Abstract
Biodegradable interpenetrating polymer network (IPN) hydrogels based on pre-vulcanized natural rubber (NR) and cassava starch (St) using sulphur (S) and glutaraldehyde (GA) as crosslinkers were developed in a solution form as a coating membrane for slow-release nitrogen fertilizer. The NR/St ratios affecting water swelling, water permeability and biodegradation were investigated. Results revealed that water swelling, water permeability and biodegradation of IPN NR/St hydrogels decreased with increasing NR content. The wax was used as outer coating shell to further improve the release characteristic of the coated urea. The urea bead (UB) coated with IPN NR/St and wax layers (W-IPN-CUB) exhibited exceptional release behavior up to 24 days in soil compared to native UB (3 days). The release mechanisms of W-IPN-CUB in both water and soil environments were a non-Fickian diffusion with n = 0.88 and 0.85, respectively, relating to the diffusion through porous membrane. Also, the W-IPN-CUB was able to slow the leaching of urea fertilizer and significantly improve the performances of corn and basil height compared to native UB (P < 0.05). Thus, the W-IPN-CUB material proved to be a good candidate material for slow release fertilizer which could be widely used in agricultural and horticultural applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that the growth of plants needs sufficient nutrients (from natural or synthetic fertilizers) to increase crop productions regarding meeting the increased populations. However, 40–70 wt% of nitrogen nutrient from urea fertilizer usually escapes to environment by ammonia volatilization or leaching to water before the plants can absorb them [1, 2]. These problems are a substantial economic loss and serious environment pollution [3, 4]. Controlled release technology could effectively resolve these problems [5]. Controlled-release urea was found to improve nitrogen use efficiencies in wheat, maize, rice, oilseed rape and potato, compared to normal urea [6,7,8]. Various types of coating materials are used for producing the slow/control released urea such as sulfur-, polymer- and superabsorbent-based materials which have the complex synthesis processing, high cost and the non-environmentally friendly preventing scale production. However, the popular one is the bio-composite materials which are relatively cheaper, biodegradable and renewable regarding starch, lignin and cellulose coating materials [5, 9, 10].
The natural starch, polysaccharide polymer, is a suitable material for agrochemical encapsulation due to its many hydroxyl groups absorbing water molecules. Rychter et al. [11] prepared potato starch film plasticized with urea thus successfully used as fertilizer on the plant growth but this film had too short release time and not satisfied from the use in field. To solve this problem, the development of coated fertilizer is developed to improve hydrophobicity by grafting the starch with a hydrophobic polymer. Chen et al. [1] grafted starch with hydrophobic poly(L-lactide) (PLLA) and encapsulated urea fertilizers within the starch matrix modified by L-lactide through in situ graft-copolymerization. The hydrophobic PLLA reduced the swellability of starch matrix and decreased urea release rate. In contrast, Riyajan et al. [12] had improved the hydrophilicity and biodegradability of natural rubber (NR) by grafting with modified cassava starch (St) (NR–g–St). They reported that the NR–g–St membrane for controlled-release urea fertilizer was degraded easily in soil but it was difficult to swell due to the grafting chemical interactions between NR and St. In addition, most hydrogels suffer from the insufficient mechanical strength to meet the requirement of other applications. However, there are only few reports dealing with the application of NR/St hydrogel solution as coating membrane for controlled-release urea fertilizer. Therefore, the development of NR/St based coating membrane to improve release characteristic and mechanical strength is still needed to be explored.
In our previous work [13, 14], we attempted to prepare crosslinked natural rubber (XNR) and cassava starch (CSt) hydrogels using interpenetrating polymer networks (IPN) method combining two or more network polymers together with high mechanical strength and good compatibility [15,16,17]. The XNR latex crosslinked with N,N′-methylene-bisacrylamide (MBA) was mixed with gelatinized cassava starch and maleic acid (MA). Finally, the mixture was casted on glass mould to form IPN XNR/CSt thick film hydrogels via crosslinked reaction at 120 °C in oven. The IPN XNR/CSt hydrogels exhibited not only a high water swelling but also good biodegradation. However, the obtained hydrogels cannot be coated on the urea bead surface due to the rigid sheet of the final form. It is known that hydrogel from NR and St is a good choice for fertilizer encapsulation but it has a short lifetime in high moisture soil which results in the decreases in efficiency of controlled-release fertilizer due to the increase of release rate with increasing imperfections and porous on the outer surface [18]. To reduce the rate of nutrient release, wax is usually used on the outer surface of slow release fertilizer to close hole, crack or imperfection [19, 20].
