Abstract
The Fe3O4–thymolblue (TB) functionalized or end capped poly(ε-caprolactone) (PCL) was synthesized by ring opening polymerization (ROP) of ε-caprolactone (CL) with Fe3O4–TB nanohybrid as an effective initiator in the presence of stannous octoate (SO) as a catalyst. The main aim of the present work is to analyze the influence of polymer chain end capping agent or functionalization agent on the structure–property relationship particularly, crystallization and thermal degradation behavior of PCL under non-isothermal condition. The structure of the polymer was analyzed using FTIR and NMR spectra. The particle size of the incorporated Fe3O4–TB nanohybrid in PCL matrix was found to be less than 20 nm by TEM. The surface morphology, skewness moment and surface roughness were analysed by AFM. The spherulitic growth of the nucleated polymer crystals was confirmed by POM. The spherulitic growth was further verified with various kinetic models. The melting and crystallization behaviors under non-isothermal condition were assessed by DSC. Various kinetic models were employed to comprehend the degradation behavior of Fe3O4–TB functionalized PCL and its associated kinetic parameters under non-isothermal condition. The energy of activation (Ea) for the crystallization and thermal degradation of PCL were found to be 132 and nearly 140 kJ/mol respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(ε-caprolactone) is a synthetic bio-degradable polyester made up of hexanoate repeating units. It receives much attention than other commercially available biodegradable polyesters owing to its semi-crystalline, oil resistance, hydrolytic degradation, biocompatible and biodegradable properties [1, 2]. The applications of PCL are innumerable such as the development of surgical sutures, implants, bio-renewable items, bio-resorbable devices and composite bags for some biomedical applications, and also in pharmaceutical and agricultural sectors [3,4,5]. PCL is also used in controlled drug delivery due to its high permeability to the wide range of drugs and biocompatibility [6]. PCL has been recognized as the safest polymer for its nontoxic nature to living organisms. The physical properties of PCL mainly depend on its molecular weight and degree of crystallinity. The ROP of CL is the promising method to obtain a polymer with high molecular weight and low polydispersity. The polymerization of CL was carried out using three different tin(II) butoxides by Dumklang et al. [7]. An Eosin Y functionalized multiwalled carbon nanotube (MWCNT) was used as an initiator for ROP of CL [8]. Zinc, magnesium, stannous alkoxides, as well as lanthanide derivatives, were reported as effective initiators for ROP of CL [9,10,11,12,13]. The ROP of CL was initiated by various chemical initiators, calcium mercaptosuccinate [14], sodium hydride [15] and isopropanol [16]. It was found that there is no report on the ROP of CL using TB decorated Fe3O4 as an initiator so far in the documented literature. The Fe3O4–TB nanohybrid was prepared and used as an effective initiator for the ROP of CL by our research team.
The potential applications of PCL are mainly in the biomedical engineering field. The crystallization and morphological properties of PCL are more important than its above-said properties for this application. The final properties of semi-crystalline PCL depend on its crystalline fraction, which gets affected by the crystallization condition. Hence, the study on the crystallization of PCL under the non-isothermal condition is being received more practical significance, as most of the processing techniques are melt based, which takes place only under non-isothermal conditions. Non-isothermal crystallization kinetics of PCL was reported in the literature [17]. The polarized optical microscope (POM) was used to confirm the spherulitic morphology of both PCL and its bio-composites. The crystallization behavior of PCL was studied under both isothermal and non-isothermal conditions [18]. The PCL and PCL/glass composites were investigated under non-isothermal condition [19]. The kinetic parameters associated with the crystallization process were determined by using Mo’s and Jeziorny methods. The crystallization mechanism, nucleation and morphology of PCL-graphene oxide composites under non-isothermal conditions were reported by many researchers [20, 21]. The crystallization and melting behavior of PCL/MWCNT composites were examined under both isothermal and non-isothermal conditions [22]. PCL undergoes slight degradation during an industrial process while processing it under the non-isothermal condition, which in turn affects the material’s properties. Therefore, the understanding of thermal degradation process of PCL and its composites under the non-isothermal condition is very useful to prepare materials for particular applications. Persenaire et al. [23] studied the mechanism and kinetics of thermal degradation of PCL. The energy of activation for the thermal decomposition process was determined. The thermal stability and decomposition kinetics of PCL/nanoclay were investigated using TGA [24]. It was reported that the activation energy associated with the thermal decomposition process was higher for pure PCL than its composites. The thermal decomposition behavior of starch-based PCL bio-composites was reported by Carmona and co-workers through non-isothermal approach [25]. Also, the associated activation energy for thermal decomposition of the prepared bio-composites was determined by the Ozawa method. The kinetic parameters in connection with the degradation process of PCL biopolymers and its composites were analyzed by a non-isothermal approach using kinetic models [26]. Su and his co-workers [27] investigated the thermal stability of PCL by TGA and its thermal decomposition mechanism by an iterative iso-conversional method. The outcome of the present work is more useful to comprehend the crystallization, melting and thermal degradation behaviors of PCL under non-isothermal condition because these parameters are related with the processing, application and thermal recycling of PCL. The newly prepared Fe3O4–TB nanohybrid is not only acting as an initiator for the ROP of CL and also acting as a nucleating agent during the crystallization process of PCL. However, the decrease in Tc can be explained on the basis of segmental mobility and the semi-crystalline nature of PCL. The present investigation brings the new idea about the role of end capping agent or functionalization group on the thermal degradation, morphological and crystallization behavior of PCL.
