB, AC, BC, A2, and D2 are critical terms in the removal process of the MO and RO16 dyes. Along these lines, the linkage between MO and RO16 removal with the examined variables was empirically fitted by the quadratic polynomial formula, as expressed in Eqs. 5 and 6, respectively.
Analysis of the diagrams inclusive of real versus predicted, where the normal probability of the residuals might be utilized to successfully verify the experimental findings of dye removal for MO and RO16. The real vs. expected graphs of MO and RO16 removal values are shown in Fig. 5a, b, where the real points were largely near the predicted points, demonstrating that the BBD model can optimize the adsorption process for these dyes. The normal probability charts of the MO and RO16 removal dyes are depicted in Fig. 5c, d. As seen from Fig. 5c, d, the typical distribution of dots around the straight-line denote the independence of the residuals [41].
Adsorption optimization of MO and RO16 dyes
3D response surfaces plots are as one of the main graphical representations that provide an understanding of the nature of the fundamental interactions among the two investigated variables and their impacts on the responses denoted by the MO removal and RO16 removal. The cumulative impacts of C–TiO2 loading and dosage (termed as AB interaction) on the MO and RO16 removal were meaningful. Figure 6a, b displays the 3D surface plot of the cumulative impacts of AB interaction on the MO and RO16 removal at the accompanying fixed circumstances: pH (7) and temperature (40 °C). The findings shown in Fig. 6a, b found that increasing the adsorbent dosage and C–TiO2 loading enhances the removal of MO and RO16 dyes. Such findings are related to the excessive surface area coming from C–TiO2 particles and the adequate quantity of reactive sites available on the CHI/C–TiO2 surface, which are attracted with MO and RO16 molecules to obtain the adsorption process.
The cumulative impacts of C–TiO2 loading vs. pH (termed as AC interaction) and dose vs. pH (termed as BC interaction) on the MO and RO16 removal were meaningful. Figure 7a–d displays the 3D surface plot of the cumulative impacts of AC and BC interactions on the MO and RO16 removal at the accompanying fixed conditions: dose (0.095 g) and temperature (40 °C) for AC interaction; and C–TiO2 loading (25%) and temperature (40 °C) for BC interaction. As demonstrated in Fig. 7a–d, the MO and RO16 (%) improved simultaneously with a pH drop from 10 to 4. As shown in Fig. 7e, pHpzc of the CHI/C–TiO2-50 equals 7.0. This observation indicates that the CHI/C–TiO2 surface acquires negative charges at pH values larger than pHpzc. As a result of the rise in H+ concentration in the solution, the CHI/C–TiO2 surface becomes positively charged at an acidic medium. Therefore, the electrostatic attractions between the sulfonate (–SO3−) groups belonging to the MO and RO16 dyes and cationic groups of CHI/C–TiO2-50, as shown in Eq. 7.
where the \({\mathrm{Dye}-\mathrm{SO}}_{3}^{-}\) indicates either the MO or the RO16 dye species.
Adsorption study
An investigation on the effect of initial concentration on the adsorption behaviour of MO and RO16 dyes by the CHI/C–TiO2-50 composite was performed at several dye adsorbate concentrations (20–200 mg/l); whereas, the mass of the adsorbent (0.095 g), volume of the dye solution (100 ml), pH (4), and temperature (40 °C) were held constant. The adsorption property of CHI/C–TiO2 towards MO and RO16 dyes as a function of contact time are shown in Fig. 8a, b. In Fig. 8a, b, the adsorption capacity of CHI/C–TiO2-50 was found to increase from 17.9 to 148.4 mg/g for MO and from 20.4 to 193.7 mg/g for RO16, across a range of dye concentration (20 to 200 mg/l). The higher concentration range for MO and RO16 provides an important driving force that contributes to the transport of MO and RO16 molecules to the available active sites on the CHI/C–TiO2-50 surface [45].
