Abstract
In this study, some physical and mechanical properties as well as antibacterial activity of sago starch films incorporated with methanol extract from Nigella sativa L. seeds (0, 1, 3, 5 and 10%) were investigated. The results showed that increasing concentration of the extract caused significant (P < 0.05) increment in thickness (0.06‒0.12 mm), moisture content (8.72‒11.19%, wet basis), water solubility (21.31‒25.62%), water absorption capacity (1.98‒3.45 g water/g dry film), and water vapor permeability (1.63 × 10− 11‒2.59 × 10− 11 g/m.s.Pa) of the films. Tensile strength and Young’s modulus changed in the range of 4.27‒6.43 MPa and 80.19‒118.55 MPa, respectively. Compared to the control, the composite films represented more flexibility and the elasticity was continually enhanced with increasing percentage of the extract. The films represented good antimicrobial activity against both the Gram-positive and Gram-negative bacteria presumably due to phenolic compounds of the extract.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primitive packaging form appeared as a result of necessity to supply food for consumption in coming days. Various types of plastics and synthetic packaging materials are dramatically practiced in different industries due to their brilliant advantages such as light weight, low cost, excellent mechanical behavior, good barrier to water, oxygen and aroma compounds properties as well as desirable heat sealability [1, 2]. In addition to packaging, synthetic plastics (SPs) are widely used in every area of life. In despite of many benefits, as a prominent example of non-biodegradable wastes, SPs cannot be decayed or decomposed by natural agents and last for many years without any degradation. With an increasing rate of 2.2%, SPs are common sources of environmental contamination with a worldwide production of about 140 million tons per year [3]. Therefore, due to adverse environmental impacts of non-biodegradable materials, it is great of interest for scientists and industries to find sustainable green solutions and develop environmentally friendly materials [4].
Partially or totally replacement of synthetic polymers by alternative edible and/or biodegradable materials could be more suitable for both manufacturers and consumers of foods [5]. Natural biodegradable polymers such as protein and polysaccharide based films, might be well-suited for utilization in packaging due to their environmental compatibility. Such films have been proposed for application in packaging of dry ingredients and ingredient delivery systems [6].
Starches have been extensively assessed by different researchers as a potential packaging film. Defined as carbohydrate polymers, starches are mainly consisted of a combination of polysaccharide amylose and branched polysaccharide amylopectin with different ratios [7]. Starches are the most biopolymer used to produce films. The popularity is mainly due to their abundance, renewability, acting as linking matrix between fillers, and low cost [8]. Furthermore, desirable physical and barrier properties have been reported for the starch films. However, hydrophilic nature and poor mechanical properties restrict the practicing of starch alone as a packaging material. Therefore, to modify and enhance the usability, it is necessary to incorporate starch with polymeric additives or plasticizers [9].
Several researchers have assessed the starch-based films made from various common sources such as cereals, potato, roots and legumes [5]. However, there is scanty scientific reports on sago starch film might due it’s relatively obscurity. Sago starch is obtained from Metroxylon sagu palm tree in Southeast Asia; and is a progressively important socioeconomic crop in the region. Sago starch has unique physicochemical characteristics but some properties of the starch are quite similar to cassava and intermediate to potato and cereal starches [10].
To the best of our knowledge, to date, no scientific study has been reported in the open literature considering the influence of extract from Nigella sativa L. seeds on properties of edible films. Therefore, due to increasing demanding for natural and safe edible films in food industry, this research was conducted to evaluate some important physical and mechanical properties of sago starch-based films incorporated with the extract from black cumin. The effectiveness of antimicrobial activity of the edible films against two gram positive and negative bacteria was also characterized.
Materials and Methods
Plant Materials
The dried Nigella sativa L. seeds were purchased from a local market in Isfahan, Iran. The samples identity was confirmed using the authentic specimens deposited at the Herbarium of the Department of Biology, Falavarjan Branch, Islamic Azad University, Isfahan. Then, using a mechanical blender, the seeds were ground to powder and stored at 4 °C until the extraction was started.