Therefore, in this work, we aimed to develop the novel coating membrane based on pre-vulcanized NR and St synthesized via IPN method using sulphur (S) and glutaraldehyde (GA) as crosslinkers to obtained IPN NR/St in a solution form. The influences of NR/St ratio on the gel fraction, water permeability and water swelling of the IPN NR/St were also evaluated. The chemical structure, microstructure and morphology of the IPN NR/St hydrogels were investigated by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and scanning electron microscopy (SEM), respectively. The biodegradation of the IPN NR/St in soil was also investigated. Finally, the solution of IPN NR/St hydrogels was applied as coating membranes to encapsulate the native urea beads. The release behaviors and release kinetics of hydrogel-coated urea beads in water and in soil environments as well as the performance of coated urea beads on the corn and basil plantation were also inspected and discussed.
Experiment
Materials
High ammonia concentrated NR latex (HA latex, 60% dry rubber content), 10% potassium hydroxide (KOH) solution, 50% Sulphur dispersion, 50% Zinc oxide (ZnO) dispersion and 50% Zinc diethyldithiocarbamate (ZDEC) dispersion were supplied by Chemical and Materials Co. Ltd., Thailand. Cassava starch (St, 19 wt% amylose) was purchased from Bigtree Intertrade Co. Ltd., Thailand. Emulvin WA as stabilizer for NR latex was obtained from Lanxess Company, Germany. GA and hydrochloric acid (HCl) were purchased from Sigma-Aldrich.
Preparation of Pre-vulcanized NR Latex as First Networks
Formulation of pre-vulcanized NR latex compound was carried out according to Maznah et al. [21]. HA latex (166.7 g) was introduced into a round bottom reactor along with 100 mL distilled water and then 10% KOH solution (3.0 g) was added and stirred at 215 rpm for 10 min at room temperature to stabilize HA latex. Dispersion additives consisting of 50% sulphur (3.0 g), 50% ZnO (0.5 g) and 50% ZDEC (3.0 g) as vulcanizing agent, activator and accelerator, respectively, were then added to the mixture and constantly stirred for 2 h. The final mixture was kept at room temperature for 72 h to obtain pre-vulcanized NR latex. The crosslink level of pre-vulcanized NR latex was determined by chloroform number test and its crosslink density was evaluated using Flory–Huggins equations. The chloroform number and crosslink density of pre-vulcanized NR latex in this research were ca. number 2 (lightly vulcanized) and 65.40 ± 3.66 mol/m3, respectively.
Preparation of IPN NR/St as Coating Solution
To synthesize the IPN NR/St hydrogels, the second networks of crosslinked St in the presence of pre-vulcanized NR latex were generated as following procedures. Firstly, to stabilize the pre-vulcanized NR latex, Emulvin WA was added. Gelatinized St was prepared by heating aqueous St suspension (25 wt%) to 80 °C and stirred at 250 rpm for 1 h. After cooling, 100 g of the gelatinized St was added by GA crosslinkers (1 g) in acidic medium (1 mL, 1 N HCl). To obtain the IPN NR/St coating solution, the gelatinized St was finally mixed with pre-vulcanized NR latex at various ratios (0–50 wt%) and the crosslinking reaction of St with GA was stirred at 50 °C for 2 h. For testing swelling, biodegradation and other properties, the final mixture was cast on a glass mould and dried at 40 °C in hot air oven.
Coating Urea Bead with IPN NR/St Coating Solution
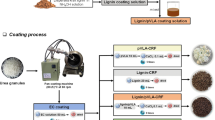
To coat urea bead with the IPN NR/St solution, the mass ratio of IPN NR/St solution to urea was fixed at 1:4. A 1 kg urea beads (UB) previously sieved to 3.6 mm in diameter and the optimal IPN NR/St coating solution (250 g) were fed into a rotary drum coater which was made from aluminum blow (20 cm diameter) and heated to 100 °C. The coater was rotated at 110 rpm to obtain the dry IPN NR/St coated urea beads (IPN-CUB) without agglomeration. Melting wax (250 g) was subsequently coated as outer shell on the IPN-CUB material using the rotary drum coater being rotating at 100 rpm at room temperature and then the IPN-CUB (W-IPN-CUB) coated by wax was finally obtained and further used for urea release experiments. The possible characteristic and structural photograph of urea bead coated by layers of IPN NR/St solution and wax were demonstrated in Fig. 1a, b, respectively. The photographs of UB, IPN-CUB and W-IPN-CUB are shown in Fig. 1c.
Characterizations
FTIR Spectral and Microstructure Analysis
The chemical structures of NR, St and IPN NR/St sheets were analyzed using a attenuated total reflectance Fourier transform infrared spectrometer (ATR-FTIR) (Spectrum Two, Perkin Elmer) equipped with a diamond head for 16 scans in the range between 4000 and 650 cm−1. The microstructure of IPN NR/St samples was measured by XRD technique using an X’Pert MPD (Philips, The Netherlands). The voltage and current used were 35 kV and 25 mA, respectively. The XRD patterns were recorded in the 2θ range of 5–50°.