SO2 capture
The removal of SO2 over CHI biopolymer in the presence and absence of variable C–TiO2 loadings was investigated. Figure 9 displays the breakthrough curves of SO2 capture over CHI biopolymer, CHI/C–TiO2-25, and CHI/C–TiO2-50. According to the breaking curves exhibited in Fig. 9, the saturation time of CHI biopolymer, CHI/C–TiO2-25, and CHI/C–TiO2-50 was 11 min, 30 min, and 35 min, respectively. These results concur with the values of SO2 adsorption capacity listed in parentheses as follows: CHI/C–TiO2-25 (0.6140 mmol/g), CHI/C–TiO2-50 (0.7622 mmol/g), and CHI biopolymer (0.1342 mmol/g). Therefore, the SO2 adsorption data revealed that SO2 capture by the CHI/C–TiO2-25 and CHI/C–TiO2-50 biomaterials was significantly improved compared to the CHI biopolymer. The improvement in adsorption performance of CHI biopolymer (without C–TiO2 particles) upon loading with C–TiO2 loading (especially 50%) for CHI/C–TiO2-50 (89.34 m2/g) can be attributed to the greater surface area effects of the C–TiO2 particles. In addition to the above, and from a chemical point of view, CHI/C–TiO2-50 possesses active adsorption sites such as Ti–O, C–O, –OH, and –NH2, which can participate in the binding of SO2 molecules [8, 20]. Fundamentally, the SO2 capture occurred at a high temperature of 360 K, which signifies that the surface sulfate-like species (SO42−) was formed from the SO2 capture process is activated by elevated temperature [46].
Kinetic study
In order to understand the adsorption behavior and determine the control mechanism responsible for the adsorption of MO and RO16 dyes on the surface of CHI/C–TiO2-50, the experimental data were evaluated by common kinetics model equations, via the pseudo-first-order (PFO) [47] and pseudo-second-order (PSO) models [48]. The terms of the kinetics including PFO and PSO were extracted by the non-linear formulas listed in Eqs. 8 and 9, respectively.
where qe (mg/g) and qt (mg/g) represent the adsorption capacities at equilibrium and time (t), respectively. k1 (1/min) and k2 (g/mg min) are the rate constants of the PFO and PSO kinetic models, respectively. The calculated kinetic parameters are recorded in Table 5, where it can be concluded that the adsorption process for both dyes are described by the PFO model over that of the PSO model. This trend is supported by the relatively high R2 values of PFO compared to the R2 values of PSO, in addition to the experimental qe values, which are closer to the calculated qe values of the PFO model, in comparison with the calculated qe values of PSO. The results reported herein support that the adsorption of the MO and RO16 dyes on CHI/C–TiO2-50 is physisorption that involves electrostatic attractions [49]. The latter occurs between the cationic groups present on the surface of CHI/C–TiO2-50 and the -SO3− groups of the MO and RO16 dyes, respectively.
Isotherm study
An account of the mode of interaction between CHI/C–TiO2-50 and the MO and RO16 dye species can be obtained by analysis of the equilibrium isotherms. The adsorption capacity of CHI/C–TiO2-50 for the uptake MO and RO16 dyes from the liquid phase was determined through the fitting of the experimental results to equilibrium isotherms like Langmuir, Freundlich, and Temkin [50,51,52]. The terms of the isotherms including Langmuir, Freundlich, and Temkin were extracted by the non-linear formulae listed in Eqs. 10–12, respectively.