Extraction
To prepare the extracts, the method described by Al-Naggar et al. [11] was practiced. In brief, about 100 g of the powdered seeds was defatted with n-hexane and then extracted in methanol using a Soxhlet apparatus. The obtained extracts were passed twice through filter paper (Whatman no. 1), dried in a rotary evaporator (at reduced pressure of 100 psi and controlled temperature of 40 °C) [12], properly protected and stored in a refrigerator at temperature of 4 °C until further experiments.
Film Preparation
To prepare film-forming, sago starch (4%, w/w) was gelatinized by adding to distilled water, heated to 90 °C and continuously stirred for 45 min to complete homogeneity. As the best heat sealability at 40% [5], a mixture of plasticizer (sorbitol:3/glycerol:1) was also added and cooled to temperature of 40–45 °C. Different concentrations of the extract (0, 1, 3, 5 and 10%; w/w) was incorporated into the mixture. To produce film-forming area, 90 g of each suspension was cast onto a 16 cm × 16 cm polycrylic plate. The films were cooled down to ambient temperature and dried in an air-ventilated oven at 40 °C for 20 h. After drying, the samples were kept at temperature of 23 ± 2 °C and relative humidity of 50 ± 5%.
Determination of Moisture Content
Moisture content of the films was determined applying standard oven method and drying the samples using an oven (Memmert type UL 40; Memmert, Schwabach, Germany) at 110 °C until constant weight. Measuring mass of the samples to the nearest 0.0001 g, Eq. (1) was used to calculate the percentage of moisture content [13]:
Thickness of Films
To measure the thickness of each film, a hand-held micrometer (Mitutoyo, Tokyo, Japan) with accuracy to the nearest ± 0.01 mm was used. The thickness was measured at eight different locations along film strips and the average value was calculated.
Solubility in Water
Water solubility of the films was assessed according to the method described by Maizura et al. [8]. The films were cut into pieces with dimensions of 2 cm × 3 cm, and stored in a desiccator for 7 days (with silica gel, 0% RH) until the experiments were started. To measure the solubility, the samples were accurately weighed to the nearest 0.0001 g, placed into beakers containing 80 ml deionized water and stirred for 1 h at 25 °C. After soaking, the beakers contents were filtered using Whatman No. 1 filter paper and the remained residues dried in an oven at temperature of 60 °C until constant weight was achieved. Finally, the solubility percentage was calculated as follows:
Water Absorption Capacity (WAC)
To determine water absorption capacity of the films, the adapted method proposed by Kiatkamjornwong et al. [14] was practiced. The films were dried in a P2O5 for one week following by dehydration in an oven at temperature of 40 °C for 24 h. Then, a piece of 2 cm × 2 cm of the dried samples was placed in a 250 mL beaker containing 100 mL of distilled water and allowed to rehydrate for 30 min. The swollen films were drained, weighted and the WAC (g water/g dried film) of the films was calculated as follows [15]:
Water Vapor Permeability (WVP)
The ASTM (1981a) procedure E96-80 [16], was practiced to measure water vapor permeability of the films. The samples were sealed in glass permeation cells containing anhydrous silica gel with relative humidity of 0 and enough space to provide the desired air gap within the cells. The permeation cells were weighted and placed in desiccators with a constant relative humidity of 0.52 (using a saturated salt solution of magnesium nitrate) maintained at temperature of 30 °C. The instantaneous weight of the cells was monitored daily for 6 days, and the water vapor permeability (WVP, g/m.s.Pa) of the films was calculated using Eq. (4) [17]:
In Eq. (4), S is saturation vapor pressure of water at test temperature (Pa), d is film thickness (m), and R1 and R2 are the relative humidity values (as a fraction) at the desiccator and permeation cell, respectively. Furthermore, WVT is rate of the water vapor transmission rate (g/m2) which was calculated from the slope of the straight line divided by the test area.