Experimental
Materials
The chemicals utilized for this experimental work were used as received without further purification. Ferrous sulphate (FeSO4), ferric chloride (FeCl3) and sodium hydroxide (NaOH) were procured from S.D. fine chemicals, India. Thymolblue (TB, initiator) was supplied by Aldrich, India. Chloroform (CHCl3), diethyl ether, ε-caprolactone (CL, monomer, 99.9% pure) and stannous octoate (SO, catalyst, 99% pure) were supplied by Merck, India.
Synthesis of Fe3O4–TB Nanohybrid
For the preparation of TB decorated Fe3O4 nanohybrid, 1.0 g of TB was dissolved in 50 mL of double distilled water. FeSO4 and FeCl3 (1:2 molar ratio) were separately mixed in a beaker. Both the separately prepared solutions were mixed together under constant stirring at room temperature until that became a homogeneous solution. Afterwards, NaOH solution was added into the homogeneous mixture until the color of the solution turned as dark black. The reaction was continued further for 2 h under constant stirring. Finally, the obtained black precipitate was separated from the supernatant solution by centrifugation at higher rpm. The separated black precipitate was kept at 100 °C in an oven for drying. Thus obtained final product was TB decorated Fe3O4 nanohybrid. The reaction is shown in Scheme 1.
Synthesis of Fe3O4–TB Nanohybrid Functionalized PCL
1.0 g CL monomer was taken into a two-way necked thermal reactor (for N2 inlet and outlet respectively), and that was mixed with the freshly synthesized Fe3O4–TB nanohybrid initiator for 10 min at 30 °C under N2 atmosphere. Here the [M/I] = 400 and the [M/C] = 1000. The homogeneous chemical mixture was kept in an oil bath at 160 °C for 2 h to initiate ROP of CL under nitrogen atmosphere. A highly viscous fluid was obtained at the end of the reaction, which was further dissolved in 15 mL of CHCl3. Afterwards, it was re-precipitated in 200 mL of diethyl ether. Thus prepared black precipitate was Fe3O4–TB functionalized PCL. For the preparation of –OH functionalized PCL, 1.0 g of Fe3O4–TB functionalized PCL was dissolved in 10 mL of dil. HCl under ultrasonic irradiation for 30 min. Now the contents were filtered to isolate the hydroxyl functionalized PCL from Fe3O4–TB nanohybrid.
Characterization of the Sample
The prepared Fe3O4–TB functionalized PCL was characterized by various analytical tools to confirm its structure–property relationship. Molecular weight determination of the polymer sample was carried out using gel permeation chromatography (GPC), Perkin Elmer Series 200 in THF solvent and poly(styrene) as an internal standard. FTIR spectral measurement was carried out on Shimadzu 8400S, Japan in the range of 400 to 4000 cm−1 to confirm the functional groups present in the Fe3O4–TB functionalized PCL. For this measurement, 2 mg of Fe3O4–TB functionalized PCL sample was made as a thin pellet by mixing it with 200 mg of KBr under the constant pressure of 7 ton. The chemical structure of the polymer sample was also examined by recording 1H and 13C-NMR spectra using a 400 MHz JEOL JNM ECS 400 spectrometer in CDCl3 solvent. The surface morphology of the sample was investigated using Park NX10 AFM instrument. The particle size and morphology of the sample was also analyzed by JEOL 2100 HRTEM instrument. The Olympus BX51 optical microscope was used to analyze the crystal growth of the prepared sample. The melting and the crystallization process of Fe3O4–TB functionalized PCL were investigated under the non-isothermal condition in the temperature range of − 30 °C to 100 °C at various heating and cooling rates of 10, 15, 20, 25 and 30 °C/min under N2 atmosphere by using Toledo DSC 822E instrument. Thermal analyser (TG/DTA 6200) was used to analyze the thermal behavior of PCL composite in the temperature range of 30 °C to 600 °C at various heating rates in steps of 5 °C/min.