Ka (L/mg), KT (L/mg), and Kf (mg/g) (L/mg)1/n, are the adsorption constants of Langmuir, Temken, and Freundlich isotherms. qmax (mg/g) is the adsorption capacity of the CHI/C–TiO2-50. n is the exponent term for the Freundlich model. bT (J/mol) is a term related to the heat adsorption. T (K) and R (8.314 J/mol K) denote the temperature and universal gas constant, respectively. The curves acquired from adsorption isotherms for MO and RO16 dyes are exhibited in Fig. 10a, b while the calculated terms of the isotherms are given in Table 6. Depending on the R2 values, it can be concluded that the adsorption of MO and RO16 dyes by CHI/C–TiO2-50 fit better with the Langmuir isotherm model, based on the highest R2 value compared to the other models. These findings signify the homogeneous character of the adsorbent surface for CHI/C–TiO2-50 with the MO and RO16 dyes [53]. The qmax values for MO and RO16 dyes on CHI/C–TiO2-50 were estimated as 196.6 mg/g and 270.5 mg/g at 40 °C, respectively. In order to get a better idea of the CHI/C–TiO2-50's adsorption capacity, it was compared with the adsorption capabilities of other materials studied for the removal of such anionic dyes (MO and RO16), according to the values listed in Table 7. These results show that the CHI/C–TiO2-50 poses a good ability to remove organic pollutants, specifically anionic dyes from polluted water.
Adsorption mechanism of MO and RO16 dyes
The surface of CHI/C–TiO2 is characterized by an enormous amount of active groups such as hydroxyl (–OH), amino (–NH2), and (Ti–OH), as per the FTIR spectrum, which is consistent with other studies [35, 38]. Because of the existence of such functional groups, the mechanism of MO and RO16 dye adsorption on the surface of CHI/C–TiO2 was deduced, as seen in Fig. 11. One of the key pathways contributing to the adsorption of MO and RO16 dyes on the surface of CHI/C–TiO2 is electrostatic forces that achieve by the attraction between the negative charge of the MO and RO16 dyes and the negatively charged groups of the CHI/C–TiO2 surface as shown in Fig. 11. The interaction between the hydrogen on the CHI/C–TiO2 surface and the nitrogen and oxygen atoms in the MO and RO16 dye structures is another probable interaction contributing to the adsorption of MO and RO16 dyes, which is called H-bonding. The n-π stacking is also implicated in the adsorption of the MO and RO16 dyes via the interface that occurs between the CHI/C–TiO2 surface (oxygen and nitrogen groups) and the aromatic rings of the MO and RO16 dyes [66].
Conclusion
A composite adsorbent with improved adsorption properties over pristine chitosan was reported herein. The composite is comprised of chitosan/C-doped TiO2 (CHI/C–TiO2) was found to be suitable for the removal of organic pollutants (methyl orange, MO; and reactive orange 16, RO16) and capture of an inorganic gas (SO2). The best experimental conditions for the design of the composite adsorbent herein and the adsorption conditions for the highest removal of MO (81.6%) and RO16 (90.4%) dyes are as follows: C–TiO2 loading = 50%, adsorbent dosage = 0.095 g, pH 4, and temperature = 40 °C. The results from the BBD model reveal that the highest MO and RO16 removal were obtained upon consideration of the following interactions: AB (C–TiO2 loading × dose), AC (C–TiO2 loading × pH), and BC (dose × pH). The results for the adsorption isotherms and the kinetic profiles showed that the adsorption process of MO and RO16 dyes onto the surface of CHI/C–TiO2-50 is primarily monolayer adsorption and physisorption in nature. The maximum adsorption capacities of CHI/C–TiO2-50 (containing 50% C–TiO2) were found to be 196.6 mg/g and 270.5 mg/g of MO and RO16 dyes, respectively. The developed biocomposite material (CHI/C–TiO2-50) was found to be an effective adsorbent for environmental applications such as the treatment of wastewater, along with gas capture, as shown for SO2.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ibrahim M, Labaki M, Giraudon JM, Lamonier JF (2020) Hydroxyapatite, a multifunctional material for air, water and soil pollution control: a review. J Hazard Mater 383:121139
