Abstract
Plastics play a very important role in our daily life. They are used for various purposes. But the disposal of these petrochemical-derived plastics causes a risk to the human and marine population, wildlife and environment. Also, due to the eventual depletion of petrochemical sources, there is a need for the development of alternate sources for the production of plastics. Biodegradable polymers produced by microorganisms can be used as substitutes for conventional plastics derived from petrochemical sources since they have similarity in their properties. Polyhydroxyalkanoate (PHA) is one such biopolymer that will be accumulated inside the cells of microorganisms as granules for energy storage under limiting conditions of nutrients and high concentration of carbon. Research on the microbial production of PHA should focus on the identification of cost-effective substrates and also identification of a suitable strain of organism for production. The major focus of this review is the production of PHA from various cost-effective substrates using different bacterial species. The review also covers the biosynthetic pathway of PHA, extraction method, characterization technique, and applications of PHA in various sectors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics are non-biodegradable polymers produced from petrochemical sources. They are an integral part of our everyday life. About 140 million tons of plastics are produced every year in the world. Plastic manufacturing has increased drastically due to its low cost, durability, good mechanical and thermal properties [1, 2]. They are used in medical applications, telecommunication, as furniture, packaging materials, shopping and garbage bags clothing, liquid containers, footwear, toys, household products, industrial products, and building materials [3, 4]. There are two major problems caused by the use of plastics. First, since they are stable, they accumulate in the environment for decades and causes several environmental and health problems. They contaminate water resources and are a threat to the life of marine animals and birds. Animals get entangled in plastics leading to their injury and ultimately death. Second, due to the inevitable decline of petroleum resources, alternate methods to produce plastics have to be developed [4,5,6]. In order to overcome the problems caused by plastics, there is a need for the development of biodegradable polymers that have the properties similar to conventional plastics. Biopolymers are biodegradable polymers produced by various microorganisms. They can be degraded aerobically and anaerobically by microorganisms. The commonly produced biopolymer is Polyhydroxyalkanoates (PHA). Different types of PHA are polyhydroxybutyrate (PHB), polyhydroxyvalerate (PHV), polyhydroxyhexanoate (PHH) and polyhydroxyoctanoate (PHO). PHB is the first class of PHA identified and studied. It has many properties similar to that of conventional plastics produced from petroleum resources [7]. PHB is synthesized by bacteria, as intracellular granules for energy storage. They are produced under the limited concentration of O, N, P, S or trace elements and high concentration of carbon. Normally nutrients present in growth medium are used for the synthesis of proteins. But when nitrogen concentration is limiting, protein synthesis ceases and PHB is synthesized [8]. Alcaligenes eutrophus is the main producer of PHB. It accumulates PHB up to 80% of its dry weight [7]. For modern plastic materials, a range of materials (both renewable and non-renewable) are used as feedstock. Figure 1 illustrates the various sources utilized as feedstock for biopolymer production. Plastics produced from non-renewable feedstock are usually petroleum-based and fortified using glass or carbon fibers. Renewable feedstock source includes microbially-grown polymers and those extracted from starch. Such materials can be reinforced by natural fibers, from plants such as hemp, jute, flax, and other cellulose sources [9]. The aim of this review is to provide an overview of PHA accumulating microorganisms and the renewable sources that can be used to produce PHB in a cost-effective manner. Biosynthetic pathways of PHB, its physicochemical properties, extraction procedures, analytical studies, degradation studies and applications in various fields are also analyzed.