Non-isothermal Crystallization Kinetics
The various kinetic parameters associated with the crystallization process under non-isothermal condition were obtained from the DSC exothermic curves of the prepared Fe3O4–TB functionalized PCL system. Moreover, the heat released during the crystallization process can be calculated by integrating the area under the exothermic curves using DSC. The evolution of crystallinity is directly related with the released heat. Therefore, the relative crystallinity (Xt) is obtained as a function of temperature by the following expression: [28]
where dHc denotes the enthalpy of crystallization, T0 and T∞ are the onset and end set crystallization temperatures respectively. The correlation between the crystallization temperature (T) and crystallization time (t) is expressed using the mathematical relation as given below:
where β-the cooling rate.
The nucleation mechanism under the non-isothermal condition is well described with the help of the Avrami equation for the PCL system. The Avrami equation is given below:
where t is the crystallization time, n is the Avrami exponent which describes nucleation and growth mechanism, Zt is the crystallization rate constant. Ozawa modified the Eq. (2) and it can also be written as: [29]
where K(T) is the crystallization rate constant and m-Ozawa index.
The Avrami and Ozawa equations were modified by Mo in order to describe the crystallization parameters under non-isothermal condition. The combined Avrami and Ozawa model suggests the following expression: [30]
where Φ-cooling rate, F(T) is the crystallization rate constant and b is the Ozawa exponent. The exponent b describes the nucleation and the type of the nucleated crystals.
The Kissinger model [31] was also applied to describe the crystallization process under the same condition. It is mainly used to determine the energy of activation for the crystallization process. The following expression was proposed for this model:
The following equation is obtained by integrating Eq. (5),
where Ea is the energy of activation, R is the gas constant and Tc is the crystallization temperature at which reaction rate reaches the maximum, and \(\varphi\) is the cooling rate.
The energy of activation is determined by the iso-conversional method. Kissinger–Akahira–Sunose (KAS) is one of the important integral iso-conversional methods. It is used to evaluate activation energies associated with the degree of conversion (α) under non-isothermal condition. This method suggests the following mathematical expression: [32, 33]
where β is the heating rate, α-degree of conversion, Tα is the temperature associated with the degree of conversion α at various heating rates. According to this method, Ea can be calculated from the slope of the plot \({\text{ln}}\left( {\upbeta /{\text{T}}_{\upalpha }^{2}} \right)\) vs 1000/Tα for each value of the degree of conversion (α) at various heating rates.
Non-isothermal Degradation Kinetics
The kinetic parameters associated with the thermal decomposition of Fe3O4–TB functionalized PCL under non-isothermal conditions were analyzed by using four reliable kinetic models. The reaction extent (α) can be proposed by the mathematical relation: [34]
where Wo is the initial weight of the sample, Wt is the weight at any specified temperature and Wf is the weight of the sample at the end of the process.
Flynn–Wall–Ozawa (FWO) Method
Flynn–Wall–Ozawa (FWO) method [27] deals with the measurement of temperature (T) corresponding to a fixed value of the degree of conversion (α) at different heating rates. The activation energy (Ea) linked with the degradation process can be measured without knowing the reaction mechanism and order using this method. This model suggests the following equation:
where E is the activation energy, β is the heating rate, R is the gas constant. The activation energy can be measured from the plot of β vs 1/T for this model.
Kissinger Model
The Kissinger model [35] assumes that the reaction rate reaches its maximum at thermal degradation temperature (Td). Hence, it implies that the degree of conversion (α) is constant at Td. But, in many cases, the degree of conversion at Td varies with the heating rate. The Kissinger model proposes the following expression:
The Ea for the degradation process of functionalized PCL can be evaluated from the plot of ln(β/T2d) vs 1/ Td.
Auggis–Bennet Model
The Auggis–Bennet model [36] is also known as a classical kinetic model. The activation energy for thermal decomposition under non-isothermal condition can also be estimated by this model. This model expresses the following relation:
where Td is the degradation temperature. The Ea can be computed by measuring the slope from the plot of ln(β/Td) vs 1/ Td.
Friedman Method
The Friedman method is one of the iso-conversional methods [27]. The thermal decomposition mechanism of PCL can be analyzed by the following Arrhenius equation-based mathematical expression:
where R is the gas constant, α is the conversion rate at time t. The plot of ln(dα/dt) vs 1/T is required to estimate Ea values at different stages of degradation reaction.
Results and Discussion
For the sake of convenience, the results and discussion part is sub-divided into three parts, namely, (i) Characterization of Fe3O4–TB functionalized PCL, (ii) Non-isothermal crystallization kinetic study of Fe3O4–TB functionalized PCL and (iii) Non-isothermal degradation kinetic study of Fe3O4–TB functionalized PCL.