Abd-Malek NN, Yousif E, Jawad AH (2020) Optimization of adsorption parameters for reactive red 4 (RR4) removal by cross-linked chitosan-epichlorohydrin using Box Behnken design. Sci Lett 14(1):83–95
Aigbe UO, Ukhurebor KE, Onyancha RB, Osibote OA, Darmokoesoemo H, Kusuma HS (2021) Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: a review. J Mater Res Technol 14:2751–2774
Márquez AA, Sirés I, Brillas E, Nava JL (2020) Mineralization of Methyl Orange azo dye by processes based on H2O2 electrogeneration at a 3D-like air-diffusion cathode. Chemosphere 259:127466
Wasim M, Sabir A, Khan RU (2021) Membranes with tunable graphene morphology prepared via Stöber method for high rejection of azo dyes. J Environ Chem Eng 9(5):106069
Li X, Wang Z, Ning J, Gao M, Jiang W, Zhou Z, Li G (2018) Preparation and characterization of a novel polyethyleneimine cation-modified persimmon tannin bioadsorbent for anionic dye adsorption. J Environ Manage 217:305–314
Braghiroli FL, Bouafif H, Koubaa A (2019) Enhanced SO2 adsorption and desorption on chemically and physically activated biochar made from wood residues. Ind Crops Prod 138:111456
Yang K, Yi H, Tang X, Zhao S, Gao F, Huang Y, Xie X (2019) Reducing the competitive adsorption between SO2 and NO by Al2O3@TiO2 core-shell structure adsorbent. Chem Eng J 364:420–427
Abdulrasheed AA, Jalil AA, Triwahyono S, Zaini MAA, Gambo Y, Ibrahim M (2018) Surface modification of activated carbon for adsorption of SO2 and NOX: a review of existing and emerging technologies. Rene Sustain Energy Rev 94:1067–1085
Xiao W, Jiang X, Liu X, Zhou W, Garba ZN, Lawan I, Yuan Z (2021) Adsorption of organic dyes from wastewater by metal-doped porous carbon materials. J Clean Prod 284:124773
Guo D, Xiao Y, Li T, Zhou Q, Shen L, Li R, Lin H (2020) Fabrication of high-performance composite nanofiltration membranes for dye wastewater treatment: mussel-inspired layer-by-layer self-assembly. J Colloid Inter Sci 560:273–283
Dewangan K, Singh D, Satpute N, Singh R, Jaiswal A, Shrivas K, Bahadur I (2022) Hydrothermally grown α-MoO3 microfibers for photocatalytic degradation of methylene blue dye. J Mol Liq 349:118202
Chanikya P, Nidheesh PV, Babu DS, Gopinath A, Kumar MS (2020) Treatment of dyeing wastewater by combined sulfate radical based electrochemical advanced oxidation and electrocoagulation processes. Sep Purif Technol 254:117570
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Inter Sci 209:172–184
Wong S, Ngadi N, Inuwa IM, Hassan O (2018) Recent advances in applications of activated carbon from biowaste for wastewater treatment: a short review. J Clean Prod 175:361–375
Nor NM, Lau LC, Lee KT, Mohamed AR (2013) Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—a review. J Environ Chem Eng 1(4):658–666
Pitchay T, Jawad AH, Johari IS, Sabar S (2022) Kinetics studies of metallic ions adsorption by immobilised chitosan. Sci Lett 16(1):137–148
Abdalla TH, Nasr AS, Bassioni G, Harding DR, Kandile NG (2022) Fabrication of sustainable hydrogels-based chitosan Schiff base and their potential applications. Arab J Chem 15(1):103511
Panda PK, Yang JM, Chang YH (2021) Preparation and characterization of ferulic acid-modified water soluble chitosan and poly (γ-glutamic acid) polyelectrolyte films through layer-by-layer assembly towards protein adsorption. Int J Biol Macromol 171:457–464
