Abstract
There is growing concern about developing treatment technologies for the hazardous Polycyclic Aromatic Hydrocarbons (PAHs), because the rising levels of these compounds in the environment by human activities. The application of laccases has been evaluated as one of the most promising treatments. Thus, laccase immobilization on polyurethane foam (PUF)—low-cost material—was evaluated for bioremediation in batch mode of simulated groundwater using a combination of 16 polycyclic aromatic hydrocarbons as model pollutants. Conditions closer to a real contaminated site (non-optimal) were considered on our experimental design, leading to the formation of new degradation intermediaries, even more degraded than the usual ones. The bioremediation of PAH (mg L−1) using immobilized laccase on PUF reached 92.35% of removal for anthracene (Ant) and 97% for benzo(a)pyrene (BaP). After the treatment, the biodegradation products were identified as diisooctyl phthalate and tetradecane. The biodegradation mechanism was proposed, where PAHs oxidation processes and aromatic ring fission led to quinone and diethyl phthalate formation. Then, through the latter processes besides, polymerization and methylation, lead to the identified biodegradation product formation. The immobilized enzyme improvement in the removal yield of 8 of the other 14 PAHs tested in μg L−1, compared to the free counterpart. Laccase immobilized on PUF achieved final anthracene concentration of 0.95 mg L−1, up to 38 μg L−1 of chrysene (77% removal) and 98 μg L−1 of pyrene (32% removal), under the intervention limits of environmental protection policies. Thus, laccase immobilized on PUF for use in bioreactors can be considered a potential approach for PAHs bioremediation for an ex-situ treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The contamination of groundwater, surface water, and drinking water by Polycyclic Aromatic Hydrocarbons (PAHs) is one of significant concern worldwide because of high ecological risks and their potential carcinogens to humans [1,2,3]. Due to these effects, the U.S. Environmental Protection Agency (USEPA) has listed 16 PAHs as priority pollutants [4]. In urban areas, processes that use fossil oil and fuels are the potentially polluting activities, such as gas stations, steel making, or coking plants of petroleum drilling and other activities [5]. It is common to observe in PAHs contaminated sites the occurrence of leaks, spills, floor, soil infiltration and release of contaminants, through infiltration and dispersion in the soil, down to lower layers of soil and groundwater [5,6,7]. Each country has its own protection and clean-up policy, which provides PAHs concentration limits, guiding the remediation management of contaminated sites (Table 1).
PAHs site remediation needs combined and integrated techniques to achieve the clean-up goals in soil and groundwater. Treatments with PAHs contaminated soils samples in batch reactors have been studied as remediation purposes, which in practical terms would need soil excavation [8,9,10]. Nevertheless, soil excavation had been carried out at a majority of PAH contaminated sites until the 1990s, and due to high cost has been replaced from others techniques. For instance, the integrated technologies [5], PAHs are transferred from soils into the aqueous phase in groundwater by surfactant flushing and soil washing, known as surfactant-enhanced remediation (SER) [7]. Once released into groundwater, PAHs can be degraded by some treatment technologies such as pump-and-treat system (ex-situ), in-situ bioremediation, or in-situ chemical oxidation (ISCO) [11]. For PAHs removal from water, electrochemical and advanced oxidation processes were also studied [12, 13]; and phytoremediation in the field of sediment bioremediation [14].
The bioremediation by enzymes, as laccases for PAHs oxidation [6, 15, 16] is a current environmental trend. Laccases are well-known for their ability to degrade organic compounds [17,18,19,20,21]. Laccases have also been reported to degrade PAHs [22,23,24] in the presence or absence of mediators, such as 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) and 1-hidroxybenzothyazole (HBT) [25,26,27,28]. There is no consensus on the use of mediators, or in the best mediators should be used. However, there is a lack in the literature that must be explored, evaluating PAHs degradation by laccases without high-cost mediator and in non-optimal pH experiment conditions.
The optimal pH range of laccases is among 4 and 5, which also is the condition where laccase has high stability [25, 29]. However, the observed pH range in groundwater detected in other studies usually is a more neutral range (pH 6 to 7) [30, 31]. Thus, the immobilization on solid materials is known as a promising technology [32] which could improve enzyme stability, specially related to pH; increase the reuse potential and also the lifetime; and reduce the required amount of enzyme [33]. Laccase immobilized on different supports has been developed for degradation of many organic compounds. The immobilization in kaolinite led to improvement on the stability at temperature, pH, inhibitors, and storage [34]. Immobilization on silica caused better pH and temperature stability, however degradation yields were slightly lower than those for free laccase [35, 36]. Polyacrylonitrile beads were used as support to immobilize laccase, showing complete degradation of nonylphenol and octylphenol [37]. One novel synthetic polymer, poly(glycidyl methacrylate), was developed as laccase support, and 90% of bisphenol A and 100% of Congo Red dye was removed in batch experiments [19]. These synthetic organic polymers used as support to immobilize laccase do have hydrophobic character, and high standard activities have been shown onto biodegradation of organic compounds.
Based on synthetic organic polymers' described properties, commercial polyurethane foam (PUF) has been shown as efficient support of other enzymes. In the case of lipases, properties as stability in pH and temperature conditions were enhanced by PUF immobilization [38, 39]. Results showed high removal capability of nonylphenol polyethoxylates by laccase from T. versicolor immobilized on PUF, due to biodegradation and adsorption on the support [40]. In this sense, the use of PUF, a low-cost material, as support for laccase immobilization can be a potential to make efficient PAHs degradation under non-optimal conditions without expensive mediators.
It is also expected to make a current trend possible to field application, considering practical aspects and environmental protection policies. Despite the variation in each protection policy, some details caught our attention, as concentration range, in μg L−1 for groundwater (Table 1). Besides, most of the state of art studies of Table 2 have been worked in an aqueous medium, presence of mediator ABTS and optimal pH conditions (range between 4 and 5); they were also evaluated in mg L−1 concentration scale. On real contaminated site remediation, high PAHs removal efficiency obtained in mg L−1 scale could not be sufficient to achieve safe concentration to protect human health and environment protection, since guide values are set to μg L−1 instead of mg L−1. In these cases, the contaminated site probably would need another complementary treatment to achieve clean-up remediation goals.