Mechanical Properties of Films
The mechanical properties of the films including tensile strength, elongation and Young’s modulus, were determined using ASTM D882 with some modification [9]. Film strips (140 m × 20 mm) were cut and kept at temperature of 23 °C and 53% RH for 48 h. The mechanical properties were measured using a TA-XT2 texture analyzer (Stable Micro System, Goldaming Surrey, UK) equipped with Texture Exponent 32 software V.4.0.5.0 (SMS). The samples were clamped between tensile grips with an initial distance of 100 mm and stretched at crosshead speed of 0.5 mm/s. During extension process, the deformation and force were recorded by the software and represented in graph format. Tensile strength and elongation at breaking as well as Young’s modulus were determined.
Evaluation of Antimicrobial Activity
To assess the antimicrobial activity of the films, the agar diffusion method according to Maizura et al. [8] was used and the inhibition zone assay on solid media was determined to evaluate the antimicrobial potential against the bacteria.
The clinical isolate of the bacteria strains including Staphylococcus aureus (gram positive) and Esherichia coli (gram negative) was obtained from Food Microbiology Laboratory, Veterinary Medicine Faculty (Islamic Azad University) of Iran. Using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) in addition to conventional morphological and biochemical tests, the bacteria strains were identified. The strains were reserved as stock strain in glycerol phosphate buffered saline (20%) at − 70 °C [18]. The films were cut into discs with diameter of 6 mm and then put on Mueller-Hinton agar plates, previously seeded with 200 µl inoculants contained about 105‒106 colony-forming units per milliliter (CFU/mL) of the bacteria; followed by incubating at 37 °C for 24 h. Then, the plates were examined for inhibition zone of the film discs.
Statistical Analysis
All the tests were performed in five repetitions and the obtained experimental data were examined by SPSS software (version 21) using analysis of variance (ANOVA). All data are presented as mean values ± standard deviation (SD) and the differences among the means were compared for significance at P < 0.05 using Duncan’s multiple range tests.
Results and Discussion
Thickness and Moisture Content
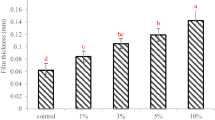
All the produced films were easily removed from the casting plate, homogenous, transparent as well as easy to manipulate and handle. Based on visual observation, the films contained no surface cracks or bubbles and had good flexibility. Figure 1 represents the average thickness for the resulting films where the value varied in the range of 0.06‒0.12 mm and significantly (P < 0.05) increased with increasing concentration of the extract.
In general, there are two different interaction types of interaction between the water and polymer including powerful polymer-water system caused by hydrogen bonds (in the moisture content below than 10%) and loose network caused by water–water interactions (in the moisture content above than 10%) [19]. Figure 2 shows the mean moisture content values determined for the films at different concentration of the extract from seeds of black cumin.
According to the results, the moisture content varied from 8.72 to 11.19% (wet basis). The statistical analysis declared meaningful influence of the extract concentration on moisture content of the films where the content enhanced with the concentration. The finding may arises from the presence of phenolic compounds in the extract of black cumin seeds having hydroxyl groups in their structure. The hydrophilic character enhanced the hygroscopic characteristics of sago starch films. The same observation has been reported by some researchers. Nouri and Mohammadi Nafchi (2014) studied sago starch film incorporated with extract from betel leaves and found that increasing percentage of the extract (in the range of 0‒30%) resulted in higher moisture content of the films. Furthermore, they reported the moisture content to be in the range of about 10.2‒12.3% (wet basis) [9]. Belibi et al. conducted a comparative study on cassava and tree cassava starch films produced by casting method with different glycerol contents (30, 35, 40 and 45% on starch dry basis. Moisture content (dry basis) of cassava and tree cassava starch films was reported to be in the ranges of 11.8‒41.1% and 15.3‒24.8%, respectively where both of them increased with increasing glycerol content) [20]. Poeloengasih and Anggraeni reported the same finding for sago starch films made with different amount of starch (9, 10 and 11% w/v) and glycerol (25‒40% w/w starch) [21]. It is worth to note that, unlike this finding, decrement in moisture content of prepared film with increasing concentration of extract has been reported by some researchers such as López-Palestina et al. for gelatin-based films containing oily tomato extract [22] and Norajit et al. for biodegradable alginate films containing ginseng extract [23]. The opposite findings may arise from differences in the hydrophilic nature of the films prepared using different components.