Biodegradable Polymers
Biodegradable polymers are defined in a variety of ways in the literature. In ASTM (American Society for Testing and Materials) D 6400-99, biodegradable polymers (plastics) are degraded by the action of naturally occurring microorganisms .such as fungi, bacteria, and algae. Compostable polymers undergo degradation by biological processes during the composting process, breaking down into CO2, H2O, inorganic compounds and biomass at a rate consistent with other compostable materials and leave no distinguishable toxic residue in the environment. According to the ISO (International Organization for Standardization) definition of biodegradable polymers, only a chemical change in the material by microorganisms is required. The DIN (German Institute for Standardization) standard, in contrast, demands the conversion of polymers into microbial metabolic products. However, the most common definition is ‘Polymers which are degraded to compostable products under normal environmental conditions (aerobic and anaerobic) within an acceptable period of time after their useful life, are termed biodegradable polymers’. These polymers degrade and decompose through the action of different types of microorganisms such as fungi, bacteria, yeasts, and actinomycetes which are present in the environment. The polymeric product is biodegraded under specific environmental conditions to a specific extent within the given time, as defined in the test method. This is an irreversible process, causing changes in structure, loss of properties and, ultimately disintegration into small fragments of non-visible, non-toxic residue for release into the environment. The polymers which degrade in the environment via several mechanisms, concluding in absolute biodegradation that leaves no evidenced residue left behind in the environment are defined as environmentally acceptable degradable polymers. Biological activity is the basis of biodegradation particularly by the action of the enzymes that direct to considerable alteration in the chemical structure of the degraded polymers. During degradation, polymers must break down completely into simple molecules such as carbon dioxide and water within a defined time period. However, thickness and geometry of the degradable products highly influence the rate of biodegradation. Although thin films may degrade quickly, thick-walled articles such as plates, food trays, and cutlery may take more than a year to degrade in the open atmosphere [10, 11].
Classification of Biodegradable Polymers
Biopolymers are biodegradable, and some are also compostable. Biodegradable polymers are broken down into water and carbon dioxide by microorganisms. Compostable biopolymers can be put into an industrial composting process and will break down by 90% within 6 months. Biopolymers are biodegradable, and some are also compostable. There are primarily two classes of biopolymers: one that is obtained from living organisms and another that is produced from renewable resources but require polymerization [12]. Bioplastics are classified into three main types based on the commercial scale of production: (1) fossil carbon source derived plastics and biodegradable; (2) biomass-derived plastics and biodegradable; and (3) biomass-derived plastics but not biodegradable. Biopolymers may also be divided into different classes [13] based on their production (Fig. 2).
-
Polymers extracted/removed directly from biomass such as polysaccharides (starch, cellulose and proteins).
-
Polymers produced by microorganisms or genetically modified bacteria. To date, this group of biobased polymers consists mainly of the polyhydroxyalkanoates, but developments with bacterial cellulose are in progress.
-
Polymers produced by classical chemical synthesis using renewable biobased monomers. A good example is a polylactic acid, a biopolyester polymerized from lactic acid monomers.
-
Polymers produced by conventional synthesis from synthetic monomers. Examples are aliphatic and aromatic polyesters.
Biopolymers from Agro-resources
Bacterial fermentation processes are performed for synthesizing the building blocks (monomers) of biopolymers from renewable resources such as organic waste, lingo cellulosic biomass (starch and cellulose) and fatty acids. These biopolymers are similar properties to that of conventional plastics. Natural biobased polymers are the other class of polymers that are found naturally, such as proteins, nucleic acids, and polysaccharides. Agropolymers preserve the petrol resources replacing conventional polymers and have common characteristics such as hydrophilicity. They are mainly extracted from plants and are compostable. Since they are produced from renewable resources, they will be an interesting alternative to nondegradable polymers for short-life range applications. Agropolymers can find biomedical applications linked with intrinsic properties and could contribute to carbon footprint reduction in the future. They are classified into polysaccharides and proteins [13,14,15].
Biopolymers from Microorganisms
Microorganisms can be used for the biosynthesis of various biopolymers such as xanthan, dextrans, pullulan, glucans, gellan, alginate, cellulose, cyanophycin, poly(gamma-glutamic acid), levan, hyaluronic acid, cellulose, organic acids, oligosaccharides, polysaccharides, and polyhydroxyalkanoates [16]. Some microorganisms are particularly capable of converting biomass into biopolymers while employing a set of catalytic enzymes. Though the fermentation procedures are costly, they are considered to be best for producing such polymers as they yield best results within the short time [17]. Microorganisms sometimes may or may not depend on a separate polymerization step during fermentation processes since the type of substrate that has to be degraded, the microbe that is involved, and the conditions that are present during the process determines the microbial fermentation processes. However, the major advantage is that biodegradable polymers are totally degraded to water, CO2, and CH4 by anaerobic microorganisms [13, 18].