Thus, the aim of the present study was to evaluate biodegradation of 16 PAHs by T. versicolor laccase immobilized on commercial polyurethane in a model solution and a groundwater sample. Different PAHs concentration scales were used in the laboratory in batch assays, without any oxidation mediator compound, in pH and temperature values close to the reality of a contaminated site. Anthracene and benzo(a)pyrene were used as model PAH for products of laccase-transformation identification.
Materials and Methods
Chemicals
Anthracene (Ant), benzo(a)pyrene (BaP), naphtalene (Nap), ABTS, Tween 80 and laccase from Trametes versicolor (activity of 0.5 U mg−1) (Sigma-Aldrich Brazil). Acetone, acetonitrile, dichloromethane and the other chemicals were purchased from (Merck Brazil). Polymeric methylene diphenyl diisocyanate (pMDI—specflex NE 134) and commercial polyol were kindly donated by Dow Chemistry.
Laccase Immobilization on Commercial Polyurethane Foam
Laccase from T. versicolor was immobilized in-situ with commercial reagents of polyurethane foam (PUF) based on Daronch [41]. The amount of enzyme was investigated for enhanced performance and fixed in this work to 0.2 wt% (data not shown). 2 mg g−1 of powder laccase in Milli-Q was added to commercial polyol and mixed with the help of a glass stick. After that, methylene diphenyl diisocyanate was added and the expansion occurred after 60 s of mechanical stirring (2,500 rpm), using 77 g of NCO per 100 g OH, according supplier instructions. The immobilized laccase was stored at 4 °C for further use.
Enzyme Activity, PAHs Determination and Statistical Analysis
The activities of the free and immobilized laccase were determined by enzymatic oxidation rate of ABTS to ABTS+ [20, 42]. For determination of the free laccase activity 0.3 mL of a laccase solution (1 mg mL−1) and 0.3 mL of aqueous ABTS (5 mM) were added to 2.4 mL of phosphate-citrate buffer (0.1 M, pH 3) in a quartz cuvette. A sample of 0.3 mL of Milli-Q water was used instead as blank.
For determination of immobilized laccase activity, a piece of foam (about 5 × 5 × 5 mm in size and 15 mg in weight) was added into 0.4 mL of 5 mM ABTS solution and 3.6 mL phosphate-citrate buffer solution (pH 3). The reaction occurred incubated at 30 °C and 250 rpm for 5 min [41]. A change in absorbance at 420 nm was monitored using a UV/Vis spectrophotometer (HACH, DR5000) and the laccase activity (U L−1) was calculated using the molar extinction coefficient of ABTS (ε420 = 36,000 M−1 cm−1) using the Eq. 1:
where ΔA is the increment of absorbance per min when it is stable, V is the total reaction volume, d is the step length, v is the sample volume and t: reaction time. One unit of laccase activity (U L−1) was defined as the amount of enzyme able to oxidize 1μmoL of substrate per minute [43]. All analysis was carried out in triplicate.
The PAHs were analyzed by a standardized gas chromatography/mass spectrometry procedure, using the recommended method by the USEPA for detection and measurements of organic pollutants in aquatic environments (USEPA method 8270). The analysis was carried out in Inova Laboratório e Engenharia (Laboratory and Engineering), whose quality assurance and analytical competence were evaluated by Supelco® quick turn proficiency test. The standard sample PE1173 from Sigma-Aldrich for PAHs was used on test, which is intended for water pollution/wastewater. A gas chromatography-mass spectrometer (Agilent 5975C, Agilent Inc., Palo Alto, USA) equipped with a HP-5MS (30 mm £ 0.25 mm) was used. The oven temperature was programmed from 100 °C with a 2-min hold and a 10 °C min−1 increment to 300 °C with a 10-min hold [44]. Sample containing PAHs (1.5 mL) was extracted three times using 1.5 mL of dichloromethane into a test-tube under agitation in a vortex mixer. After this step, the extract was evaporated at room temperature and redissolved in 1.5 mL acetone, adapted from USEPA method 8270.
The obtained ions in FULL (SCAN) mode of MS scan analysis were compared according to the similarity (m/z) of the data available in the National Institute of Standards and Technology library (NIST, 2009). The match factor values were used for compound identification based on qualities of the mass spectra determined by the NIST Match and Reverse Match (R. Match) factor methods [45].
Statistical evaluations were performed by one-way analysis of variance (ANOVA), and differences at a significant level p < 0.05 were considered.
Tests for PAHs Removal
The ability of immobilized laccase to remove anthracene, and benzo(a)pyrene in mg L−1 concentration range was investigated at three conditions; in buffer solution (pH 7), real groundwater sample (pH 6.82), and in the presence of a mixture of 14 PAH in μg L−1 concentration (PAHs mixture condition, pH 7.1). The same conditions were evaluated using free laccase, as shown in Fig. 1. Both PAHs in mg L−1 range were used as model PAH for biodegradation products of laccase-transformation identification and mechanism assessment. The removal of anthracene, and benzo(a)pyrene in mg L−1 and a mixture of 14 PAHs in μg L−1 were measured in PAH mixture condition. The behavior of laccase in front of different pH was investigated before and after the immobilization procedure using ABTS as substrate, and it was observed that the maximum activity values of the free laccase was at pH 3 and for immobilized enzyme was obtained at pH 4 (data not shown).
All conditions of batch removal experiments were performed in triplicate at 25 ± 2 °C in 125 mL Erlenmeyer flasks, with 20 mL reactants during five days in an orbital shaker. Surfactant Tween 80 (1% v/v) and PAHs dissolved in acetone was added to make the final concentrations 12.48 mg L−1 of Ant and 5.00 mg L−1 of BaP [44]. While in free laccase tests 150 U L−1 were used, in immobilized laccase tests ten pieces (about 5 × 5x5 mm in size and 20 mg in weight each), making 7 U L−1, were used. Initial laccase activity in tests using immobilized enzymes was lower than free laccase due to limitations on maximum supportable amounts, as enzymes in the support and lower specific activity. Thus, most of the previous study conditions of the group [44] were maintained, including 150 U L−1 in free laccase tests, however, agitation was increased to 250 rpm in all conditions due to make easy immobilized enzymes treatability.
Buffer condition was carried out in citrate–phosphate buffer (pH 7) and PAHs mixed condition in Municipal Potable Water (pH 7.1) with a standard solution containing others 14 PAHs dissolved in acetone, to make the final concentrations (μg L−1): naphthalene (Nap) 1000, acenaphthene (Ace) 426, acenaphthylene (Acy) 72, fluorene (Flu) 84, phenanthrene (Phe) 1000, fluoranthrene (Fla) 162, pyrene (Pyr) 144, benzo(a)anthracene (BaA) 150, chrysene (Chr) 165, benzo(b)fluoranthene (BbF) 131, benzo(k)fluoranthene (BkF) 60, dibenzo(a;h)anthracene (DaA) 88, benzo(g;h;i)perylene (BgP) 72 and indeno(1,2,3-cd)pyrene (IcP) 202.