Solubility and Water Absorption Capacity
Most of the biopolymers are sensitive to water. As an important factor indicates biodegradability of films, solubility in water could be considered to define applications for their composite films. It is worth to note that higher solubility would represents poorer water resistance. In term of edible films, discussion and conclusion about the usefulness or disadvantage of this feature is subject to much debate. Since, on the one hand, a lower solubility of edible films is desirable throughout storage. However, on the other hand, for cooking food products coated with the films, a high solubility would be beneficial [9].
The experimental values obtained for water solubility of the sago starch films incorporated with extract from seeds of black cumin are represented in Fig. 3.
As the results show, the solubility changed from 21.31 to 25.62% which is comparable with the values reported for bio-nanocomposite films based on potato starch/halloysite nanoclay (about 24‒35%) [4], cassava (23.0‒32.1%) and tree cassava (19.3‒23.1%) starch films containing different glycerol contents [20], sago starch films made with glycerol (15.89‒28.81%) [21], partially hydrolyzed sago starch‒alginate edible film containing lemongrass oil (about 25‒45%) [8], and for edible film from mushrooms (L. edodes and F. velutipes) byproducts (15.26‒35.96%) [17]. In addition to the components of film, the test conditions mainly including water temperature and the process duration could effectively influenced the solubility where, in general, increasing both of the factors lead to more solubility in water of the films. In comparison with the control (film without extract), incorporation of the extract into the film resulted in higher water solubility of the film. Furthermore, statistical analysis revealed that the solubility was (P < 0.05) significantly enhanced with increasing concentration of the extract in the practice range. In the open literature, the same observation in term of the influence of extract concentration on the solubility has been reported by Nouri and Mohammadi Nafchi [9], Norajit et al. [23], Emam-Djomeh et al. [24], Maizura et al. [8], and Silva et al. [25] for other plant extract.
It is believed that the water absorption behavior of a hydrogel film is affected by a number of factors such as existence of functional groups could be ionized or protonated, chain relaxation effects, crosslinking degree, and hydrophilic-hydrophobic interactions [26]. Water capacity of the films are presented in Fig. 4.
Based on the experimental data and statistical analysis, introducing extract from seeds of black cumin to sago starch matrix resulted in significant increment in the water absorption capacity of the starch. Interaction between the extract and starch in the film structure could be the main reason for this observation where diffusion of water into the structure has been enhanced by decreasing the hydrogen bonds in the film matrix. Due to this fact, free water molecules interacts strongly with the composite film. This finding not only agree well with the results of the film solubility in water but also is confirmed by the observation reported by different researchers. Maizura et al. indicated that the addition of lemongrass oil into hydrolyzed sago starch-alginate film led to more flexible structure in the polymer and contributed to enhanced water absorption in the film [8]. Pereira et al. studied the influence of Aloe vera on water absorption of alginate hydrogel films and found that increasing portion of Aloe vera exhibits a significant increment in the water absorption capacity [15].
Water Vapor Permeability
Water vapor permeability is of high importance when developing food packaging materials where efficient barrier properties are desired to minimize moisture transfer between outside packaging environment and packaged food. The average water vapor permeability values for the sago starch films studied in this work changed from 1.63 × 10− 11 to 2.59 × 10− 11 g/m.s.Pa. The minimum water vapor permeability belonged to the films without extract (control) where the maximum value was seen in the film containing 10% extract from the black cumin seeds. Based on the obtained results, the permeability was continually increased with increasing concentration of the extract incorporation in the films. The variance analysis showed that, in comparison with control, the WVP is not significantly different (P > 0.05) for films containing 1% (1.78 × 10− 11 g/m.s.Pa) but meaningfully (P < 0.05) increased in film containing 3% extract. Furthermore, the experimental results represented that as the extract concentration increased from 3 to 5%, the WVP of the film enhanced from 2.19 × 10− 11 to 2.43 × 10− 11 g/m.s.Pa.