Biopolymers from Biotechnology
An environmentally conscious alternative for petroleum-derived plastics is to design/synthesize polymers that are biodegradable. The presence of hydrolyzable or oxidizable linkage on the polymer main chain, the presence of suitable substituents, correct stereo configuration, balance of hydrophobicity and hydrophilicity, and conformation flexibility contribute to the biodegradation of hydrolysable polymers, which proceeds in a diffuse manner, with the amorphous regions degrading prior to the degradation of the crystalline and cross-linked regions. In this context, the biotechnological approaches are being increasingly recognized as a key to developing better biodegradables and low-cost biopolymers. Fully biodegradable synthetic polymers, such as polylactic acid (PLA), polycaprolactone (PCL), and polyhydroxybutyrate-valerate (PHBV), have been commercially available since 1990. However, these synthetic polymers from natural resources are usually more expensive than petroleum-based polymers [19]. For decades, scientists have been fascinated with microbial production of PHB [20, 21]. As an alternative approach, microorganisms have been engineered to produce hydroxyalkanoates (HAs) [22, 23]. Besides wild-type strains, recombinant strains are also being developed. Various types of recombinant Escherichia coli strains are able to synthesize PHA to high intracellular levels and some are amenable to genetically mediated lysis systems to facilitate the release of the PHA granules [13, 24].
Biopolymers from Synthetic Monomers
A large number of biodegradable polyesters are based on petroleum resources, obtained chemically from synthetic monomers. According to the chemical structures, we can distinguish polycaprolactone, aliphatic copolyesters, and aromatic copolyesters. All these polyesters are soft at room temperature. These are polymers that do not occur in nature as such, and, therefore, when they end up in the natural environment, they represent a durable foreign object, since they cannot be incorporated into the natural cycle. It is estimated that the discarded plastic bottle remains in the natural environment for 450 years. Among these polymers, degradability is otherwise achieved with the integration of hydrolytically unstable bonds into the polymer (e.g., esters, amide groups) [13, 25].
Polyhydroxyalkanoates
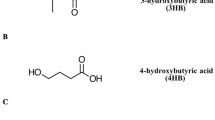
Polyhydroxyalkanoates (PHAs) are among the most investigated biodegradable polymers in recent years. They are superior to other biodegradable polymers because of the large number of different monomer constituents that are incorporated. These polyesters have chemical and physical properties similar to conventional plastics, which add to their biodegradability and biocompatibility [26]. PHAs are mainly produced from renewable resources by fermentation. A wide variety of prokaryotic organisms accumulate PHA from 30 to 80% of their cellular dry weight. PHAs are considered biodegradable and thus suitable for, e.g., short-term packaging, and also considered as biocompatible in contact with living tissues and can be used for biomedical applications [27]. PHAs are polyesters of hydroxyalkanoates with the general structural formula as shown in Fig. 3, where n varies from 600 to 35,000 and if R = hydrogen Poly(3-hydroxypropionate) R = methyl Poly(3-hydroxybutyrate) R = ethyl Poly(3-hydroxyvalerate) R = profile Poly(3-hydroxyhexanoate) R = pentyl Poly(3-hydroxyoctanoate) R = nonyl Poly(3-hydroxydodecanpate) PHAs are generally classified into short-chain-length PHAs (sCL-PHAs) and medium-chain-length PHAs (mCL-PHAs) by the different number of carbons in their repeating units. For instance, sCL-PHAs contain four or five carbons in their repeating units, while mCL-PHAs contain six or more carbons in the repeating units. Accumulation of PHA by microorganisms can be stimulated under unbalanced growth conditions, for example, when nutrients such as nitrogen, phosphorus, or sulfate become limited; when the oxygen concentration is low; or when the C: N ratio of the feed substrate is higher [28]. During starvation, PHA serves as a carbon and energy source and is rapidly oxidized thereby retarding the degradation of