(i) Characterization of Fe3O4–TB Functionalized PCL
FTIR Spectra and GPC Study
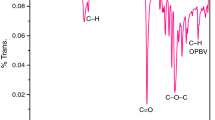
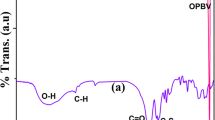
The FTIR spectra of Fe3O4, Fe3O4–TB nanohybrid and Fe3O4–TB functionalized PCL are shown in Fig. 1. Figure 1a indicates the FTIR spectrum of pure Fe3O4. The spectrum shows two peaks corresponding to the O-H stretching (3446 cm−1) and the metal oxide (M–O) stretching (598 cm−1) of Fe3O4. The FTIR spectrum of Fe3O4–TB nanohybrid is given in Fig. 1b. The –OH stretching vibration is identified at 3420 cm−1. The characteristic peaks of C–H symmetric stretching and anti-symmetric stretching vibrations of TB are seen at 2892 and 2952 cm−1 respectively. The aromatic C–H stretching is witnessed at 2620 cm−1. The other assigned peaks at 1410 cm−1 and 1173 cm−1 are related to C–S and C–O–C stretching vibrations of TB respectively. The C=C, C–S and –SO2 stretching of TB were observed at 1462 cm−1, 1176 cm−1 and 1238 cm−1 respectively. The M–O stretching is noticed at 602 cm−1 [37]. The FTIR spectrum of Fe3O4–TB functionalized PCL is illustrated in Fig. 1c. The O-H stretching vibration of PCL has appeared at 3336 cm−1. The peaks observed at 2863 and 2943 cm−1 are assigned to the C–H symmetric and asymmetric stretching vibrations respectively. The carbonyl group (C=O) of PCL is noticed at 1718 cm−1 [37]. The C–O–C stretching vibration of PCL is found to be at 1174 cm−1. The peak at 722 cm−1 is linked with the C–H out of plane bending vibration of PCL. The M–O stretching has appeared at 596 cm−1. However, the presence of C–H and C=O stretching characteristics peaks are mainly used to confirm the functionalities of PCL.
The chemical polymerization of CL in the presence of Fe3O4–TB nanohybrid was confirmed by GPC measurement. The GPC profile of pure PCL and Fe3O4–TB functionalized PCL are portrayed in Fig. 1d, e respectively. The results of the profiles revealed that the weight average molecular weight (Mw) of Fe3O4–TB functionalized PCL was considerably higher than pure PCL. The rise in the molecular weight of the Fe3O4–TB functionalized PCL is due to the attached nanohybrid end groups with PCL. The GPC data are listed in Table 1.
NMR Study
The chemical structure of Fe3O4–TB functionalized PCL was examined with the help of 1H- and 13C-NMR spectra. The 1H-NMR spectrum of Fe3O4–TB functionalized PCL is displayed in Fig. 2a. The solvent peak of CDCl3 is observed at 7.3 ppm. The alkoxy proton peak is seen at 4.17 ppm in the 1H-NMR spectrum [38]. The signal at 2.30 ppm is associated with the methylene proton of PCL located to the near by carbonyl group. The assigned signals between 1.37 and 1.7 ppm are witnessed to other methylene protons of PCL. The structure of PCL was confirmed by the above discussed peaks. The 13C-NMR spectrum of Fe3O4–TB functionalized PCL is mentioned in Fig. 2b. The solvent signals are observed between 76.5 and 77.4 ppm in the 13C-NMR. A peak at 173.1 ppm is referred to the carbon signal of the carbonyl group of PCL [34]. The alkoxy carbon signal peak is assigned at 64.4 ppm. The signal for methylene carbon adjacent to the carbonyl group of PCL is seen at 34.01 ppm. The chemical structure of Fe3O4–TB functionalized PCL was confirmed by carbon NMR spectrum.
HRTEM and AFM Study
The HRTEM micrograph of Fe3O4–TB functionalized PCL is represented in Fig. 3a. The morphology of the incorporated Fe3O4–TB nanohybrid in the PCL matrix is found to be more or less spherical in shape. The size of the particles is varied from 5 to 20 nm. The Fe3O4–TB nanohybrids are interconnected with each other in the PCL matrix. Also, the particles of Fe3O4–TB nanohybrid seem to be embedded well in the PCL matrix. It also confirms the good interaction between the nanohybrid and PCL. The SAED pattern of the prepared sample is given in Fig. 3b.The appearance of well organized concentric circles in the SAED pattern reveals the semi-crystalline nature of PCL.