Considering the results obtained for the films in this work, it could be concluded that higher moisture content in the film resulted in higher WVP. The observation agrees well with the finding reported by several researchers such as Nouri and Maohammdi Nafchi for sago starch film incorporated with betel leaves extract [9], Benavides et al. studied the influence of oregano essential oil on properties of alginate film [27], and Olivas and Barbosa-Cánovas for alginate-calcium film as affected by plasticizer [28]. It is reported that water shows a plasticizing role reducing intermolecular bonds matrix between the polymer chains in the polymer and facilitates transferring of water vapor through the film [27].
Mechanical Properties
The results experimentally obtained for mechanical properties, including tensile strength, elongation at break and Young’s modulus, of the sago starch film incorporated with different percentage of extract from black cumin seeds are given in Table 1.
Ultimate tensile strength, often shortened to tensile strength, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials the ultimate tensile strength is close to the yield point, whereas in ductile materials the ultimate tensile strength can be higher. The ultimate tensile strength is usually found by performing a tensile test and recording the engineering stress versus strain. The strength is of vital criteria for food packaging applications since it can allow the packaging films to withstand the normal stress encountered during food handling, shipping, and transportation. As shown (Table 1), tensile strength of the film was significantly (P < 0.05) decreased with increasing concentration of the extract. The tensile strength is an intensive property may be dependent on some factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. On the contrary, the opposite effect on elongation of the film was observed where the higher percentage of the extract increased flexibility of the film. The observation is maybe related to lower intermolecular forces resulting from chain-to-chain interaction due to addition of the extract. Therefore, increasing free volume and mobility of the polymer chain reduces the glass transition temperature of film and enhances the flexibility [21]. The finding is consistent with several studies reported in the open literature for different biopolymers. Ibrahim et al. studied cornstarch-based films as affected by different concentrations (0‒55%) of various plasticizers (including fructose, sorbitol and urea) and found that regardless of plasticizer type, the tensile stress and Young’s modulus of plasticized films decreased as the plasticizer concentrations were raised [3]. In addition, Nouri and Mohammadi Nafchi [9], Poeloengasih and Anggraeni [21], Nemazifard et al. [29], and Szymańska-Chargot et al. [30] reported the same observations.
Young’s modulus represents the elastic properties of material where the higher value indicating less deformation in the material under tensile or compressive stress. From the results represented in Table 1, highest Young’s modulus in the present work was obtained for pure sago starch film (control). Furthermore, increasing the extract percentage in the range of 1‒10% caused significant (P < 0.05) increment in elasticity of the composite film indicating that extract acts similar to plasticizer.
Antibacterial Activity
Antimicrobial assay of the films against both Gram-positive (Staphylococcus aureus) and Gram-negative (Esherichia coli) bacteria was performed through the inhibition zone test and the obtained results are represented in Table 2.
Analysis of the inhibition zone revealed that, in general, incorporating sago film with the extract resulted in effective antibacterial activity against the studied microorganism strains. From the results, S. aureus was the most sensitive and E. coli and was the most impervious strain to the extract where an inhibition influence for the bacteria was observed to be in the range of 3.5‒13.6 mm and 2.3‒9.4 mm, respectively. The reason for this difference could be related to morphological differences between the microorganisms. In general, a phospholipidic membrane exists in outer layer of Gram-negative bacteria carrying the structural lipopolysaccharide constituents and makes the cell wall strong and impermeable to lipophilic solutes. On the contrary, the Gram-positive organisms are more delicate since they possesses only an outer peptidoglycan layer could not obstructs effectively the solutes penetration. Due to hydroxyl groups existent in phenolic compounds and disabling the cytoplasmic membrane, the extract acts as a proton exchanger and reduces the pH gradient through the cytoplasmic membrane. The phenomenon results in disrupting of the electron flow and proton motive force and consequently leads to discharge of the phosphoanhydride bonds in adenosine triphosphate (ATP) and cell death [9].