cellular components, combating the adverse conditions as in rhizosphere [29]. Besides functioning as a carbon and energy storage compound, other possible functions of PHA have been gaining interest. Studies on the ability of a microbial cell to take up genetic material from an external medium (known as competence) have led to the identification of another type of PHA that is a constituent of cytoplasm and cytoplasm membranes [30]. PHA production in bacterial cells was initially described by Beijerinck and colleagues [31]. PHA inclusions within the cells were first described as lipids by biochemists; however, in 1925 Lemoigne determined the inclusion bodies to be polyhydroxybutyrate (PHB). Lemoigne first reported the discovery of PHB as a component of the bacterium Bacillus megaterium in 1926 [32]. An improved fermentation process, a more efficient recovery/ purification process, and the use of inexpensive carbon sources for the production of PHB have also been found to substantially reduce the cost of production [33]. Microbial PHA is a potential renewable biopolymer with properties closely resembling some common petrochemical plastics. Because of the vast range of structurally different monomers that can be polymerized by microbes, a wide range of material properties can be achieved [34]. PHA production starts in response to stress imposed on cells, usually by nitrogen or phosphorus limitation, in the presence of an abundant carbon source. Under these conditions (PHA accumulation phase), the cells do not grow or divide but instead divert their metabolites toward the biosynthesis of hydroxyalkyl-CoA (HA-CoA). HA-CoA is an activated monomeric precursor that is polymerized by the enzymatic action of PHA synthase to form PHA polyester. Being insoluble in water, PHA begins to form amorphous and nearly spherical granules that gradually fill the cells and force them to expand. The most widely produced PHA is PHB, which is water insoluble and relatively resistant to hydrolytic degradation. PHB is soluble in chloroform and other chlorinated hydrocarbons. Further, PHB is suitable for medical applications like bone plates, nails, screws, and in the treatment of osteomyelitis. PHB has its melting point at 175 °C and glass transition temperature of 15 °C [27]. Polyhydroxyalkanoates are different types and they can be structurally classified on the basis of a number of carbon atoms and the types of monomers (Fig. 3). They can be classified into three:
General structure of PHA, n = 1, 2, 3 to several thousand [35]
-
Short chain length PHAs (scl-PHAs): the monomers consist of 4–5 carbon atoms. Examples include poly (3-hydroxy butyrate) and poly (4-hydroxy butyrate) [36].
-
Medium chain length PHAs (mcl-PHAs): they consist of 6–14 carbon atoms. Examples include poly(3-hydroxy hexanoate) and poly(3-hydroxy octanoate) [37].
-
Scl-mcl PHA copolymer consists of monomers with 4–12 carbon atoms [38].
-
Long chain length PHA (lcl PHA): they consist of more than 15 carbon atoms [39].
Biosynthetic Pathways for PHA Production
Synthesis of PHA can be carried out by chemical or biological methods. High molecular weight PHA can be obtained when they are synthesized by biological approaches. But the structure of PHA cannot be predicted when they are obtained biologically [40]. There are almost 250 different bacterial species that can produce PHB. But only a few of them like Alcaligeneslatus, B. megaterium, C. necator, and P. oleovoranscan biosynthesize PHB [41]. There are several pathways for PHA generation and they mainly depend on the strain of microorganisms employed [42]. The biosynthetic pathways of polyhydroxyalkanoate are completely associated with the central metabolic pathways of bacteria like Glycolysis, Citric acid cycle or Krebs cycle, β-oxidation pathway, de novo fatty acid synthesis pathway, Calvin cycle and serine pathway. Under nutrient excess conditions, large amounts of coenzyme-A will be produced from the Citric acid cycle and this blocks the PHA synthesis by suppressing 3-ketothiolase. When the nutrients are limiting, the amount of coenzyme A produced will not be sufficient for inhibition of 3-ketothiolase and hence acetyl CoA will be guided towards PHA synthesis pathway [35].