The other test condition contained an unpolluted groundwater sample collected from an abandoned gas station area and stored at 4 °C until the use. The samples were initially passed through a vacuum filtration system (25 μm) to remove the sample's suspended solids. The following physic-chemical properties were read in-situ by HI 9828HANNA multiparameter probe (Hanna Instruments, Woonsocket, USA): 50 ORP (oxidation–reduction potential), 197.7 μSm−1 (conductivity), 25.3 NTU (turbidity) 20.5 °C (temperature) and pH 6.82. The observed properties in groundwater were similar to those detected in other studies [30, 31].
Tests for PAHs removal by free and immobilized laccase on PUF in 3 conditions (buffer pH 7, groundwater and PAHs mixture) were performed in triplicates for 120 h, with 1.5 mL of medium samples collected every 24 h for the GC–MS analysis.
Results and Discussion
Removal Capability of Ant and BaP in mg L−1
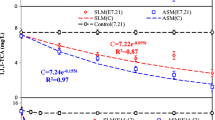
Although many studies suggest the importance of redox mediators in the oxidation of PAHs by laccases, this work pursues closer to field reality of groundwater conditions and avoid operating at high-cost conditions. Experiments were carried out in non-optimal conditions, without any mediator compound. Because of their higher capacity to be degraded by laccase, anthracene, and benzo(a)pyrene have been chosen as models PAHs for carrying out the mg L−1 concentration range. The removal efficiencies of PAHs reacting by free and immobilized laccase on PUF for 120 h, were determined at 24 h intervals in 3 experimental conditions: Buffer pH 7, PAHs mixture and Groundwater (Fig. 2).
Figure 2 shows a significant effect on both PAHs removal by immobilized laccase on PUF compared with free laccase. The results showed that BaP removal rate already reached the maximum after 24 h for all three conditions, and there were no significant differences after 48 h (p < 0.05). Maximal Ant removal by laccase on PUF was 81.6% in groundwater condition and 92.4% for simulated PAHs condition (PAHs mixture). The highest removal rate reached 97.1% of Ant and 99.2% of BaP by laccase on PUF in condition Buffer pH 7, where the final 0.952 mg L−1 achieved Ant concentration was lower than New Zealand guide values showed in Table 1 (1.000 mg L−1), and close to the Brazilian limit (900 mg L−1).
For all three conditions using free laccase, the remaining relative activity (120 h) was almost 77%, and the mediator compound's dependency was evidenced by the effects of BaP degradation, which may have negatively influenced. No significant elimination of BaP without ABTS has been related to other studies [25]. A positive percentage was detected after 96 h only in buffer pH 7 condition, where 16.55% of BaP removal by free enzyme was reached; but lower than obtained in our previous work, which was above 30% [44]. After 120 h in all three tested conditions, an average of 35% removal of Ant, without significant difference among then. Theses Ant removal percentages by free laccase were lower than those reported in other studies observed without mediators [35, 46]. However, the above-mentioned works operate at adjusted optimal pH conditions (range between 4 and 5) where laccase have high activity. Under neutral pH range between 6.8 and 7, as in this work, anthracene degradation by free laccase presents a different behavior, as the presence of several compounds in medium (groundwater sample and PAHs mixture condition) enhanced the removal, the presence of citrate and phosphate ions of buffer solution affected negatively Ant removal until 96 h.
The presence of other compounds in both other conditions, PAHs mixture and groundwater, seems to also decrease both free and immobilized laccase activity. While 47% of free laccase relative activity was observed in groundwater condition, only 26% of remaining relative laccase activity were detected for laccase on PUF in groundwater and PAHs mixture; which was the same apply for free laccase in PAHs mixture. However, compared with Fig. 2, PAHs removal yields by free and immobilized laccase had slight relation to laccase activity, although significant, as observed by Wu et al. [28].
Furthermore, in the first 24 h, almost all benzo(a)pyrene was removed by immobilized laccase at all conditions. In contrast, anthracene removal was 73.1% in buffer pH 7 condition and approximately 50% in the other two, which could be associated with the ring number of the PAH. Some authors [47] also observed higher removal of 5 and 6 rings PAHs, more than 95%, in comparison to 3 rings PAHs, more than 80% for Ant using immobilized laccase, attributed to the support adsorption. The removal associated to adsorption will be investigated after biodegradation product identification as product identification of anthracene and benzo(a)pyrene biodegradation.
Removal Capability of the 14 PAHs Mixture in μg L−1
The degradation of simulated PAHs which are listed as priority pollutants were also evaluated in condition PAHs mixture but μg L−1 range. The purpose here was to observe their influence on the transformation of benzo(a)pyrene and anthracene, which likely occurs due to competitiveness and inhibition of the enzyme metabolism [23, 48]. According to some authors listed in state of the art, laccase also has the capability to degrade other PAHs, besides Ant and BaP, and were included them in the medium in μg L−1 range to evaluate their removal.
Guidelines for assessing and managing the contaminated site of PAHs fixed limits in μg L−1 for groundwater contaminants. Usually, these limits are different according to each country and policy decisions regarding tolerable levels of risk for the derivation of groundwater acceptance criteria, as shown in Table 1. In this direction, it is applicable to work in μg L−1 range when the objective is to envision and associate the under developing process to applications in the remediation of contaminated PAHs sites.
In the present study, 7 of 14 PAHs tested were degraded, achieving more than 50%, by free laccase, mainly naphthalene (100%) and phenanthrene (85.8%). As shown in Fig. 3, the results were positive, since 8 of 14 compounds had the removal percentage enhanced when immobilized laccase was used, when compared to free laccase. Removal of 53.6% of BaA by free laccase and 68.8% of BbF by laccase on PUF were consistent with other previous results, also operating without mediators using free laccase as well, but at pH 4.2 [46]. Immobilized laccase removed 77.3% of chrysene up to 34 μg L−1, and 32.1% of pyrene up to 98 μg L−1, whose final concentration values are lower than acceptance criteria of Brazilian [49] and New Zealand [50] law, respectively.