The surface texture of Fe3O4–TB functionalized PCL was analyzed by AFM. The nature of the material surface can be confirmed with the help of the AFM image. The functional performance of the material is related to the material’s surface texture. The surface roughness and grain orientation of PCL was examined using 2D and 3D micrographs (Fig. 3a, b). The presence of well-scattered sharp peaks on the sample surface confirms the interaction of Fe3O4–TB with PCL matrix. Moreover, the grains are found to have different size and spacing. The particle size of the Fe3O4–TB nanohybrid is found to be less than 20 nm (Fig. 3c). The root mean square and average surface roughness were measured as 7.1 nm and 5.6 nm respectively. The surface is having both peaks and valleys. However, the surface has more planar region than peaks which is confirmed by the negative Skewness moment (− 0.12). The distribution of peak’s height is symmetrical in the 3D micrograph.
(ii) Non-isothermal Crystallization Kinetic Study of Fe3O4–TB Functionalized PCL
DSC Profile
The melting and crystallization of PCL were examined under non-isothermal condition by DSC measurement. The DSC heating scan of pure PCL heated at 10 °C/min is displayed in Fig. 4. The Tm of pure PCL (62.7 °C) was lower while compared with Tm of Fe3O4–TB functionalized PCL (66.4 °C) heated at the same heating rate. The increase in Tm is mainly due to the end capped Fe3O4–TB nanohybrid in PCL matrix. The melting and crystallization behaviors of Fe3O4–TB functionalized PCL were examined by the non-isothermal approach. The Fe3O4–TB functionalized PCL was heated in the temperature range of -30 °C to 100 °C at five different heating rates (from 10°C/min to 30 °C/min in steps of 5 °C/min) in N2 atmosphere (Fig. 5a–e). The sample heated at 10°C/min exhibits a broad peak at 66.4 °C which is corresponding to the Tm of PCL. It was noticed that the Tm of PCL shifts toward higher temperature in accordance with the heating rates. This is in good agreement with the literature report [39]. The increase in Tm with the increase in heating rate was understood from the plot of heating rate vs Tm for Fe3O4–TB functionalized PCL (Fig. 5f). The amount of heat energy required for melting the substance was reduced under fast scanning process. The DSC cooling scans of Fe3O4–TB functionalized PCL recorded at five different cooling rates are shown in Fig. 5g–k. The crystallization temperature was identified at 30.2 °C for the sample heated at 10 °C/min. It was understood that the Tc value was notably decreased from 30.2 to 24.4 °C while increasing the cooling rates [40]. The plot of cooling rate against Tc is shown in Fig. 5l. The decreasing trend in Tc can be understood from the plot. It concludes that the Tc value of Fe3O4–TB functionalized PCL was disturbed while changing the cooling rate. The obtained DSC data is compiled in Table 2.
Non-isothermal Crystallization Study
The crystallization process of Fe3O4–TB functionalized PCL was inspected to assess the crystallization rate, nucleation mechanism and the type of crystal growth by the non-isothermal approach. The structure and properties of semi-crystalline PCL depend on the above-said parameters. The prepared PCL was cooled at various cooling rates from 10 °C/min to 30 °C/min in steps of 5 °C/min to comprehend its crystallization behavior. The effect of cooling rates on the crystallization enthalpy (∆Hc), the degree of crystallinity (XC) and crystallization peak temperature (Tc) were examined from the crystallization curves as shown in Fig. 4g–k. The crystallization curve becomes broader and the Tc value decreases with the increase in cooling rates. During the cooling process (from the melting stage), the polymer chains are arranged in an ordered and tightly packed fashion. The stereo regular arrangement can be influenced by the chain end capping or functionalization agent. In the present case, the ferrite-TB nanohybrid is an end capping agent. The chain mobility of PCL is restricted by the chain end capping agent. At the higher cooling rate, the PCL chains are tightly packed and arranged in a fast manner. As a result, the Tc values were decreased. Generally, the crystallization process occurs slowly at a lower cooling rate. Therefore, the Tc value shifts toward a higher temperature. While cooling the specimens quickly, more super cooling is required to initiate the crystallization process since the motion of the PCL molecules does not follow the cooling rate.