The general biosynthetic pathway of polyhydroxybutyrate involves three different enzymes catalyzing three different reactions. The three enzymes are coded by three different genes. In the first reaction, two acetyl coenzyme A (acetyl -CoA) molecules condense to form acetoacetyl CoA [38, 42]. This reaction is catalyzed by the enzyme β-ketothiolase, coded by phbA gene and it catalyzes the formation of a carbon–carbon bond [43]. In the second reaction, acetoacetyl CoA A is reduced to (R) – 3 hydroxybutyryl CoA by the enzyme acetoacetyl CoA reductase, which is NADPH dependent. Acetoacetyl CoA reductase is coded by phb B gene. In the third reaction, PHB synthase coded by phb C gene catalyzes the polymerization of R-3-hydroxybutyryl-CoA to PHB [35, 38, 43]. The genes coding the enzymes responsible for PHB biosynthesis are arranged in an operon phbCAB.The reactions are shown in Figs. 4 and 5. The key molecule that provides the substrate 3-hydroxyalkanoyl—CoA for PHA synthesis is acetyl CoA. The substrate 3-hydroxyalkanoyl-CoA can also be obtained from the β-oxidation of fatty acids. Acyl CoA produced in the β-oxidation pathway pass into the PHA synthesis process. The precursor for PHA synthesis 3-hydroxy acyl CoA is supplied in the process by the action of various enzymes. Those enzymes are 3-keto acyl CoA reductase, epimerase, (R)-enoyl-CoA hydratase/enoyl CoA hydratase 1, acyl CoA oxidase and enoyl CoA hydratase 1 [35]. Most of the Pseudomonas species use this pathway of PHA synthesis. PHA is also synthesized from many other precursors and these reactions are catalyzed by different enzymes. In recombinant E. Coli, PHA precursor 3-hydroxyacyl-CoA is supplied by the action of the enzymes 3-hydroxyacyl-ACP-CoA transferase and malonyl-CoA-ACP-transacylase and the reaction is catalyzed by the enzyme PHA synthase. In pathway seen in Rhizobium, (S)-(+)-3-hydroxybutyryl-CoA is oxidized to PHB by NADH dependent acetoacetyl CoA reductase. 4-hydroxybutyrate containing PHA in the organism Clostridium kluyveri is synthesized from 4-hydroxybutyryl-CoA by the enzymes succinic semialdehyde dehydrogenase, 4-hydroxy butyrate dehydrogenase, and 4-hydroxy butyrate- CoA:CoA transferase. In mutants and recombinants of Alcaligenes eutrophus, 4,5-alkanolactone is converted to the PHA precursor 4,5-hydroxy acyl CoA by the enzymes lactonase and hydroxy acyl-CoA synthase. 4-hydroxybutyrate is produced by the oxidation of 1,4-butanediol in A. hydrophila 4AK4 and it is then converted to 4-hydroxy butyryl CoA. The reactions are catalyzed by the enzyme alcohol dehydrogenase. Pathway in Acinetobacter species and Brevibacterium species involves the conversion of 6-hydroxy hexanoate to 6-hydroxyhexanoate-containing PHA by the enzymes cyclohexanol dehydrogenase, cyclohexanone monooxygenases, caprolactone hydrolase, 6-hydroxyhexanoate dehydrogenase, 6-oxohexanoate dehydrogenase, semialdehyde dehydrogenase, putative 6-hydroxy hexanoate dehydrogenase and putative hydroxy acyl-CoA synthase [40].