Soluble and Gel Fraction Tests
Soluble fraction of the IPN NR/St samples was performed by extraction of 0.1 g of the IPN NR/St samples in excess distilled water with mild stirring at 250 rpm for 3 h to reach swelling equilibrium. The swollen sample was then filtered by filter papers and dried in oven until weight was constant. The sample weight loss is the soluble fraction. The gel fractions of IPN NR/St samples were calculated by Eq. (1) [22]:
where Sol and Gel are soluble fraction (%) and gel fraction (%) of sample, respectively.
Water Swelling Ratio (SR)
The IPN NR/St sheets (20 mm × 20 mm × 0.5 mm) were immersed in distilled water at room temperature, removed at every 24 h and weighed each swollen sample. The water swelling ratio (SR) was calculated as follows:
where W1(g) and W2(g) are the weight of the dried and swollen samples, respectively.
Water Permeability of IPN NR/St Film
To choose the suitable IPN NR/St formula for coating on urea bead, water permeability of the IPN NR/St films was tested by a method adapted from Han et al. [23]. The water permeability was performed using 6 cm—diameter permeable cups containing silica gel sealed by the IPN NR/St film. The cups were then stored at room temperature for 24 h and weighed. The water permeability (WP) was calculated by Eq. (3):
where WP unit is g/m2 h−1, Δm is the increasing weight of silica gel (g), t is 24 h and the water permeable area or S = 2.826 × 10−3 m2.
Release Behavior and Kinetics of W-IPN-CUB in Water and Soil
Native urea bead (UB) and W-IPN-CUB of 7 g were immersed in 100 mL distilled water at room temperature (31 °C). A 5.0 mL solution was taken out to evaluate the urea contents at a certain interval time and then added the same volume of fresh water. The release of urea from W-IPN-CUB in soil was adapted from Liang et al. [24]. The release of urea from UB and W-IPN-CUB in agricultural field was tested in a soil column. The sandy soil used in experiment was washed 10 times with distillation water and air-drying.
Polypropylene (PP) pots of 25 cm internal diameter and 22 cm height were used to hold soil. Loaded UB and W-IPN-CUB (7.0 g) was well mixed with 1.0 kg dry soil and then placed in PP pot equipped with the filter paper No. 5 in the bottom. Water (200 mL) was added slowly and the filtered water was then centrifuged to separate sandy soil particles from water. The temperature of soil used in this experiment was also measured to be 31 °C.
According to Ehrlich reaction, the released urea from the UB and W-IPN-CUB in water and soil was added 4-(Dimethylamino) benzaldehyde (DMBA) as indicator and was measured at 440 nm by UV–Vis spectrophotometer (UV 2600, Shimadzu). All the release experiments were done in duplicate, and their results were averaged. Moreover, the kinetics of urea release from native UB and W-IPN-CUB in both water and soil environments were also studied and discussed.
Scanning Electron Microscopy (SEM)
A scanning electron microscope (SEM, JSM-5410LV, JEOL, Japan) was used to study the surface morphologies of the IPN NR/St film specimens before and after burial test, including swollen sample of IPN-CUB and W-IPN-CUB previously freezed drying process. All specimens were mounted onto stubs and coated with gold plasma. The SEM images of coated samples were then taken.
Biodegradation in Soil Test
To examine the biodegradation of IPN NR/St hydrogels, the analysis procedure was slightly modified from Riyajan et al. [12]. The specimen (20 mm × 20 mm × 0.5 mm) was buried under soil at 7 cm from top soil (Warinchamrap, Ubon Ratchathani, Thailand). The water was added every week for 90 days. Each month, specimen was carefully taken out, washed with distilled water and dried at 45 °C until weight constant before being weighed. The biodegradability was determined by measuring weight loss of the specimens. The reported values were averaged from five specimens.
Evaluation of Plant Growth
Pot culture experiments on the corn and basil plants were conducted under the same laboratory condition with the treatment of native UB (control) and W-IPN-CUB. The corn and basil babies were firstly seeded in PP pots (25 cm diameter and 22 cm height) containing 2 kg loamy soil. When the corn and basil babies aged 20 days with the same height, 5 g of native UB and W-IPN-CUB were put in the soil of each samples and water (500 mL) was added every day. The changing of corn and basil heights was recorded from the feeding start of fertilizer to 6 weeks. The values of changing of corn and basil heights were averaged from five pots and analyzed by one-way ANOVA with P < 0.05.