The nucleation mechanism and growth geometry of the crystals were studied under non-isothermal crystallization condition for the prepared Fe3O4–TB functionalized PCL using two different models. According to the proposed Avrami expression in Eq. (3), the Avrami exponent (n) and crystallization rate constant (Zt) were determined from the slope and intercept of the plot of ln[− ln(1 − Xt)] vs. ln(t) as shown in Fig. 6a-e. The n and Zt values represent the rate of nuclei formation and the type of growth respectively. The obtained n values were varied from 2.96 to 3.15, which suggested the 3D spherulitic growth [39, 41]. The measured Zt values were varied from 0.37 to 1.25 for various cooling rates. The crystallization rate increases with the increase in cooling rates. It was concluded that the crystallization rate constant is inversely proportional to crystallization time. The kinetics of Fe3O4–TB functionalized PCL was performed by Mo by combining the Avrami and Ozawa equation as given in Eq. (4). The plot of ln(Φ) vs ln(t) was made to calculate the values of F(T) and Ozawa exponent (b) respectively (Fig. 6f). It can be seen that the plot yielded a linear relationship for a given Xt and confirmed that the Mo model can successfully explain the non-isothermal crystallization of ferrite-TB functionalized PCL. In the case of pristine PCL, attainment of such a linear relationship is somewhat difficult because of the absence of functionalization agent or nucleating agent chemically attached with the PCL chains. This explained the role of an end capping agent on the structure–property relationship of PCL. This can be further confirmed by determining the various parameters. The F(T) and b values were obtained as 1.18 and 3.09 respectively. The value of b is above 3, which confirmed the 3D spherulitic crystal growth [37,38,39]. Hence, both the models authentically confirmed the 3D spherulitic growth as well as the same range of crystallization rate constant for the PCL. The polarized optical micrograph (POM) is illustrated in Fig. 6g. All the grown crystals are in 3D spherulitic shape. Hence, the kinetic results are matched with the experimental result. The POM micrograph shows more number of spherulites per unit area. As a result, one cannot clearly view the growth of the polymer crystals. Moreover, PCL contains more number of chains with ferrite-TB as a chain end capping agent. This induced the crystallization process, at the same time the chain mobility is arrested to some extent. Due to these reasons, the PCL crystal growth is not clearly observed.
The energy required for the crystallization process was determined by Kissinger model. The plot of ln(Φ/Tc2) vs 1/Tc (Fig. 7a) was used to determine the Ea value for the crystallization process of Fe3O4–TB functionalized PCL. The Ea value was evaluated as 132 kJ/mol. The Ea value was observed to be lower when compared with pure PCL [42]. This is due to the incorporated Fe3O4–TB nanohybrid in PCL matrix which considerably enhances the transportation ability of polymer chains during the crystallization process. The crystallization rate coefficient (CRC) was obtained as − 0.396 from the plot of crystallization temperature (Tc) vs. cooling rate (Φ) as shown in Fig. 7b. The crystallization rate is higher for polymers with short repeating units than the polymers with longer repeating units. The plot of Tm against Tc (Fig. 7c) reveals a linear relation between them.
The Ea for the crystallization process was also determined by the iso-conversional method. The KAS method was employed to evaluate activation energies associated with a crystallized fraction under non-isothermal condition. The graph of crystallization fraction (α) as a function of temperature was drawn to determine the temperature associated with the degree of conversion for various heating rates as shown in Fig. 8a–e. The obtained α values are listed in Table 3. The Ea value was determined from the plot of ln\((\beta /{\text{T}}_{\upalpha }^{2})\) vs 1000/Tα as shown in Fig. 8f–o. The obtained Ea values are given in Table 3. It is well known that ferrite nanocrystal is a good nucleating agent and resulting with an increase in crystallinity and Tc. But in the present study, the ferrite nanocrystals are decorated with TB dye and attached with PCL chains. Hence, the nucleating activity of ferrite is decreased. During the cooling process, the PCL chain mobility is restricted to some extent by the nanohybrid. More number of spherulites are grown but disturbed by the PCL chain mobility. This can be evidenced by the POM image. From this one can come to the conclusion that smaller the size of nucleating agent the better will be the nucleating activity.
(iii) Non-isothermal Degradation Kinetic Study of Fe3O4–TB Functionalized PCL
TGA Profile
The TG thermograms of pure PCL and Fe3O4–TB functionalized PCL heated at 10 °C/min are portrayed in Fig. 9a, b respectively. A single step degradation process was perceived for both the systems. The degradation temperature of pure PCL and Fe3O4–TB functionalized PCL was determined as 394.8 and 395.5 °C respectively. The thermal stability of the functionalized PCL was slightly enhanced while compared with pure PCL. The thermal stability of Fe3O4–TB functionalized PCL was studied under non-isothermal condition. The TG thermograms of Fe3O4–TB functionalized PCL was heated in the range of 30 to 600 °C at five different heating rates in the air atmosphere and are shown in Fig. 10a–e. All the thermograms exhibited a single step degradation process in the given temperature range for Fe3O4–TB functionalized PCL. The decomposition temperature of PCL was found to be shifted to a higher temperature in accordance with the heating rate. This is in good agreement with the literature report [30, 31]. The initial degradation temperature (Tid) and the final degradation temperature(Tfd) were observed at 240 and 418.3 °C for the functionalized PCL heated at 10 °C/min, which were observed to be increased with the increase in heating rates. The derivative thermograms of Fe3O4–TB functionalized PCL for all the heating rates are shown in Fig. 10f–j. The increase in Td was noticed from the derivative thermograms in accordance with the heating rates. The PEMA-co-PHEMA-g-PCL showed two-step degradation processes with initial degradation temperatures at 243 °C and 361 °C respectively [36]. Hence, the Fe3O4–TB functionalized PCL is thermally stable while compared with the previously reported one.