Properties of PHA
Most of the PHAs have properties identical to that of petroleum-based polymers like polypropylene, polystyrene, and polyethylene [44, 45]. Polyhydroxyalkanoates are high molecular weight, biodegradable compounds that are insoluble in water and soluble in chlorinated hydrocarbons and chloroform. They are UV resistant but have poor resistance against acids and bases. They are biocompatible and non-toxic. The properties of PHB can be compared with that of conventional plastics because they have an almost same degree of crystallinity, melting temperature, tensile strength and degradation temperature [45,46,47] (Table 1). The family of polyhydroxyalkanoates (PHA) exhibits a wide variety of mechanical properties from hard crystalline to elastic, depending on the composition of monomer units [24]. Solid-state PHB is a compact right-handed helix with a two-fold screw axis (i.e. two monomer units complete one turn of the helix) and a fibre repeat of 0.596 nm [26]. The stereoregularity of PHB makes it a highly crystalline material. Its melting point is around 177 ~ close to that of polypropylene, with which it has other similar properties, although the biopolymer is stiffer and more brittle. The densities of crystalline and amorphous PHB are 1.26 and 1.18 g/cm3, respectively [24]. PHB is water-insoluble and relatively resistant to hydrolytic degradation. This differentiates PHB from most other currently available bio-based plastics which are either moisture or water soluble. Mechanical properties of PHB like Young’s modulus and tensile strength are close to that of polypropylene though an extension to break is markedly lower than that of polypropylene [24, 48]. However, due to the high stereoregularity of biologically produced macromolecules, PHB is a highly crystalline polymer that is stiff and brittle. It is also thermally unstable during processing [49]. The molecular weight of PHB degrades significantly at temperture just above the Tm. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) combines the thermo-mechanical properties of PE (strength, flexibility, ductility, toughness, elasticity) with the physical–chemical properties (compatibility) of polyesters (printability, dyeability, barrier performance) [50].
Microorganisms Producing PHA
Both prokaryotic and eukaryotic type of microorganisms can produce different types of PHAs. PHAs have also been reported in blood and tissues of human and animals, and are used to control of seizure, metabolic disease, and increasing cardiac efficiency. Different bacteria produce a different type of PHAs [53]. They are synthesized by some wide range of Gram-positive and Gram-negative bacteria in aerobic and anaerobic environments. PHAs are accumulated as inclusions in the bacterial cytoplasm in response to inorganic nutrient limitations when the microbes are cultured in the presence of an excess carbon source. At present, PHAs are classified into two major classes: short chain length PHAs (scl-PHAs) with C4–C5 monomers and medium chain length PHAs (mcl-PHAs) with C6–C14 monomers. They were first described by Lemoigne, who observed that Bacillus megaterium produced an intracellular polymer that contained hydroxybutyrate monomers, later called polyhydroxybutyrate (PHB), which is the most widely produced scl-PHA by bacteria. Several decades later, other related biopolyesters with longer side chains (mcl-PHAs) began to be seen. Their production in the laboratory was first reported for Pseudomonas putida GPo1 (formerly known as Pseudomonas oleovorans GPo1). Depending on the organism, PHA production can reach levels as high as 90% of the cell dry weight. When the environment becomes more hospitable, the PHAs are biodegraded to the corresponding monomers, which are used as carbon and energy sources. First investigations of purified scl-PHA granules from Bacillus and mcl-PHA granules from P. putida GPo115 demonstrated that the granules contained protein and lipid materials besides PHA. It was clear that PHAs, being highly hydrophobic polymers, must be separated from the cell cytoplasm, suggesting that the lipids found associated with the PHA probably derived from membrane structures that surrounded the PHA. The nature of the PHA–lipid membrane interaction and the structure of this membrane have been the subject of considerable debate in the PHA field. Freeze-fracture electron micrographs of slowly growing P. putida GPo1 have shown that the boundary (lipid) layer around the large PHA granules formed in such cells is too thin to be a bilayer membrane, leading to the conclusion that PHA granules must be surrounded by a lipid monolayer. When such cells are fractured, fracture faces arise due to the separation of this phospholipid monolayer from the hydrophobic polyester granule. Similar observations have been reported for freeze-fractured PHA granules of Bacillus cereus. In some microbial strains, PHA accumulation can also appear in parallel to biomass production. This ‘growth-associated’ PHA accumulation is known for Alcaligenes latus, Methylobacterium sp. ZP24, Bacillus mycoides RLJ B-017 and recombinant E. coli. Additionally, a hyperproduction of PHA after a period of carbon starvation was described for Pseudomonas 2F. For PHA-producing microbial cells, PHAs serve as reserve materials for carbon and energy. Under conditions of starvation, these reserve materials can be mobilized, thus providing the cell with an advantage for survival [54]. Table 2 provides an overview of the PHA-producing genera that have been reported in the literature.