Identification of Ant and BaP Biodegradation Products
The oxidation of PAHs by laccase has been reported, and the oxidation products were detected by gas chromatography-mass spectrometry (GC–MS) analysis. Our results showed that Ant and BaP removal increased in experiments with laccase immobilized in PU foam compared to free laccase, in all three evaluated conditions. The condition of free laccase in pH 7 buffer solution in time 96 h was chosen to detect what compounds where formed by degradation of both PAHs. Chromatogram were indicated in Fig. 4, containing peaks of anthracene and benzo(a)pyrene biodegradation by free enzyme.
The biodegradation products observed after removing of anthracene and benzo(a)pyrene by free and immobilized laccase were evaluated by match quality test after GC–MS scan analysis. The mass spectra (m/z) of the formed products in the chromatograms were compared with the National Institute of Standards and Technology library data (NIST, 2009). After this procedure, based on Koo et al. method [45], identified compounds with fair and poor match factors values were eliminated. Table 3 shows some parameters for 1,2 benzenedicarboxylic acid diisooctyl ester (diisooctyl phthalate) and tetradecane, which were identified as Ant and BaP biodegradation products, obtained after GC–MS scan analysis and match quality test.
However, other possible pathways have also been proposed based on biodegradation products of PAHs by several microorganisms and enzymes. Although these specifically studies were not found quinones, many of the biodegradation products identified here have previously been identified in other studies. Quin et al. [51] have proposed one pathway for biodegradation of BaP, where pyrene and phenanthrene were identified as biodegradation products, whose successive loss of aromatic ring from BaP to Phe happens similarly. On the basis of identified biodegradation products after GC–MS, the degradation pathway for anthracene and benzo(a)pyrene was proposed. This possible pathway is represented in Fig. 5.
As shown in Fig. 5, the laccase oxidation of anthracene occurs in 9th and 10th carbons, resulting in anthraquinone (anthracene-9,10-dione) [27, 36, 52]. After the oxidation and ring fission of this biodegradation product, diethyl phthalate is formed, and subsequently, phthalate acid [53, 54]. The laccase degradation of benzo(a)pyrene results in the oxidation and ring fission, resulting in pyrene [51]. After that, the pyrene is oxidized and the ring fission occurs, forming diethyl phthalate, a common biodegradation product of Ant and BaP degraded, probably due to the similarity on molecule structure and ionization potential of anthracene and benzo(a)pyrene [25, 51]. The pyrene degradation can also be followed by the formation of benzene ethanol, that is also oxidized by laccase and may lead to ring fission, polymerization, and methylation, forming aliphatic compounds, pathway similar to benzo(a)anthracene’s biodegradation [55, 56]. Once laccase oxidation of compounds starts by forming a radical, a wide variety of compounds may be formed [57, 58].
Once the pathway was proposed, the abundance peaks of biodegradation products were considered (Table 3) in condition buffer pH 7 by free laccase, where 16.55% of BaP was degraded; thus, to confirm of the occurrence of BaP (98.7%) biodegradation by immobilized laccase on PUF under the same condition. While abundances of approximately 719,079 of Tetradecane (III) and 9302 for diisooctyl phthalate were detected in samples treated with immobilized laccase, for free laccase peaks of 85,525 and 20,472 (Table 3), respectively, were detected for those compounds. This result is a positive indicator for the occurrence of BaP degradation by immobilized laccase, and also Ant, according to the purposed pathway. At this pH range between 6 and 7, free laccase is known for having lower PAH’s oxidation yield, specifically for BaP [27, 47]. However, these factors were not have observed for BaP biodegradation through immobilized laccase, not even in the absence of mediators and presence of other substances (biodegradation products) that could compete or have priority of laccase metabolism.
Besides biodegradation another effect could also influence the BaP and Ant removal rates by laccase on PUF. Niu et al. [47] noted an improvement in PAHs removal when they used encapsulated laccase in loading spider type reactor fabricated by poly(d,l-lactide-co-glycolide). Their results showed that higher rates were achieved by PAHs degradation combined with the adsorption onto the surface of the fibers. The same effect linked to adsorption influence was observed in the removal of nonylphenol polyethoxylates by immobilized laccase on PUF, in continuous flow circular bioreactor, due to the hydrophobic character of PUF [40]. In addition to biodegradation, this adsorption phenomenon probably influenced PAHs removal by immobilized laccase in our experiments but in a lighter way.
Feasible Perspectives on Bioremediation of PAH’s by Immobilized Laccase on PUF
The use of laccase for PAHs degradation has been studied in the presence of expensive mediators such as ABTS, on controled pH, and temperature conditions, for bioremediation purposes (Table 2). The significant challenge for make still study bioremediation processes able in the future for on-field application is to consider some issues associated with practical aspects, process cost and attendance to environmental protection policies. Therefore, these are exactly the considerations and care adopted in this study that proposed a potential bioremediation strategy of polycyclic aromatic hydrocarbons by immobilized laccase on PUF.
The protection and cleanup policies of PAHs contaminated sites sets concentration limits and intervention values in μg L−1 range for groundwater [49, 50, 59, 60]. These could be one essential issue that had not considered in most studies of PAHs degradation by laccase when evaluated in model solutions in mg L−1 range when the legal limits were disposed of in μg L−1 range for groundwater. Contextualizing our results with safe groundwater levels for human health protection and resource conservation, the final concentration of anthracene in mg L−1 range, chrysene and pyrene in μg L−1 range was detected lower than those listed in Table 1. Although it was obtained higher removal yields of BaP by laccase on PUF, the final concentration achieved was insufficient to reduce to values listed in protection and cleanup policies for groundwater. Thus, in this case another treatment must be necessary to reduce until the legal limits of legislation.
For those cases in practical terms, the process must be divided into two treatment phases. In the first phase most PAHs are removed and in the second phase the residue is polished and removed until acceptable policy concentration. In bioremediation, it is common to use integrated technology with surfactant enhanced or chemical oxidation treatment to achieve the cleanup objectives [11, 61]. Soil excavation has been done at major PAH contaminated sites until the 1990s. However, soil flushing (in-situ) and surfactant enhanced technology has replaced this outdated technique in the soil PAHs contaminated sites. Once PAHs are transferred from soil to groundwater, treatment can happen in-situ or ex-situ. Pump-and-treat is a known technique where the removal of PAHs from groundwater could occur in aqueous medium from the installation of pumping wells and external reactor for biotransformation (ex-situ).