Results and Discussion
Synthesis and Characterization of IPN NR/St Hydrogels
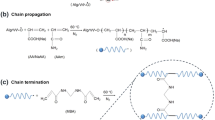
In order to prepare IPN of NR and St, two polymer networks of pre-vulcanized NR and crosslinked St must be synthesized consecutively. The pre-vulcanized NR latex as first network were carried out by mixing NR latex and KOH buffer with dispersion additives including sulphur, ZnO and ZDEC as vulcanizing agent, activator and accelerator, respectively, and constantly stirred for 2 h. The sulphur (S8) was activated by activator and accelerator at room temperature and formation of accelerator-polysulphide (see Scheme 1a). The accelerator-polysulphides are capable of sulphurating rubber chains and considered as sulphurating agent of the type-monomeric polysulphides. The polysulphides sulphurating agent molecules react with rubber molecules to form sulphurated rubber molecules of the type-polymeric polysulphides and release accelerator molecules. Then, the sulphurated rubber decomposes into radicals which are the crosslink forerunners and combine with another rubber molecule to form crosslinked rubber (see Scheme 1b). The formation of crosslinked St as second networks was subsequently performed by addition of gelatinized St and GA (4% of St) in acidic medium (1 mL, 1 N HCl) into the NR latex first networks. Then, the crosslinking reaction of St with GA was stirred at 50 °C for 2 h. The crosslinking of St molecules with GA was carried out through nucleophilic addition of hydroxyl group to the carbonyl group in order to form hemi-acetal linkages as shown in Scheme 1c [25] and finally this led to the successful formation of IPN NR/St solution (see Scheme 1d).
The chemical structures of NR, St and IPN NR/St hydrogel were characterized by ATR-FTIR as shown in Fig. 2a. NR exhibits the characteristic bands of C=C (stretching) at 1664 cm−1 and C–H (stretching) at 2997, 1473 and 1376 cm−1 and C=C (bending) at 853 cm−1. In St, there are main bands at 3770–3300 cm−1 (–OH stretching) and three bands at 1165, 1084 and 987 cm−1 (C–O–C stretching). The peak at 1636 cm−1 is attributed to the adsorbed water in the amorphous region of starch [26]. For the IPN NR/St, the broad band of –OH group in St is observed at 3313 cm−1 and the bands at 1655, 1539 and 1452 cm−1 are attributed to the C=C and C–O stretching and –CH3 deformation, respectively, which is similar to our previous work [14]. The new peaks at 1246 and 1083 cm−1 relating to asymmetric and symmetric bending of C–O–C, respectively, as well as a new peak at 703 cm−1 (C–O bending) were also observed which indicates the reaction between GA and St portions.
The microstructures of pure NR, pure St and the IPN NR/St samples were also evaluated by XRD as shown in Fig. 2b. It is seen that a NR pattern shows broad peak at 2θ = 18.6° which corresponds to an amorphous structure. While, XRD pattern of St exhibits five peaks at 2θ = 15.1, 17.1, 18.1, 19.5 and 23.3°, respectively, which are attributed to the semi-crystalline structure of St [27]. For the IPN NR/St showed the different XRD pattern from the pure St and pure NR but it closed to the combination between both parent polymers that confirmed the formation of new polymer (IPN). It exhibits a broad peak with weak intensity at 18.1° of NR fraction and the less intensity peaks of St portion at 2θ = 15.0, 17.0, 19.8 and 23.1° were observed. This pattern exhibited decrease in the crystallinity compared to St that indicates the crosslinks between St and GA [28]. Moreover, the slightly shifted peaks in the IPN NR/St XRD pattern may indicate the good compatibility between the NR and St networks through the IPN method [14]. The IPN formation between NR and St networks confirmed by FTIR and XRD results could improve their compatibility and provide a good water absorption and yield a longer service times for slow-release fertilizer.
Effect of NR/St Ratio on Gel and Soluble Fractions of IPN NR/St Hydrogel
The IPN NR/Starch (St) hydrogels were synthesized from hydrophilic polymer (St) and hydrophobic polymer (NR) by using IPN technology to combine both excellent properties from St and NR components. Generally, the natural starch consisting of abundant hydroxyl groups is easily soluble in water but our work aimed to solve this weak point through the IPN technology in order to enhance its application ranges. The effect of NR/St ratio on gel and soluble fractions of IPN NR/St sample is presented in Table 1. It is apparent that the soluble fractions of IPN NR/St trend to increase with increasing St content. The values of soluble fractions increase from 4.5 to 10.4% when St contents increased from 10 to 50 wt%, while the gel fraction decreases gradually. This result is similar trend with previous work that the NR/St IPN hydrogels were synthesized using MBA and MA as crosslinking agents [13, 14]. However, we found that the gel fractions of the IPN NR/St in this work was slightly lower than the previous work in all series [13]. This probably suggested that the IPN NR/St hydrogels have higher porous network structures.