Table 4 showed a comparative TGA data of pristine PCL and Fe3O4–TB functionalized PCL systems. The onset, 50% and maximum Td of Fe3O4–TB functionalized PCL system exhibited higher values due to the influence of TB decorated ferrite nanohybrid.
Non-isothermal Degradation Study
The thermal degradation behavior and its associated kinetic parameters under non-isothermal condition were analyzed by four different kinetic models for Fe3O4–TB functionalized PCL. The prepared PCL was heated under various heating rates in air atmosphere to determine its degradation behavior more accurately. The TGA patterns of Fe3O4–TB functionalized PCL and its derivative plots for all the five heating rates are given in Fig. 10a–j respectively. The increase in degradation temperature (Td) with the increasing heating rates can be understood from the non-isothermal TG and DTG curves. Moreover, the peak area of the DTG curves becomes wider with the increasing heating rates. The plot of Td vs heating rate (Fig. 11a) revealed the gradual increase in the Td with the increasing heating rates. This is because of the fast scanning process while heating the specimen. The methodology of finding out Ea for the decomposition process varies with different models. Flynnwall-Ozawa model (the plot of lnβ vs 1000/Td; Fig. 11b), Auggis-Bennet method (the plot of ln(β/Td) vs 1000/Td; Fig. 11c) and Kissinger method (\({\text{ln}}(\upbeta /{{\text{T}}^{\text{2}}}_{{\text{d}}})\) vs 1000/Td; Fig. 11d) were employed to estimate the Ea values for the decomposition process of the PCL [29]. The Ea values were obtained as 146.9 kJ mol−1,141.3 kJ mol−1 and 138.8 kJ mol−1 by Flynnwall–Ozawa, Auggis–Bennet and Kissinger models respectively. The obtained Ea values were in the acceptable error limit only. The Ea value for Kissinger model was lower while comparing with other models.
The Friedman model was implemented to determine the Ea value for the degradation process of Fe3O4–TB functionalized PCL. The Ea value was determined from the slope of the plot of ln(dα/dt) vs 1/T (Fig. 12a–e). A decreasing trend was noticed for all the plots. The calculated Ea values were in the range of 99 to 117.7 kJ mol−1 for different heating rates. The plot of Ea vs reaction extent (Fig. 12f) reveals a linear relation between them. The Ea values calculated by Kissinger and Friedman models for Fe3O4–TB functionalized PCL were observed to be lower while compared with the reported Ea values (171 kJ mol−1 for Kissinger and 177 kJ mol−1 for Friedman models) of pure PCL. It was reported that the decrease in Ea was noticed for the PCL/poly(trimethylene carbonate) blend system [34]. Our result is in accordance with the literature report.
The present investigation proved that the size and length of the end capping or functionalization agent can alter the thermal properties of the PCL. When compared with the pristine PCL, the Tc as well as Td of the Fe3O4–TB nanohybrid functionalized PCL has higher values. This is due to the nucleating activity of ferrite nanocrystals. Unfortunately, there is a restriction in the segmental mobility of the PCL offered by the nanohybrid. As a result, there appeared more number of spherulites per unit area.
Conclusion
The Fe3O4–TB functionalized PCL was successfully prepared by ROP technique in the presence of Fe3O4–TB nanohybrid initiator. The chemical structure of PCL was verified with the help of the observed carbonyl stretching (1720 cm−1) and C–O–C (1179 cm−1) stretching of ester linkage peaks in the FTIR spectrum. The assigned signals at 4.17 ppm and 2.30 ppm in the 1H-NMR spectrum were related to the alkoxy and methylene protons of PCL. The particle size of the incorporated Fe3O4–TB nanohybrid was determined to be less than 20 nm. The spherulitic crystal growth was investigated by POM. The Avrami (n) and Ozawa (b) exponents confirmed the 3D spherulitic growth. Hence, the theoretical study was matched with the experimental results obtained. The Tc and Td of Fe3O4–TB functionalized PCL were altered while increasing the heating and cooling rates. The Ea for the decomposition of PCL was evaluated by four different kinetic models. The obtained values were found to be reliable with each other. This study proved that the polymer chain end capping agent can influence the Tc and Td of the PCL. And it also clearly explained that the size and length of the end capping agent have a definite role while predicting the structure–property relationship of a polymer.