Production of PHA
PHA production involves strain development, shake flask optimization, lab and pilot fermentor studies and then industrial scale-up. Effective microbial production of PHA depends on several factors, including the final cell density, bacterial growth rate, percentage of PHA in cell dry weight (CDW), time taken to reach high final cell density, substrate to product transformation efficiency, price of substrates, and a convenient and cheap method to extract and purify the PHA. So far, only a few PHA is produced in large scale for commercial exploitations. All of these PHAs are produced using a fed-batch process to achieve high cell density growth. However, continuous processes are important for reducing the cost of PHA production. Increasingly, it becomes attractive to produce PHA using mixed cultures [56].
Fed-Batch Process
For most of the SCL PHA, CDWs can reach around 100 g/L CDW after 48–60 h of fermentation containing approximately 80% PHA in CDW. When Ralstonia eutropha is used, around 200 g/L CDW containing > 80% PHA, namely PHBV can be achieved. While mCL-PHA is most difficult to reach high cell density due to the heavy demand for oxygen by Pseudomonas sp. that are obligate aerobes. For example, when Pseudomonas entomophila was used to make PHA using fatty acids as substrates, CDW went down to around 20 g/L due to the intensive foaming problem resulted from fatty acids. Reduction in aeration to avoid foaming led to reducing CDW.
Although fed-batch processes are efficient to achieve high cell density fermentation in most cases, it suffers from having to interrupt the fermentation process and cleaning up the entire fermentation system including re-sterilization. All these operations increase the complexity of the process, leading to high PHA production cost [56].
Continuous Process
The continuous process offers the advantages of maintaining growth conditions constantly, allowing the cells to grow to relatively high density and maintaining that density for a long period of time. Since conditions are constant, CDW, PHA content, and PHA monomer compositions can be maintained relatively stable and reproducible during the continuous processes. However, microbial contamination is a setback especially for continuous long fermentation processes that are more prone to attract infections. To avoid contamination, it is important to select microorganisms that are robust in growth and that growth conditions selectively favor the production strains. Recently, the author’s lab found that some Halomonas sp. were able to grow to a high CDW in the presence of high salt concentrations such as 35–80 g/L NaCl and high pH of 8–11. Since most non-halophilic bacteria are not able to grow under the high NaCl conditions and high pH, these PHA-producing strain grow to over dominate others.
Continuous fermentation processes were conducted using the Halomonas species TD01 for at least two weeks without contaminated by other microbes. At the end of the fermentation, CDW reached over 80 g/L containing around 75% PHA. The process has been optimized by the industries in the presence of seawater instead of NaCl aqueous solution to reach over 100 g/L CDW containing over 80% of PHA (unpublished results). Most importantly, the continuous fermentation process was run for at least two weeks without bacterial contamination under 60 g/L NaCl and pH 8–9 and under open (unsterile) conditions. Such a process can save not only fresh water but also energy cost as well as intensive labors for cleaning the fermentation systems. In the future, such process should be developed to use cellulose as a substrate to avoid food versus fuels or food versus chemicals disputes [56].