The results of our study demonstrated that in the first 24 h, almost all Ant and BaP (mg L−1) were removed by immobilized laccase in PUF from the medium in all three conditions. However, the adsorption onto the support's surface, similarly observed in the laccase reverse micelles system [47] and immobilized on PUF [40], could also be occurred. Even though the hydrophobic character of the PUF surface could influence PAHs adsorption, that probably happened; higher peaks of identifying Ant and BaP metabolites also were good indicators that there had been much biodegradation.
So, if possibly adsorption into PUF could have more influence on PAH removal, one question could be made, could not be applied PUF directly without enzyme immobilization? Although the adsorption kinetics and mechanisms were not objected to investigation in this work, those influences deserve to be briefly commented on and discussed, aiming for future studies and possibilities of application in remediation processes.
PUF is a useful adsorbent of chemicals present in oils and fuels, such as petroleum hydrocarbons and other organic compounds. Some PUF sponges have been developed using commercial materials for oil-spill remediation. According to the authors, PUF is an environmentally benign, low-cost, scalable design of efficient and reusable oil sorbents [62]. Other recent research observed 100% removal of two and three rings PAHs by PUF, include anthracene, however, only 63,8% of chrysene (four rings) was removed [63]. Most actually study, which also worked with immobilized laccase in PUF, revealed that PUF alone is an excellent adsorbent of nonylphenol polyethoxylates [40].
Considering that PUF is widely used as an insulation and lining material for refrigerators, after the product's lifetime the PUF waste turn is a problem related to waste management because they are considered toxic waste due to toxic flame-retardant chemicals [64, 65]. That must have the correct destination by landfilling, recycling, or incineration [66]. On the other hand, commercially PUF has been related as an efficient adsorbent of PAHs and other petroleum hydrocarbons, as reported in the literature. Considering that PUF waste use to be designated to industrial landfilling in under developing countries, these could be a potential waste material for reuse application as adsorbent. Thus, PUF with more PAHs adsorbed onto the surface, a reuse application could be made, and nothing will change thinking about the waste destination to landfilling. Indeed, the adsorbent potential of PUF waste must be studied, if maybe it could be used in a first phase treatment for remediation purposes.
In this work, a new perspective based on pump-and-treat and biotransformation was brought, which could be applied in the second phase treatment, where the residue is polished and removed until acceptable policy concentration. After the analysis of GC–MS of buffer condition with free laccase, two Ant and BaP biodegradation products were identified, and the degradation mechanism was proposed. The abundance values of those biodegradation products were recorded and associated with the percentage of removal of the respective PAH by free laccase. In this sense, higher amount of identified biodegradation product of BaP (tetradecane) was formed by immobilized than free laccase, based on abundance peaks.
Based on our biodegradation products’ analysis by free and immobilized laccase in non-optimal pH range without expensive mediator ABTS (> $ 65 g−1 – Sigma-Aldrich) we could verify that in batch experiments with vigorous agitation, PAHs degradation was observed.. Ant, Chr and Pyr were achieved safe levels for groundwater protection in this work, in practical terms of contaminated area management, for those three PAHs specifically on remediation level, could be considered achieved the cleanup goal, the remediation may be stopped and could initiate the monitoring level until rehabilitee. The challenge for feasible this ex-situ bioremediation process in the future on-field applications is to evaluate if using commercial laccases (≈ $ 2 kg−1—Alibaba); the obtained results will be maintained. The expectation is that process cost can be reduced, eliminating high-cost mediators, chemicals, and enzymes, upon using low-cost support for enzyme immobilization. Indeed, the first step has been taken in this direction.
Conclusions
For any PAHs contaminated site cleanup, costs have played an essential role in selecting site remedial alternatives. However, PAHs treatment by laccase evaluated in controlled conditions with high-cost reagents (ABTS and pure enzymes) are in the reverse path. Combination of bioprocess with commercial materials could reduce significantly remediation cost. However, efficiency must be tested and approved, before application in the field. Laccase immobilized on commercial polyurethane foam was evaluated under non optimal conditions without mediator to remove PAHs and its potential for practical bioremediation application. Shortly reviewing state of the art and environmental policies led us to realize that PAHs' range concentration is an essential factor and must be considered. In comparison with free laccase, was achieved high removal of anthracene and benzo(a)pyrene in mg L−1 range by immobilized laccase on PUF and a brief increase of others 8 PAHs removal in μg L−1 range. The prospective biodegradation mechanism was proposed based on the match quality analysis of formed diisooctyl phthalate and tetradecane. The comparison of the biodegradation products abundance peaks with percentage Ant and Bap removal, allowed us to conclude that more degradation happened. In contrast to protection and cleanup policies, anthracene in mg L−1 range, chrysene and pyrene in μg L−1 range were reduced in the medium under safe concentration, lower than the intervention values of protection policies. It was positive in the continuous development of a viable technique closer to field application so that a new approach ex-situ bioremediation was brought based on pump-and-treat and biotransformation. The cost has already reduced, eliminating high-cost mediators and other chemicals for controlled conditions, using low-cost support for enzyme immobilization. Finally, this process's last challenge is to evaluate if using commercial laccases (E.g. ≈ $ 2 kg−1 laccase powder from Alibaba supplier); the obtained results will be maintained being able for field application.