Fan XD, Zhang XK (2013) Simultaneous removal of SO2 and NO with activated carbon from sewage sludge modified by chitosan. Appl Mech Mater 253:960–964
Ali HE, Nasef SM, Gad YH (2022) Remediation of Astrazon blue and Lerui acid brilliant blue dyes from waste solutions using amphoteric superparamagnetic nanocomposite hydrogels based on chitosan prepared by gamma rays. Carbohydr Polym 283:119149
Jawad AH, Rangabhashiyam S, Abdulhameed AS, Syed-Hassan SSA, Alothman ZA, Wilson LD (2022) Process optimization and adsorptive mechanism for reactive blue 19 dye by magnetic crosslinked chitosan/MgO/Fe3O4 biocomposite. J Polym Environ 30(7):2759–2773
Mohammadi E, Daraei H, Ghanbari R, Athar SD, Zandsalimi Y, Ziaee A, Yetilmezsoy K (2019) Synthesis of carboxylated chitosan modified with ferromagnetic nanoparticles for adsorptive removal of fluoride, nitrate, and phosphate anions from aqueous solutions. J Mol Liq 273:116–124
Jawad AH, Abdulhameed AS, Selvasembian R, Alothman ZA, Wilson LD (2022) Magnetic biohybrid chitosan-ethylene glycol diglycidyl ether/magnesium oxide/Fe3O4 nanocomposite for textile dye removal: Box-Behnken design optimization and mechanism study. J Polym Res 29(5):1–15
Vela N, Calín M, Yáñez-Gascón MJ, Garrido I, Pérez-Lucas G, Fenoll J, Navarro S (2018) Photocatalytic oxidation of six pesticides listed as endocrine disruptor chemicals from wastewater using two different TiO2 samples at pilot plant scale under sunlight irradiation. J Photochem Photobiol A Chem 353:271–278
Goldstein S, Behar D, Rabani J (2008) Mechanism of visible light photocatalytic oxidation of methanol in aerated aqueous suspensions of carbon-doped TiO2. J Phys Chem C 112(39):15134–15139
Shehzad H, Ahmed E, Sharif A, Farooqi ZH, Din MI, Begum R, Nawaz I (2022) Modified alginate-chitosan-TiO2 composites for adsorptive removal of Ni (II) ions from aqueous medium. Int J Boil Macromol 194:117–127
Cui HF, Wu WW, Li MM, Song X, Lv Y, Zhang TT (2018) A highly stable acetylcholinesterase biosensor based on chitosan-TiO2-graphene nanocomposites for detection of organophosphate pesticides. Biosens Bioelectron 99:223–229
Qu L, Chen G, Dong S, Huo Y, Yin Z, Li S, Chen Y (2019) Improved mechanical and antimicrobial properties of zein/chitosan films by adding highly dispersed nano-TiO2. Ind Crops Prod 130:450–458
Rasoulzadeh H, Motesaddi Zarandi S, Massoudinejad M, Amini MM, Sheikhmohammadi A (2021) Investigation into the influencing factors and adsorption characteristics in the effective capture of carbon dioxide in flue gas by chitosan grafted Leca biocomposite. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.2004408
Zhang W, Huang T, Ren Y, Wang Y, Yu R, Wang J, Tu Q (2021) Preparation of chitosan crosslinked with metal-organic framework (MOF-199)@ aminated graphene oxide aerogel for the adsorption of formaldehyde gas and methyl orange. Int J Biol Macromol 193:2243–2251
Aboushouk MI, El-Zomrawy AA, Salem AM (2022) Electrochemical detection of H2S gas based on chitosan extracted from shrimp shells loaded cadmium ions. Egypt J Chem 65(5):185–192
Zhao J, Xi X, Ouyang H, Yang J, Wang Y, Yi L, Zhao L (2021) Acidic and alkaline gas sensitive and self-healing chitosan aerogel based on electrostatic interaction. Carbohydr Polym 272:118445
Bayram A, Özbek C, Şenel M, Okur S (2017) CO gas sorption properties of ferrocene branched chitosan derivatives. Sen Actuators B: Chem 241:308–313
Abdulhameed AS, Mohammad AT, Jawad AH (2019) Application of response surface methodology for enhanced synthesis of chitosan tripolyphosphate/TiO2 nanocomposite and adsorption of reactive orange 16 dye. J Clean Prod 232:43–56