Water Swelling and Permeability of IPN NR/St Hydrogels
Starch is usually chosen as biodegradable materials for several products even though their poor water resistance due to high water sensitivity limits the application of starch-based material for controlled-release fertilizers. To overcome this, we tried to use NR as a hydrophobic substance to improve the water resistance of starch. By increasing the hydrophobic of the polymer blend membrane, the water resistance of the matrix could be improved and thus the urea release rate could be reduced with increasing NR content in the IPN NR/St membrane [1, 14]. In the application of urea controlled release, water permeability and water absorption which are the important parameters for fertilizer-coated materials were thus investigated in this work. The effect of NR/St ratio on the water permeability and water swelling of IPN NR/St hydrogel was evaluated and displayed in Table 1. It is seen that water permeability and water swelling of IPN NR/St hydrogel tend to decrease with increasing NR portions which is similar to our previous works [13]. This phenomena resulted from the increase of hydrophobicity of polymer matrix which directly affected on the water transport reduction and retardation of water ingress to the matrix [29]. Moreover, the decrease of –OH hydrophilic groups in the IPN NR/St membrane could be the main reason for the decrease of water absorbency [23].
Biodegradation of IPN NR/St Hydrogels
The principal goal of coated or slow-release fertilizers is to provide nutrients in a slowed/delayed manner to meet with the sequential nutrient requirement of plant in order to increase crop yields [8]. Although starch (St) is an effective material for fertilizer encapsulation, it degrades too quickly due to the abundant hydroxyl groups absorbing and ingressing water molecules to the matrix [1]. Therefore, it cannot efficiently control the release of nutrients in soil for long time. In this work, NR with a slower biodegradable process and a large amounts in Thailand has thus been chosen to blend with St for reducing the St degradation rate and prolonging their service times.
Surface morphologies of the IPN NR/St films degraded in soil for 30 and 90 days were examined using SEM as shown in Fig. 3 (column I) and (column II), respectively, which in the first, second and third rows are the IPN NR/St of 90/10, 70/30 and 50/50 ratios, respectively. After 30 days, SEM of the IPN NR/St hydrogel of 90/10 ratio showed a perforation on the membrane surface (Fig. 3Ia) while in 70/30 (Ib) and 50/50 (Ic) ratios, the number of holes increased with increasing St ratio. Moreover, with increasing buried time to 90 days, the number of holes in all IPN NR/St samples increased significantly and the rapture structures of pores/holes were clearly seen in Fig. 3IIa–c. This indicates that the degradation of IPN NR/St sample increased with increasing time which was comparable to our previous works [13, 14]. This result may be attributed to the more hydrophilic behavior of St resulting in the increase in the hygroscopic characteristics of the blends and promotion of the growth of microorganisms during degradation as well as the increases in weight loss of IPN NR/St hydrogels [30].
This result also suggested that the IPN NR/St coating membrane could reduce the environment pollutant in soil when it was used as a coating membrane on the fertilizer surfaces. The reduction of weight in pure NR and a series of IPN NR/St hydrogels decomposed in soil at 90 days was presented in Table 1. It is found that the degradation of IPN NR/St hydrogels increased with the increasing St contents. The weight retention of IPN NR/St samples decreased from 83 to 48% with increasing St content from 50 to 90% which was analogous to previous work [13]. This implies that NR molecule can be biodegraded by the help of St. Abraham et al. [31] also reported that the degradation of NR was an oxidative cleavage of the double bond in the NR backbone due to various bacterials but it can be realized that the biodegradation rate of NR was very slow. From the results of gel fraction, water swelling and permeability, biodegradation (see Table 1), and SEM morphology, in summary, we believed that the NR/St ratio providing the suitable IPN NR/St hydrogel with good water permeability and an acceptable degradation rate was 70/30 ratio.
Morphology of W-IPN-CUB Surface Before and After Water Immersion
Figure 4 presents the optical and SEM micrographs of IPN-CUB and W-IPN-CUB. It is seen from Fig. 4a that the urea bead (UB) was completely covered by the IPN NR/St membrane which was almost uniform but some rougher surfaces were still observed. After further coating with two layers of wax, W-IPN-CUB showed a smoother surface and more uniform structure (Fig. 4b). This suggests that the wax can be used to make more uniform structure and smoother surface of encapsulation process [19, 20].
The SEM cross-sectional area of IPN-CUB and W-IPN-CUB surfaces were displayed in Fig. 4c, d, respectively. It is seen that the cover thickness of W-IPN-CUB material was about two times larger than that of IPN-CUB. The cover thickness of IPN-CUB and W-IPN-CUB was ca. 108.3 ± 3.0 and 193.5 ± 3.2 µm, respectively. This data obviously confirm that the cover layer of W-IPN-CUB material is actually comprised of IPN NR/St and wax.
The application of coated fertilizer is to retard the release of nutrient from fertilizer. The morphologies of IPN-CUB and W-IPN-CUB materials before and after immersion in water for 24 h were selected randomly and observed with optical microscopy and SEM techniques as shown in Fig. 5. Before immersion in water, the structures of IPN-CUB (Fig. 5a) and W-IPN-CUB (Fig. 5b) materials exhibited a permanently spherical shape with a smooth surface. However, after swelling in water for 24 h, the structure of IPN-CUB was collapsed (see Fig. 5a) but that of W-IPN-CUB was still remained as a spherical shape (Fig. 5b). This indicated that W-IPN-CUB materials has more stable structure due to the help of wax to retain the structure of coating membrane [19, 20].