References
Abdel-Shafy HI, Mansour MSM (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Petrol 25:107. https://doi.org/10.1016/j.ejpe.2015.03.011
Eom SY, Yim DH, Moon SI, Youn JW, Kwon HJ, Oh HC, Yang JJ, Park SK, Yoo KY, Kim HS, Lee KS, Chang SH, Kim YD, Kang JW, Kim H (2013) Polycyclic aromatic hydrocarbon-induced oxidative stress, antioxidant capacity, and the risk of lung cancer: a pilot nested case-control study. Anticancer Res 33:3089
Ball A, Truskewycz A (2013) Polyaromatic hydrocarbon exposure: an ecological impact ambiguity. Environ Sci Pollut Res Int 20:4311. https://doi.org/10.1007/s11356-013-1620-2
Llobet JM, Falcó G, Bocio A, Domingo JL (2006) Exposure to polycyclic aromatic hydrocarbons through consumption of edible marine species in Catalonia. Spain J Food Prot 69:2493. https://doi.org/10.4315/0362-028X-69.10.2493
Kuppusamy S, Thavamani P, Venkateswarlu K, Lee YB, Naidu R, Megharaj M (2017) Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: technological constraints emerging trends and future directions. Chemosphere 168:944. https://doi.org/10.1016/j.chemosphere.2016.10.115
Liang X, Guo C, Liao C, Liu S, Wick LY, Peng D, Yi X, Lu G, Lin Z, Dang Z (2017) Drivers and applications of integrated clean-up technologies for surfactant-enhanced remediation of environments contaminated with polycyclic aromatic hydrocarbons (PAHs). Environ Pollut 225:129. https://doi.org/10.1016/j.envpol.2017.03.045
Sun Y, Zhang S, Lan J, Xie Z, Pu J, Yuan D, Yang H, Xing B (2019) Vertical migration from surface soils to groundwater and source appointment of polycyclic aromatic hydrocarbons in epikarst spring systems, southwest China. Chemosphere 230:616. https://doi.org/10.1016/j.chemosphere.2019.05.007
Peluffo M, Rosso JA, Morelli IS, Mora VC (2018) Strategies for oxidation of PAHs in aged contaminated soil by batch reactors. Ecotoxicol Environ Saf 151:76. https://doi.org/10.1016/j.ecoenv.2017.12.067
Xie H, Liu H, Xie Y, Yang M, Guo S, Zhou Z, Xu H (2015) Fabrication of a novel immobilization system and its application for removal of anthracene from soil. Biochem Eng J 97:8. https://doi.org/10.1016/j.bej.2015.01.011
Gray MR, Banerjee DK, Fedorak PM (1994) Biological remediation of anthracene-contaminated soil in rotating bioreactors. Appl Microbiol Biotechnol 40:933. https://doi.org/10.1007/BF00174002
Lamichhane S, Krishna KCB, Sarukkalige R (2017) Surfactant-enhanced remediation of polycyclic aromatic hydrocarbons: a review. J Environ Manag 199:46. https://doi.org/10.1016/j.jenvman.2017.05.037
Ajab H, Isa MH, Taqub A (2020) Eletrochemical oxidation using Ti/RuO2 anode for COD and PAHs removal from aqueous solution. Sust Mater Technol 26:e00225. https://doi.org/10.1016/j.susmat.2020.e00225
Lai X, Ning X, Li JC, Zhang Y, Yuan Y (2020) Comparison of the Fe2+/H2O2 and Fe2+/PMS systems in simulated sludge: removal of PAHs, migration of elements and formation of chlorination by-products. J Hazard Mater 398:122826. https://doi.org/10.1016/j.jhazmat.2020.122826
Verâne J, Santos NCP, Silva VL, Almeida M, Oliveira OMC, Moreira ITA (2020) Phytoremediation of polycyclic aromatic hydrocarbons (PAHs) in mangroove sediments using Rhizophora mangle. Mar Pollut Bull 160:111687. https://doi.org/10.1016/j.marpolbul.2020.111687
Aydin S, Karaçay HA, Shahi A, Gökçe S, Ince B, Ince O (2017) Aerobic and anaerobic fungal metabolism and Omics insights for increasing polycyclic aromatic hydrocarbons biodegradation. Fungal Biol Rev 31:61. https://doi.org/10.1016/j.fbr.2016.12.001
Mohan SV, Kisa T, Ohkuma T, Analy RA, Shimizu Y (2006) Bioremediation technologies for treatment of PAH-contaminated soil and strategies to enhance process efficiency. Rev Environ Sci Biotechnol 5:347. https://doi.org/10.1007/s11157-006-0004-1
Pereira CS, Kelbert M, Daronch NA, Michels C, Oliveira D, Soares HM (2020) Potential of enzymatic process as an innovative technology to remove anticancer drugs in wastewater. Appl Microbiol Biotechnol 104:23. https://doi.org/10.1007/s00253-019-10229-y
Kelbert M, Pereira CS, Daronch NA, Cesca K, Michels C, Oliveira D, Soares HM (2020) Laccase na efficacious approach to remove anticancer drugs: a study of doxorubicin degradation, kinectic parameters, and toxicity assessment. J Hazard Mater . https://doi.org/10.1016/j.jhazmat.2020.124520
Bayramoglu G, Karagoz B, Arica MY (2018) Cyclic-carbonate functionalized polymer brushes on polymeric microspheres: immobilized laccase for degradation of endocrine disturbing compounds. J Ind Eng Chem 60:407. https://doi.org/10.1016/j.jiec.2017.11.028
García-Morales R, García-García A, Orona-Navar C, Osma JF, Nigam KDP, Ornelas-Soto N (2018) Biotransformation of emerging pollutants in groundwater by laccase from P. sanguineus CS43 immobilized onto titania nanoparticles. J Environ Chem Eng 6:710. https://doi.org/10.1016/j.jece.2017.12.006
Uhnáková B, Petricková A, Biedermann D, Homolka L, Vejvoda V, Bednár P, Papousková B, Sulc M, Martínková L (2009) Biodegradation of brominated aromatics by cultures and laccase of Trametes versicolor. Chemosphere 76:826. https://doi.org/10.1016/j.chemosphere.2009.04.016
Baldantoni D, Morelli R, Belino A, Prati MV, Alfani A, Nicola F (2017) Anthracene and benzo(a)pyrene degradation in soil is favoured by compost amendment: perspectives for a bioremediation approach. J Hazard Mater 339:395. https://doi.org/10.1016/j.jhazmat.2017.06.043
Haritash AK, Kaushik CP (2009) Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): a review. J Hazard Mater 169:1. https://doi.org/10.1016/j.jhazmat.2009.03.137
Pang R, Li M, Zhang C (2015) Degradation of Phenolic compounds by laccase immobilizes on carbon nanomaterials: diffusional limitation investigation. Talanta 131:38. https://doi.org/10.1016/j.talanta.2014.07.045