Garg VK, Kumar R, Gupta R (2004) Removal of malachite green dye from aqueous solution by adsorption using agro-industry waste: a case study of Prosopis cineraria. Dyes Pigm 62(1):1–10
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of reactive blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J Magn Magn Mater 404:179–189
Mohammad AT, Abdulhameed AS, Jawad AH (2019) Box-Behnken design to optimize the synthesis of new crosslinked chitosan-glyoxal/TiO2 nanocomposite: methyl orange adsorption and mechanism studies. Int J Biol Macromol 129:98–109
Mostafa MH, Elsawy MA, Darwish MS, Hussein LI, Abdaleem AH (2020) Microwave-assisted preparation of Chitosan/ZnO nanocomposite and its application in dye removal. Mater Chem Phys 248:122914
Noorimotlagh Z, Kazeminezhad I, Jaafarzadeh N, Ahmadi M, Ramezani Z, Martinez SS (2018) The visible-light photodegradation of nonylphenol in the presence of carbon-doped TiO2 with rutile/anatase ratio coated on GAC: effect of parameters and degradation mechanism. J Hazard Mater 350:108–120
dos Santos JM, Pereira CR, Pinto LAA, Frantz T, Lima ÉC, Foletto EL, Dotto GL (2019) Synthesis of a novel CoFe2O4/chitosan magnetic composite for fast adsorption of indigotine blue dye. Carbohydr Polym 217:6–14
Melián EP, Díaz OG, Méndez AO, López CR, Suárez MN, Rodríguez JD, Peña JP (2013) Efficient and affordable hydrogen production by water photo-splitting using TiO2-based photocatalysts. Int J Hydro Energy 38(5):2144–2155
Jawad AH, Abdulhameed AS, Wilson LD, Hanafiah MAKM, Nawawi WI, Alothman ZA, Rizwan Khan M (2021) Fabrication of Schiff’s base chitosan-glutaraldehyde/activated charcoal composite for cationic dye removal: optimization using response surface methodology. J Polym Environ 29(9):2855–2868
Çatlıoğlu F, Akay S, Turunç E, Gözmen B, Anastopoulos I, Kayan B, Kalderis D (2021) Preparation and application of Fe-modified banana peel in the adsorption of methylene blue: process optimization using response surface methodology. Environ Nanotechnol Monit Manage 16:100517
Jawad AH, Abdulhameed AS, Bahrudin NN, Hum NNMF, Surip SN, Syed-Hassan SSA, Sabar S (2021) Microporous activated carbon developed from KOH activated biomass waste: surface mechanistic study of methylene blue dye adsorption. Water Sci Technol 84(8):1858–1872
Langhammer D, Kullgren J, Mitev P, Österlund L (2018) SO2 adsorption on rutile TiO2 (110): An infrared reflection-absorption spectroscopy and density functional theory study. Surf Sci 677:46–51
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Vet Akad Handl 24:1–39
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
de Souza APN, Licea YE, Colaço MV, Senra JD, Carvalho NM (2021) Green iron oxides/amino-functionalized MCM-41 composites as adsorbent for anionic azo dye: kinetic and isotherm studies. J Environ Chem Eng 9(2):105062
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Frenudlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:327–356
Salem MA, Khan AM, Manea YK, Wani AA (2022) Nano chromium embedded in f-CNT supported CoBi-LDH nanocomposites for selective adsorption of Pb2+ and hazardous organic dyes. Chemosphere 289:133073
Hussain S, Kamran M, Khan SA, Shaheen K, Shah Z, Suo H, Ghani U (2021) Adsorption, kinetics and thermodynamics studies of methyl orange dye sequestration through chitosan composites films. Int J Boil Macromol 168:383–394
Sriram G, Bendre A, Altalhi T, Jung HY, Hegde G, Kurkuri M (2022) Surface engineering of silica based materials with Ni–Fe layered double hydroxide for the efficient removal of methyl orange: Isotherms, kinetics, mechanism and high selectivity studies. Chemosphere 287:131976