Figure 5c–f show the SEM morphologies of cover surface of IPN-CUB and W-IPN-CUB before (Fig. 5c, e) and after swelling (Fig. 5d, f) in water for 24 h. Before swelling in water, some pores/holes in the surface of IPN-CUB material were seen and no pore/hole was observed in the surface of W-IPN-CUB membrane because wax covered such cracks or holes [19, 20]. However, after swelling in water for 24 h, a number of holes with bigger pore size were clearly observed in the IPN-CUB cover surface. These holes could probably be the channels that water molecules diffuse into the internal fertilizer to dissolve urea and then urea can be released quickly.
On the other hand, the surface of W-IPN-CUB appeared only a few holes due to the improvement of hydrophobic surface of W-IPN-CUB by introduction of wax [19, 20]. As a result, the release rate of urea under W-IPN-CUB could be reduced significantly. In other words, the W-IPN-CUB was able to slow the leaching of urea fertilizer. From these results, wax has thus been used to improve the hydrophobic surface of several hydrophilic materials [32, 33] due to a high content in esters of long-chain fatty alcohols and acid, as well as long-chain alkanes that have low-energy surfaces. Furthermore, wax has been chosen to be the top-coated fertilizer products [19, 20] providing for the controlled release because the wax outer layer protects the internal phase from liquid water [34, 35].
Release Behavior and Kinetics Study of W-IPN-CUB in Water and Soil
The influences of W-IPN-CUB on the rate of urea release in water and soil environments are demonstrated in Fig. 6a. At the same weight of UB, the native UB was completely released in water within 2 h, while the urea release rate of W-IPN-CUB in water had been certainly slowed down and completely released in 72 h, which was totally different compared to the work of Bortoletto-Santos et al. reported that uncoated urea completely released up to 30 days [36]. In soil test, the urea release of native UB and W-IPN-CUB was completely released in 3 (72 h) and 24 (576 h) days, respectively. The longer release time in soil (576 h) than in water (72 h) could be due to lesser water molecules surrounded the W-IPN-CUB. The significant improvement of release time for W-IPN-CUB suggested that it could able to slow the release of urea fertilizer and thereby decreasing nitrogen oxide (NOx) and dinitrogen (N2) emissions [37]. Therefore, the W-IPN-CUB could be an alternative membrane for using in agriculture and horticulture applications.
To determine the release behavior of urea from the W-IPN-CUB in both water and soil environments, the modified Peppas’s model [36, 38] was used to interpret and obtain the release process kinetic parameters:
where t is the time, ti is initial release time (the material retention time for release), k is the diffusion constant depending on the type of material and the permeation medium, n is the diffusional exponent suggesting the type of transport mechanism for a given solute; and Mt and \({M_\infty }\) are the urea mass released at a time t and at the steady state, respectively. This relationship could be only applied in the release range from starting to about 60%. By plotting graphs of ln(Mt/\({M_\infty }\)) versus ln(time) for each release test, the n and k values can thus be determined from slope and intercept, respectively, as shown in Fig. 6b.
The typical n parameter (diffusion process) ranges from 0 to 1, where n = 0.5 (Fickian release) contributes to a diffusional release through a semipermeable membrane, but 0.5 < n < 1 indicates the non-Fickian behavior describing the diffusion through the pores of coating membrane, whereas n = 1 characterizes a zero-order kinetic process commonly interpreting as a process where the diffusion barrier (i.e., the coating) is dynamically modifying the pore sizes or the chain organization [36, 38]. From Fig. 6b, n values are determined as 0.88 and 0.85 for the release of W-IPN-CUB in water and soil, respectively. The obtained n values in this work is also similar to other reports [36, 39]. For these cases, the urea release from W-IPN-CUB in both water and soil environments is considered to non-Fickian behavior. This may indicates that the diffusion occurs from the pores on the coating membrane and is regulated by the constant permeation through the W-IPN-CUB membrane [36]. In other words, the urea was released from W-IPN-CUB by the combine mechanisms of pure diffusion controlled and swelling controlled release [39]. Moreover, the k values in both water and soil are found to be 7.58 × 10−2 and 8.54 × 10−3 h−1, respectively. This result indicates that diffusion constant of urea through W-IPN-CUB membrane in soil is about 89% slower than in water. This may resulted from the less water entering into UB via W-IPN-CUB membrane in soil compared to in water.