Conclusions
The effect of methanol extract from Nigella sativa L. seeds with different concentrations on the physical, mechanical and antibacterial properties of sago starch films was evaluated. The experimental results revealed that the extract could be successfully incorporated in to the films. The extract content was found to affect significantly (P < 0.05) the assessed characteristics of the specimens. The incorporation of extract improved all the evaluated physical properties of the films. Although extract concentration affected the film solubility in water, sago starch film generally revealed low water solubility (21.31‒25.62%) indicating that sago starch film could be used in developing of capsule shell material, mainly capsule shell for special target. Meaningful decrements in both tensile strength and elastic modulus values of composite sago starch film were found compared to the control film without extract. The methanol extract could be an effective additive for the films to improve antibacterial properties due to its hydroxyl groups existent in phenolic compounds. Finally, it is suggested to develop of sago starch film formulation with other compatible biopolymers to improve the functional characteristic of the film.
References
Ehivet FE, Min B, Park M-K, Oh J-H (2011) Characterization and antimicrobial activity of sweet potato starch-based edible film containing origanum (Thymus capitatus) oil. J Food Sci 76:178–184
Sorrentino A, Gorrasi G, Vittoria V (2007) Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci Tech 18:84–95
Ibrahim MIJ, Sapuan SM, Zainudin ES, Zuhri MYM (2019) Physical, thermal, morphological, and tensile properties of cornstarch-based films as affected by different plasticizers. J Food Prop 22:925–941
Sadegh-Hassani F, Mohammadi Nafchi A (2014) Preparation and characterization of bionanocomposite films based on potato starch/halloysite nanoclay. Int J Biol Macromol 67:458–462
Mohammadi Nafchi A, Cheng LH, Karim AA (2011) Effects of plasticizers on thermal properties and heat sealability of sago starch films. Food Hydrocoll 25:56–60
Jagannath JH, Nanjappa C, Das Gupta DK, Bawa AS (2003) Mechanical and barrier properties of edible starch–protein-based films. J Appl Polym Sci 88:64–71
Fazilah A, Maizura M, Abd Karim A, Bhupinder K, Rajeev B, Uthumporn U, Chew SH (2011) Physical and mechanical properties of sago starch–alginate films incorporated with calcium chloride. Int Food Res J 18:1027–1033
Maizura M, Fazilah A, Norziah MH, Abd Karim A (2007) Antibacterial activity and mechanical properties of partially hydrolyzed sago starch–alginate edible film containing lemongrass oil. Food Chem Toxicol 72:324–330
Nouri L, Mohammadi Nafchi A (2014) Antibacterial, mechanical, and barrier properties of sago starch film incorporated with betel leaves extract. Int J Biol Macromol 66:254–259
Tie APL, Karim AA, Manan DMA (2008) Physicochemical properties of starch in sago palms (Metroxylon sagu) at different growth stages. Starch/Starke 60:408–416
Al-Naggar TB, Gómez-Serranillos MP, Carretero ME, Villar AM (2003) Neuropharmacological activity of Nigella sativa L. extracts. J Ethnopharmacol 88:63–68
Rezaei M, Ghasemi Pirbalouti A (2019) Phytochemical, antioxidant and antibacterial properties of extracts from two spice herbs under different extraction solvents. J Food Meas Charact 13:2470–2480
Monjazeb Marvdashti L, Koochaki A, Yavarmanesh M (2019) Characterization, release profile and antimicrobial properties of bioactive polyvinyl alcohol-alyssum homolocarpum seed gum-nisin composite film. Food Biophys 14:120–131
Kiatkamjornwong S, Chomsaksakul W, Sonsuk M (2000) Radiation modification of water absorption of cassava starch by acrylic acid/acrylamide. Radiat Phys Chem 59:413–427