Collins PJ, Kotterman MJJ, Field JA, Dobson ADW (1996) Oxidation of anthracene and benzo[a]pyrene by laccases from Trametes versicolor. Appl Environ Microbiol 62:4563. https://doi.org/10.1128/AEM.62.12.4563-4567.1996
Johannes C, Majcherczyk A, Hüttermann A (1996) Degradation of anthracene by laccase of Trametes versicolor in the presence of different mediator compounds. Appl Microbiol Biotechnol 46:313. https://doi.org/10.1007/s002530050823
Li X, Cheng WuY, Feng Y, Liu W, Lin X (2014) Influencing factors and product toxicity of anthracene oxidation by fungal laccase. Pedosphere 24:359. https://doi.org/10.1016/S1002-0160(14)60022-9
Wu Y, Teng Y, Li Z, Liao X, Luo Y (2008) Potential role of polycyclic aromatic hydrocarbons (PAHs) oxidation by fungal laccase in the remediation of an aged contaminated soil. Soil Biol Biochem 40:789. https://doi.org/10.1016/j.soilbio.2007.10.013
Majcherczyk A, Johannes C, Hüttermann A (1998) Oxidation of polycyclic aromatic hydrocarbons (PAH) by laccase of Trametes versicolor. Enzyme Microb Tech 22:335. https://doi.org/10.1016/S0141-0229(97)00199-3
Adeniji AO, Okoh OO, Okoh AI (2019) Levels of polycyclic aromatic hydrocarbons in the water and sediment of buffalo river estuary, south africa and their health risk assessment. Arch Environ Con Tox 76:657. https://doi.org/10.1007/s00244-019-00617-w
Li J, Li F, Liu Q (2017) PAHs behavior in surface water and groundwater of the Yellow River estuary: evidence from isotopes and hydrochemistry. Chemosphere 178:143. https://doi.org/10.1016/j.chemosphere.2017.03.052
Bautista LF, Morales G, Sanz R (2010) Immobilization strategies for laccase from Trametes versicolor on mesostructured silica materials and the application to the degradation of naphthalene. Bioresour Technol 101:8541. https://doi.org/10.1016/j.biortech.2010.06.042
Kim J, Grate JW, Wang P (2006) Nanostructures for enzyme stabilization. Chem Eng Sci 61:1017. https://doi.org/10.1016/j.ces.2005.05.067
Dodor DE, Hwang H, Ekunwe SIN (2004) Oxidation of anthracene and benzo[a]pyrene by immobilized laccase from Trametes versicolor. Enzyme Microb Tech 35:210. https://doi.org/10.1016/j.enzmictec.2004.04.007
Bautista LF, Moralez G, Sanz R (2015) Immobilization strategies for laccase from Trametes versicolor on mesostructured silica materials and the application to the degradation of naphthalene. Chemosphere 136:273. https://doi.org/10.1016/j.biortech.2010.06.042
Hu X, Wang P, Hwang H (2009) Oxidation of anthracene by immobilized laccase from Trametes versicolor. Bioresour Technol 100:4963. https://doi.org/10.1016/j.biortech.2009.03.089
Catapane M, Nicolucci C, Menale C, Mita L, Rossi S, Mita DG, Diano N (2013) Enzymatic removal of estrogenic activity of nonylphenol and octylphenol aqueous solutions by immobilized laccase from Trametes versicolor. J Hazard Mater 248–249:337. https://doi.org/10.1016/j.jhazmat.2013.01.031
Bresolin D, Estrella AS, Silva JRP, Valerio A, Sayer C, Araújo PHH, Oliveira D (2019) Synthesis of a green polyurethane foam from a biopolyol obtained by enzymatic glycerolysis and its use for immobilization of lipase NS-40116. Bioproc Biosyst Eng 42:213. https://doi.org/10.1007/s00449-018-2026-9
Nyari NLD, Fernandes IA, Bustamante-Vargas CE, Steffens C, Oliveira D, Zeni J, Rigo E, Dallago RM (2016) In situ immobilization of Candida antarctica B lipase in polyurethane foam support. J Mol Catal B 124:52. https://doi.org/10.1016/j.molcatb.2015.12.003
Stenholm A, Hedeland M, Arvidsson T, Pettersson CE (2020) Removal of nonylphenol polyethoxylates by adsorption on polyurethane foam and biodegradation using immobilized Trametes versicolor. Sci Total Environ 724:138159. https://doi.org/10.1016/j.scitotenv.2020.138159
Daronch NA (2020) Polyurethane Foam as Matrix for One-Step Laccase Immobilization. Federal University Of Santa Catarina [dissertation], Florianópolis, Brazil: https://repositorio.ufsc.br/bitstream/handle/123456789/216319/PENQ0877-D.pdf?sequence=-1&isAllowed=y. Accessed 12 Aug 2020
Rahmani K, Faramarzi MA, Mahvi AH, Gholami M, Esrafili A, Forootanfar H, Farzadkia M (2015) Elimination and detoxification of sulfathiazole and sulfamethoxazole assisted by laccase immobilized on porous silica beads. Int Biodeterior Biodegrad 97:107. https://doi.org/10.1016/j.ibiod.2014.10.018
Childs RE, Bardsley WG (1975) The steady state kinetics of peroxidase with 2,2’ azino di (3 ethylbenzthiazoline 6 sulphonic acid) as chromogen. Biochem J 145:93. https://doi.org/10.1042/bj1450093
Perini BLB, Bitencourt RL, Daronch NA, Schneider ALS, Oliveira D (2020) Surfactant-enhanced in-situ enzymatic oxidation: a bioremediation strategy for oxidation of polycyclic aromatic hydrocarbons in contaminated soils and aquifers. J Environ Chem Eng 8:104013. https://doi.org/10.1016/j.jece.2020.104013
Koo I, Kim S, Zhang X (2013) Comparative analysis of mass spectral matching-based compound identification in gas chromatography-mass spectrometry. J Chromatogr A 1298:132. https://doi.org/10.1016/j.chroma.2013.05.021
Xu P, Du H, Peng X, Tang Y, Zhou Y, Chen X, Fei J, Meng Y, Yuan L (2020) Degradation of several polycyclic aromatic hydrocarbons by laccase in reverse micelle system. Sci Total Environ 708:134970. https://doi.org/10.1016/j.scitotenv.2019.134970
Niu J, Dai Y, Guo H, Xu J, Shen Z (2013) Adsorption and transformation of PAHs from water by a laccase-loading spider-type reactor. J Hazard Mater 248–249:254. https://doi.org/10.1016/j.jhazmat.2013.01.017
Rao MA, Scelza R, Acevedo F, Diez MC, Gianfreda L (2014) Enzymes as useful tools for environmental purposes. Chemosphere 107:145. https://doi.org/10.1016/j.chemosphere.2013.12.059