The possible mechanism of control urea release coating with IPN-CUB and W-IPN-CUB is schematically illustrated in Fig. 7a, b, respectively. When the IPN-CUB and W-IPN-CUB were immersed in water environments, the water molecules diffuse through the membranes and dissolve urea to solution. Then the solute as urea solution will move throughout the coating membrane by diffusion process to outside of the membranes [1, 40,41,42]. As seen in Fig. 7a, the urea in IPN-CUB membrane could be released at higher rate due to the higher water volume entering into the membrane compared to the urea release in W-IPN-CUB covered by IPN NR/St and wax layers (Fig. 7b). This is because the wax layer was expected to retard the rate of water molecules entering to the IPN NR/St membrane before reaching the native UB. This schematic model probably matches with the urea release profiles as shown in Fig. 6a. For the W-IPN-CUB release in soil environment, it may be explained similarly as follows; firstly, the moisture in soil around the W-IPN-CUB permeates through the coating membrane on urea bead surface and dissolves urea solid to urea solution. The urea solution inside of W-IPN-CUB is then diffused outside to the soil which has higher moisture condition and that can be absorbed by the root of plants.
Evaluation of Plant Growth
The effect of W-IPN-CUB on the plant growth was also investigated using two plants; corn and basil. The growth rate of plants can normally be analyzed using several methods such as measurement the dry mass of plant, mass (dry and green) of the seeding or fruit of plant and so on [6, 11]. However, the measurement of plant height is an easy method and used in this work. The plant growth test measured the change of corn and basil height was performed and compared after the addition of urea bead types (native UB and W-IPN-CUB) in soil up to 6 weeks as shown in Fig. 8.
The change of corn and basil height without urea (control) was little and the highest of corn and basil height was only 6 and 9 cm for 6 weeks, respectively. After treating with the same weight of fertilizers; UB and W-IPN-CUB, the change of corn and basil height were affected obviously. During 1–3 weeks, both corn and basil plants added with UB and W-IPN-CUB showed a steadily growth rate with no significant difference but changed considerably when compared to control plants. After that, the height of corn added W-IPN-CUB was slightly higher than that of corn added UB (P < 0.05) and the highest of corn height added UB and W-IPN-CUB at 6 weeks was 42 and 60 cm, respectively, while the highest of basil height with same condition was 21 and 24 cm, respectively. The small value of P < 0.05 in statistical analysis suggested that corn and basil height with W-IPN-CUB have developed further due to the slow release of urea nutrient, minimum nutrient losses to environment and not a chance [43, 44]. Thus the height of corn and basil with added W-IPN-CUB was increased continuously.
The development of corn and basil trees added with different types of urea bead was presented in Fig. 9. The corn and basil height increased from the beginning of the experiment and the leaves of corn and basil changed from light green to dark green after addition urea bead. This indicates that nitrogen nutrient from urea fertilizer accelerated the growth of plant in the first planting by accelerating the growth up of leaf size, increasing the height and mass of plant. The control release of nutrients in fertilizer by coating membrane (W-IPN-CUB) caused the increase not only in corn and basil height but also in size of corn ears and basil leaves as obviously shown in Fig. 9b, e, respectively. It was found that the ears of corn (Fig. 9c) and leaves of basil (Fig. 9f) added with W-IPN-CUB were larger than those of corn and basil added with native UB. This is probably because the nitrogen nutrient from W-IPN-CUB was controlled on sufficient releasing, effectiveness and consistency for growth up of baby corn and basil to mature and thus improving crop yields.
Conclusion
The IPN NR/St coating membrane based on pre-vulcanized NR and crosslinked gelatinized St was developed by IPN method using sulphur and GA as crosslinkers. Soluble fraction, water swelling and water permeability of IPN NR/St hydrogel increased with St ratio, while gel fraction decreased. The biodegradation of IPN NR/St hydrogel improved significantly with increasing St content. The optimum IPN NR/St was 70:30. The urea release of W-IPN-CUB material could prolong up to 24 days compared to native UB (3 days). The release mechanism of urea from W-IPN-CUB in both water (n = 0.88) and soil (n = 0.85) environments was a non-Fickian diffusion relating to the diffusion through the porous membrane. The performance of encapsulated urea bead (W-IPN-CUB) on corn and basil plantation was significantly higher than using native UB. These results demonstrated that the biodegradable IPN NR/St hydrogel could be used as coating membrane in slow-release fertilizer application for improving the effectiveness of fertilizer and reducing environmental pollution and could be a promising material in agriculture and horticulture applications.