Niu C, Zhang N, Hu C, Zhang C, Zhang H, Xing Y (2021) Preparation of a novel citric acid-crosslinked Zn-MOF/chitosan composite and application in adsorption of chromium (VI) and methyl orange from aqueous solution. Carbohydr Polym 258:117644
Verma M, Tyagi I, Kumar V, Goel S, Vaya D, Kim H (2021) Fabrication of GO-MnO2 nanocomposite using hydrothermal process for cationic and anionic dyes adsorption: kinetics, isotherm, and reusability. J Environ Chem Eng 9(5):106045
Zhu W, Jiang X, Liu F, You F, Yao C (2020) Preparation of chitosan-graphene oxide composite aerogel by hydrothermal method and its adsorption property of methyl orange. Polymer 12(9):2169
Allouche FN, Yassaa N, Lounici H (2015) Sorption of methyl orange from aqueous solution on chitosan bomass. Proc Earth Planet Sci 15:596–601
Kim TY, Lee JW, Cho SY (2015) Application of residual brewery yeast for adsorption removal of Reactive Orange 16 from aqueous solution. Adv Powder Technol 26(1):267–274
Ramachandran P, Vairamuthu R, Ponnusamy S (2011) Adsorption isotherms, kinetics, thermodynamics and desorption studies of reactive Orange 16 on activated carbon derived from Ananas comosus (L.) carbon. J Eng Appl Sci 6(11):15–26
Abd Malek NN, Jawad AH, Ismail K, Razuan R, Alothman ZA (2021) Fly ash modified magnetic chitosan-polyvinyl alcohol blend for reactive orange 16 dye removal: adsorption parametric optimization. Int J Boil Macromol 189:464–476
Abd Malek NN, Jawad AH, Abdulhameed AS, Ismail K, Hameed BH (2020) New magnetic Schiff’s base-chitosan-glyoxal/fly ash/Fe3O4 biocomposite for the removal of anionic azo dye: an optimized process. Int J Biol Macromol 146:530–539
Jawad AH, Malek NNA, Abdulhameed AS, Razuan R (2020) Synthesis of magnetic chitosan-fly ash/Fe3O4 composite for adsorption of reactive orange 16 dye: optimization by Box–Behnken design. J Polym Environ 28(3):1068–1082
Ecer Ü, Zengin A, Şahan T (2021) Magnetic clay\zeolitic imidazole framework nanocomposite (ZIF-8@ Fe3O4@ BNT) for reactive orange 16 removal from liquid media. Colloids Surf A 630:127558
Singh SK, Das A (2015) The n → π* interaction: a rapidly emerging non-covalent interaction. Phys Chem Chem Phys 17(15):9596–9612
Acknowledgements
The authors would like to acknowledge Faculty of Applied Sciences, Universiti Teknologi MARA, Shah Alam, for facilitating this research work. The authors would like to thank Universiti Teknologi MARA (UiTM) and Universitas Indonesia (UI) for supporting this research project by UiTM-UI Strategic Research Partnership grants with grants numbers 100-RMC 5/3/SRP 052/2021 (UiTM) and NKB-674/UN2.RST/HKP.05.00/2021 (UI).
Funding
This research project is funded by the Universiti Teknologi MARA (UiTM) and Universitas Indonesia (UI) under UiTM-UI Strategic Research Partnership grants with grants numbers 100-RMC 5/3/SRP 052/2021 (UiTM) and NKB-674/UN2.RST/HKP.05.00/2021 (UI).
Author information
Authors and Affiliations
Contributions
ASA: Formal analysis, Validation, Data Curation, Writing—Original; AHJ: Project administration, Formal analysis, Validation, Data Curation, Writing—Original, Validation, funding acquisition, Project administration; MR: Project administration, Conceptualization, funding acquisition. TK: Validation, Data Curation. LDW: Writing—Review & Editing. ZMY: Formal analysis, Validation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdulhameed, A.S., Jawad, A.H., Ridwan, M. et al. Chitosan/Carbon-Doped TiO2 Composite for Adsorption of Two Anionic Dyes in Solution and Gaseous SO2 Capture: Experimental Modeling and Optimization. J Polym Environ 30, 4619–4636 (2022). https://doi.org/10.1007/s10924-022-02532-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02532-z