References
Alamri H, Zhao J, Pahovnik D, Hadjichristidis N (2014) Polym Chem 5:5471
Baji ASC, Wong TS, Srivatsan GO, Mathur G (2006) Mater Manuf Process 21:211
Castillo RV, Mueller AJ (2009) Prog Polym Sci 34:516
Wei X, Gong C, Gou M, Fu S, Guo Q, Shi S, Luo F, Guo G, Qiu L, Qian Z (2009) Int J Pharm 381:1
Woodruff MA, Hutmacher DW (2010) Prog Polym Sci 35:1217
Fan H, Jin Z (2015) Chem Commun 51:15114
Dumklang M, Pattaong N, Punyodom W, Meepowpan P, Molloy R, Hoffman M (2009) Chiang Mai J Sci 36:136
Chen H-H, Anbarasan R, Kuo L-S, Chen PH (2011) Mater Chem Phys 126:584
Chamberlain BM, Cheng M, Moore DR, Ovitt TM, Lobkovsky EB, Coates GW (2001) J Am Chem Soc 123:3229
Shueh ML, Wang YS, Huang BH, Kuo CY, Lin C (2004) Macromolecules 37:5155
Storey RF, Sherman JW (2002) Macromolecules 35:1504
Kricheldorf HR (2004) J Polym Sci Part A Polym Chem 42:4723
Guillaume SM, Schappacher M, Soum A (2003) Macromolecules 36:54
Kannammal L, Palanikumar S, Meenarathi B, Anbarasan R (2017) J Thermoplast Compos Mater 30:1056
Monelave M, Contreras JM, Laredo E, Carrasquero FL (2010) Express Polym Lett 7:431
Darcos V, Habnouni SE, Nottelet B, Ghaoui AE, Coudane J (2010) PolymChem 1:280
Yang CT, Lee LT, Wu TY (2018) Polymers 10:543
Dhanvijay PU, Shertukde VV, Kalkar AKJ (2012) J Appl Polym Sci 124:1333
Oliveira Pires LS, Vaz Fernandes MHF, Marques de Oliveira JM (2018) J Thermal Anal Calorim 134:2115
Zhang J, Qiu Z (2011) Ind Eng Chem Res 50:13885
Lv Q, Wu D, Qiu Y, Chen J, Yao X, Ding K. Wei N (2015) Thermochim Acta 612:25
Tzong-Ming W, Erh-Chiang C (2006) Polym Eng Sci 46:1309
Persenaire O, Alexandre M, Degee P, Dubois P (2001) Biomolecules 2:288
Hadj-Hamou AS, Metref F, Yahiaoui F (2017) Polym Bull 74:3833
Carmona VB, Campos AD, Marconcini JM, Capparelli Mattoso LH (2014) J Therm Anal Calorim 115:153
Vangala SPK, Chaudhary A, Tiwari P, Katiyar V (2017) J Energ Environ Sustain 3:10
Su TT, Jiang H, Gong H (2008) Polym Plast Technol Eng 47:398–403
Mucha M, Tylman M, Mucha J, (2015) Polimery 60:11
Tzong MW, Chen EC (2006) Polym Eng Sci 46:1309
Li Y, Han C, Yu Y, Xiao L, Shao Y (2018) J Therm Anal Calorim 131:2213
Achla S, Maiti N, Jacob J (2017) Int J Polym Anal Ch 22:222
Vyazovkin S, Sbirrazzuoli N (2003) J Therm Anal Calorim 72:681
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N (2016) Thermochim Acta 520:1
Marquez Y, Franco L, Puiggali J (2012) Thermochim Acta 550:65
Reddy MM, Vivekanandhan S, Misra M, Bhatia SK, Mohantty AK (2013) Prog Polym Sci 38:1653
Demir P (2017) J Sci Engg 17:73
Meenarathi B, Palanikumar S, Kannammal L, Anbarasan R (2015) Spectrochim Acta Part A 35:93
Meenarathi B, Chen HH, Chen PH. Anbarasan R (2014) Int J Plast Technol 18:135
Mahalakshmi S, Alagesan T, Parthasarathy V, Tung KL, Anbarasan R (2018) J Therm Anal Calorim https://doi.org/10.1007/s10973-018-7514-2. (In Press)
Ahmed J, Luciano G, Schizzi I, Ali Arfat Y, Maggiore S, Lidia Arockia Thai T (2018) Thermochim Acta 659:96
Kawazu K, Nakagawa S, Ishizone T, Nojima S, Arai D, Yamaguchi K, Nakahama S (2017) Macromolecules 50:7202
Vyazovkin S, Sbirrazzuoli N (2006) Macromo Rapid Commun 27:1515
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest among the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahalakshmi, S., Parthasarathy, V., Tung, KL. et al. Non-isothermal Crystallization and Degradation Kinetics of Fe3O4–Thymolblue Functionalized Poly(ε-caprolactone). J Polym Environ 27, 1259–1272 (2019). https://doi.org/10.1007/s10924-019-01401-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01401-6