Pereira RF, Carvalho A, Gil MH, Mendes A, Bártolo PJ (2013) Influence of Aloe vera on water absorption and enzymatic in vitro degradation of alginate hydrogel films. Carbohydr Polym 98:311–320
ASTM (1981a) Standard test methods for water vapor transmission of materials, method E96–80. American Society for Testing and Materials, Philadelphia
Zhang K, Wang W, Zhao K, Ma Y, Cheng S, Zhou J, Wu Z (2020) Producing a novel edible film from mushrooms (L. edodes and F. velutipes) byproducts with a two-stage treatment namely grinding and bleaching. J Food Eng. https://doi.org/10.1016/j.jfoodeng.2019.109862
Daneshzadeh MS, Abbaspour H, Amjad L, Mohammadi Nafchi A (2020) An investigation on phytochemical, antioxidant and antibacterial properties of extract from Eryngium billardieri F. Delaroche. J Food Meas Charact 14:708–715
Lourdin D, Coignard L, Bizot H, Colonna P (1997) Influence of equilibrium relative humidity and plasticizer concentration on the water content and glass transition of starch materials. Polym 38:5401–5406
Belibi PC, Daou TJ,. Ndjaka JMB, Nosm B, Michelin L, Durand B (2014) A comparative study of some properties of cassava and tree cassava starch films. Phys Procedia 55:220–226
Poeloengasih CD, Anggraeni FD (2014) Exploring the characteristics of sago starch films for pharmaceutical application. Starch/Starke 66:1103–1108
López-Palestina CU, Aguirre-Mancilla CL, Raya-Pérez JC, Ramirez-Pimentel JG, Vargas-Torres A, Hernández-Fuentes AD (2019) Physicochemical and antioxidant properties of gelatin-based films containing oily tomato extract (Solanum lycopersicum L.). CYTA-J Food 17(1):142–150
Norajit K, Kim KM, Ryu GH (2010) Comparative studies on the characterization and antioxidant properties of biodegradable alginate films containing ginseng extract. J Food Eng 98:377–384
Emam-Djomeh Z, Moghaddam A, Yasini Ardakani SA (2015) Antimicrobial activity of pomegranate (Punica granatum L.) peel extract, physical, mechanical, barrier and antimicrobial properties of pomegranate peel pxtract-incorporated sodium caseinate film and application in packaging for ground beef. Packag Technol Sci 28:869–881
Silva OA, Pellá MG, Pellá MG, Caetano J, Simões MR, Bittencourt PRS, Dragunski DC (2019) Synthesis and characterization of a low solubility edible film based on native cassava starch. Int J Biol Macromol 128:290–296
Bajpai SK, Daheriya P, Ahuja S, Gupta K (2016) Water absorption and antimicrobial behavior of physically cross linked poly (vinyl alcohol)/carrageenan films loaded with minocycline. Des Monomers Polym 19:630–642
Benavides S, Villalobos-Carvajal R, Reyes JE (2012) Physical, mechanical and antibacterial properties of alginate film: effect of the crosslinking degree and oregano essential oil concentration. J Food Eng 110:232–239
Olivas GI, Barbosa-Cánovas GV (2008) Alginate–calcium films: Water vapor permeability and mechanical properties as affected by plasticizer and relative humidity. LWT-Food Sci Technol 41:359–366
Nemazifard M, Kavoosi G, Marzban Z, Ezadi N (2017) Physical, mechanical, water binding and antioxidant properties of cellulose dispersions and cellulose film incorporated with pomegranate seed extract. Int J Food Prop 20:1501–1514
Szymańska-Chargot M, Chylińska M, Pertile G, Pieczywek PM, Cieślak KJ, Zdunek A, Frąc M (2019) Influence of chitosan addition on the mechanical and antibacterial properties of carrot cellulose nanofibre film. Cellulose 26:9613–9629
Acknowledgements
Authors are deeply grateful to the Islamic Azad University, Damghan Branch and Islamic Azad University, Falavarjan Branchfor the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ekramian, S., Abbaspour, H., Roudi, B. et al. Influence of Nigella sativa L. Extract on Physico‒Mechanical and Antimicrobial Properties of Sago Starch Film. J Polym Environ 29, 201–208 (2021). https://doi.org/10.1007/s10924-020-01864-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01864-y