Board Decision 045/2014/E/C/I, Provides for the approval of the Guiding Values for Soils and Groundwater in the State of São Paulo (2014) São Paulo State Environment Agency (CETESB), São Paulo, Brazil. https://cetesb.sp.gov.br/solo/wp-content/uploads/sites/18/2014/12/DD-045-2014-P53.pdf. Accessed 5 Mai 2020
Guidelines for Assessing and Managing Contaminated Gasworks Sites in New Zealand (1997) Ministry for the Environment (NZME), Wellington, New Zealand. https://www.mfe.govt.nz/sites/default/files/gas-guide-aug97-final.pdf. Accessed 12 July 2020
Quin W, Fan F, Zhu Y, Huang X, Ding A, Liu X, Dou J (2018) Anaerobic biodegradation of benzo(a)pyrene by a novel Cellulosimicrobium cellulans CWS2 isolated from polycyclic aromatic hydrocarbon-contaminated soil. Braz J Microbiol 49:258. https://doi.org/10.1016/j.bjm.2017.04.014
Arca-Ramos A, Eibes G, Feijoo G, Lema JM, Moreira MT (2015) Coupling extraction and enzyme catalysis for the removal of anthracene present in polluted soils. Chem Eng J 93:289. https://doi.org/10.1016/j.bej.2014.10.015
Swaathy S, Kavitha V, Pravin AS, Mandal AB, Gnanamani A (2014) Microbial surfactant mediated degradation of anthracene in aqueous phase by marine Bacillus licheniformis MTCC 5514. Biotechnol Rep 4:161. https://doi.org/10.1016/j.btre.2014.10.004
Tarafdar A, Sinha A, Masto RE (2017) Biodegradation of anthracene by a newly isolated bacterial strain, Bacillus thuringiensis AT.ISM.1, isolated from a fly ash deposition site. Lett Appl Microbiol 65:327. https://doi.org/10.1111/lam.12785
Abo-State MA, Saleh Y, Partila AM (2013) Identification of polycyclic aromatic hydrocarbon degrading bacterial strain and its ability to degrade pyrene. World Appl Sci J 23:515. https://doi.org/10.5829/idosi.wasj.2013.23.04.13078
Partila AM, Mohammed MR (2019) Evaluation of the benzo[a]anthracene biodegradation by animal bioassays. Bull Natl Res Cent 43:72. https://doi.org/10.1186/s42269-019-0115-9
Daronch NA, Kelbert M, Pereira CS, Araújo PHH, Oliveira D (2020) Elucidating the choice for a precise matrix for laccase immobilization: a review. Chem Eng J 397:125506. https://doi.org/10.1016/j.cej.2020.125506
Zeng Y, Hong PKA, Wavrek DA (2000) Integrated chemical-biological treatment of benzo[a]pyrene. Environ Sci Technol 34:854. https://doi.org/10.1021/es990817w
Dutch Target and Intervention Values (2000) Ministry of Housing, Spatial Planning and the Environment (VROM), The Hague, Netherlands. https://www.esdat.net/environmental%20standards/dutch/annexs_i2000dutch%20environmental%20standards.pdf. Accessed 21 July 2020
Towards Setting Guideline Values for the Protection of Groundwater in Ireland (2003) Environment Protection Agency (EPA) Wexford, Ireland. https://www.epa.ie/pubs/reports/water/ground/EPA_ground_water_ guideline_values_interim_report.pdf. Accessed 12 July 2020
Valderrama C, Alessandri R, Aunola T, Cortina JL, Gamisans X, Tuhkanen T (2009) Oxidation by Fenton’s reagent combined with biological treatment applied to a creosote-contaminated soil. J Hazard Mater 166:594. https://doi.org/10.1016/j.jhazmat.2008.11.108
Barry E, Mane AU, Libera JA, Elam JW, Darling SB (2017) Advanced oil sorbents using sequential infiltration synthesis. J Mater Chem A 5:2929. https://doi.org/10.1039/C6TA09014A
Frescura LM, Pereira HA, Silva FV Jr, Menezes BB, Hilgemman M, Lazzaretti AP Jr (2018) A comparative study between high density polyethylene, polyurethane foam and amberlite XAD-2 in the removal of different PAHs. Polycycl Aromat Comp. https://doi.org/10.1080/10406638.2018.1545680
Liang S, Neisius M, Mispreuve H, Naescher R, Gaan S (2012) Flame retardancy and thermal decomposition of flexible polyurethane foams: structural influence of organophosphorus compounds. Polym Degrad Stabil 97:2428. https://doi.org/10.1016/j.polymdegradstab.2012.07.019
Duan H, Yu D, Zuo J, Yang B, Zhang Y, Niu Y (2016) Characterization of brominated flame retardants in construction and demolition waste components: HBCD and PBDEs. Sci Total Environ 572:77. https://doi.org/10.1016/j.scitotenv.2016.07.165
Stancin H, Ruzicková J, Mikulcic H, Raclavská H, Kucbel M, Wang X, Duic N (2019) Experimental analysis of waste polyurethane from household appliances and its utilization possibilities. J Environ Manag 243:105. https://doi.org/10.1016/j.jenvman.2019.04.112
Cho S, Park SJ, Lim J, Rhee YH, Shin K (2002) Oxidation of polycyclic aromatic hydrocarbons by laccase of Coriolus hirsutus. Biotechnol Lett 24:1337. https://doi.org/10.1023/A:1019896316366
Koschorreck K, Richter SM, Swierczek A, Beifuss U, Schmid RD, Urlacher VB (2008) Comparative characterization of four laccases from Trametes versicolor concerning phenolic C-C coupling and oxidation of PAHs. Arch Biochem Biophys 474:213. https://doi.org/10.1016/j.abb.2008.03.009
Arca-Ramos A, Eibes G, Moreira MT, Feijoo G, Lema JM (2014) Vegetable oils as NAPLs in two phase partitioning bioreactors for the degradation of anthracene by laccase. Chem Eng J 240:281. https://doi.org/10.1016/j.cej.2013.11.076
Acknowledgements
The authors wish to thank the Brazilian Agency CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for the financial support and to UNIVILLE-Universidade da Região de Joinville, where the experiments were carried out.
Author information
Authors and Affiliations
Contributions
BP, DO and AS conceived and designed research. BP and ND conducted experiments. RB contributed analytical tools. BP, CA and DO wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Perini, B.L.B., Daronch, N.A., Bitencourt, R.L. et al. Application of Immobilized Laccase on Polyurethane Foam for Ex-Situ Polycyclic Aromatic Hydrocarbons Bioremediation. J Polym Environ 29, 2200–2213 (2021). https://doi.org/10.1007/s10924-020-02035-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-02035-9