References
Chen L, Xie Z, Zhuang Z, Chen X, Jing X (2008) Carbohydr Polym 72:342
Kottegoda N, Sandaruwan C, Priyadarshana G, Asitha Siriwardhana A, Rathnayake UA, Arachchige DMB, Kumarasinghe AR, Dahanayake D, Karunaratne V, Amaratunga GAJ (2017) ACS Nano 11:1214
Tang J, Hong J, Liu Y, Wang B, Hua Q, Ying D (2017) J Polym Environ. https://doi.org/10.1007/s10924-017-1074-6
Chatterjee R (2009) Environ Sci Technol 43:1659
Azeem B, KuShaari K, Man ZB, Basit A, Thanh TH (2014) J Control Release 181:11
Gao X, Li C, Zhang M, Wang R, Chen B (2015) Field Crops Res 181:60
Geng J, Sun Y, Zhang M, Li C, Yang Y, Liu Z, Li S (2015) Field Crops Res 184:65
Zheng W, Zhang M, Liu Z, Zhou H, Lu H, Zhang W, Yang Y, Li C, Chen B (2016) Field Crops Res 197:52
Shang Y, Guo K, Jiang P, Xu X, Gao B (2018) Int J Biol Macromol 109:524
Norgren M, Edlund H (2014) Curr Opin Colloid Interface Sci 19:409
Rychter P, Kot M, Bajer K, Rogacz D, Siskova A, Kapusniak J (2016) Carbohydr Polym 137:127
Riyajan SA, Sasithornsonti Y, Phinyocheep P (2012) Carbohydr Polym 89:251
Vudjung C, Chaisuwan U, Pangan U, Chaipugdee N, Boonyod S, Santawitee O, Saengsuwan S (2014) Energy Proc 56:255
Vudjung C, Saengsuwan S (2017) J Elastom Plast 49:574
Dragan ES (2014) Chem Eng J 243:572
Berrebi M, Fabre-Francke I, Lavédrine B, Fichet O (2015) Eur Polym J 63:132
Wang Y, Huang Z, Zhang L (2006) Trans Nonferrous Met Soc China 16:517
Azeema B, KuShaaria KZ, Man Z (2016) Proc Eng 148:282
Mohd Ibrahim KR, Babadi FE, Yunus R (2014) Particuo 17:165
Naz MY, Sulaiman SA (2016) J Control Release 225:109
Maznah KS, Baharin A, Hanafi I, Azhar ME, Hakim MMR (2008) Polym Test 27:823
Zohuriaan-Mehr MJ, Kabiri K (2008) Iran Polym J 17:451
Han X, Chen S, Hu X (2009) Desalin 240:21
Liang R, Yuan H, Xi G, Zhou Q (2009) Carbohydr Polym 77:181
El-Tahlawy K, Venditti RA, Pawlak JT (2007) Carbohydr Polym 67:312
Ngwabebhoh FA, Gazi M, Oladipo AA (2016) Chem Eng Res Des 112:274
Liu C, Shao Y, Jia D (2008) Polymer 49:2176
Minhas M, Ahmad M, Ali L, Sohail M (2013) DARU J Pharm Sci 21:44
Gajdosova M, Pecek D, Sarvasova N, Grof Z, Stepanek F (2016) Int J Pharm 500:136
Jumaidin R, Sapuan SM, Jawaid M, Mohamad R, Ishak MR, Sahari J (2017) Int J Bio Macro 99:265
Abraham E, Elbi PA, Deepa B, Jyotishkumar P, Pothen LA, Narine SS, Thomas S (2012) Polym Degrad Stab 97:2378
Zhang W, Lu P, Qian L, Xiao H (2014) Chem Eng J 250:431
Al-Mohamadawi A, Benhabib K, Dheilly R, Goullieux A (2016) Constr Build Mater 102:94
Wittenbrook LS, Scheiderer EL (1978) US Patent 4,082,533 April 1978
Lubkowski K (2014) Environ Eng Manag 13(10):2573
Bortoletto-Santos R, Ribeiro C, Polito WL (2016) J Appl Polym Sci 133:43790
Chen J, Lu S, Zhang Z, Zhao X, Li X, Ning P, Liu M (2018) Sci Total Environ 613–614:829
Ritger PL, Peppas NA (1987) J Control Release 5:37
Jamnongkan T, Kaewpirom S (2010) J Polym Environ 18:413
An D, Liu B, Yang L, Wang T, Kan C (2017) Chem Eng J 311:318
Yang L, An D, Wang T, Kan C, Jin Y (2017) Particuo 30:73
Zhang S, Yang Y, Gao B, Wan Y, Li YC, Zhao C (2016) J Agric Food Chem 64:5692
Liu Q, Chen Y, Liu Y, Wen X, Liao Y (2016) Soil Tillage Res 157:1
Yang Y, Tong Z, Geng Y, Li Y, Zhang M (2013) J Agric Food Chem 61:8166
Acknowledgements
This work was supported by Grants from National Research Council of Thailand (NRCT, 2558A11702208) and Center of Excellence for Innovation in Chemistry (PERCH-CIC), Office of the Higher Education Commission, Ministry of Education (OHEC). Faculty of Science, Ubon Ratchathani University was also acknowledged for some financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Vudjung, C., Saengsuwan, S. Biodegradable IPN Hydrogels Based on Pre-vulcanized Natural Rubber and Cassava Starch as Coating Membrane for Environment-Friendly Slow-Release Urea Fertilizer. J Polym Environ 26, 3967–3980 (2018). https://doi.org/10.1007/s10924-018-1274-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1274-8