Mixed Cultures
Many studies on PHA production have been focused on industrial biotechnology-based production methods using pure culture technology and genetically modified microorganisms. Due to the high costs of sterilizing equipment and the substrate, as well as the batch-wise processing, PHA production is complicated and expensive. To lower the PHA production cost, some studies used a strategy called microbial community engineering for the enrichment of PHA-producing biomass. They require nonsterile substrate and operational conditions. PHA-producing microorganisms selected from the natural environment, instead of a certain type of modal bacterium, are used for PHA production. Additionally, the PHA production process can be operated continuously. Several studies have investigated the possibility of implementing the microbial community engineering process for PHA production using synthetic wastewater. It has proven possible to enrich a stable microbial community for PHA production while reaching a comparable productivity in terms of maximum PHA content (90% of CDW) and biomass-specific productions rate to pure culture process. The results demonstrate a possibility to produce valuable chemicals while treating wastewater. Agro-industrial waste streams instead of artificial substrates for PHA production were also used, including effluents of sugar factories, oil mills, wood mills, paper mills, or municipal wastes. However, the PHA storage capacity obtained from these studies was still significantly lower than the microbial enrichments selected on synthetic feedstock, reaching only PHA content around 55% of the dry weight. A study conducted by Albuquerque et al. obtained the currently best PHA storage capacity from real wastewater of 75% of the CDW. Nevertheless, the process should be further optimized to increase the PHA storage capacity of enrichments selected from agro-industrial wastewater. PHA production from wastewater opens a new way for not only low-cost material production but also for low-cost production of PHA-based biofuels including 3-hydroxybutyrate methyl ester (3HBME) and 3-hydroxyalkanoate methyl esters (3HAME). Palm oil could also be a raw material resource for PHA production. Plant oils are known to generate higher PHA yields due to higher carbon content per gram of oil compared to sugars. Among various oils, palm oil is being studied extensively for the production of various types of PHA. It has been confirmed that high yield production of PHA could be realized from palm oil and its by-products. The studies provide preliminary results on the efficiency of palm oil bioconversion into PHA and future implementation of these substrates for PHA production systems [56].
Renewable Substrates for PHA Production
The commercialization of PHA reminds unsuccessful till now even though there are numerous advantages of using biodegradable plastics. PHA could not currently compete with the bulk production of petrochemical plastics because of their high production cost. Significant exertions have been dedicated to decrease those processing cost through the improvement of proficient bacterial strains, fermentation and recovery processes. The expense of the substrate is the major cost in the PHA production. Therefore, appropriate selection of carbon substrate becomes a significant step that determines the overall proficiency of the bacterial fermentation and final product cost. Thus, adopting renewable, inexpensive and most readily available carbon substrates that might accept both the microbial growth and PHA production is the simplified approach. Different carbon sources can be utilized by the microorganism starting from inexpensive, complex waste effluents to plant oils, fatty acids, alkanes and as well as simple carbohydrates for the production of PHA. Waste materials are discharged in huge amount from food processing and agricultural industries throughout the year which can be an excellent renewable feedstock for PHA production. Exploiting these waste materials for PHA production as a carbon source not just reduces the substrate cost as well as recovers the waste disposal cost [41]. For cost-effective production of PHA, agro-wastes like fruit peels, bagasse and deoiled cakes were screened as a sole source of carbon. Halomonas campisalis MCM B-1027, which was isolated from one of the extreme environment, i.e. Lonar Lake, India, was explored for the production of PHA using fruit peels and bagasse having fermentable sugars. Among the agro-wastes tested, 1% (v/v) aqueous extract of bagasse was found to be the optimum carbon source with 47% PHA production on dry cell weight basis. Significant amount of total sugars are utilized and converted into cell mass and PHA, e.g. 62% sugar utilized from bagasse extract, 84% from orange peel extract and 71% from banana peel extract as compared to 51% in case of maltose. Hence the cost of production would be positively reduced [57]. Production of poly(3-hydroxyalkaonates) (PHA) by Pseudomonas aeruginosa 42A2 from agro-industrial oily wastes was studied. PHA accumulation, throughout the cell cycle, was observed as intracellular accumulation associated to polyphosphate granules. A 54.6% PHA accumulation was obtained when technical oleic acid (TOA) was used as carbon source. Molecular weight of the polymer was 54.7 Da. PHA accumulation ranged between 66.1% when waste-free fatty acids from soybean oil (WFFA) were used as carbon substrate, 29.4% when waste frying oil (WFO) was used and 16.8% when glucose was used [58]. Nutrients such as ammonium, nitrite, and phosphate reached as low as zero within 15 days of incubation, indicating the system’s bioremediation capability while yielding valuable cyanobacterial biomass for PHB extraction. Maximum PHB accumulation in A. fertilissima was found in sedimented fish pond discharge at 20 cm culture depth with stirring and an initial inoculum size of 80 mg dry cell weight (dcw)/liter. Under optimized conditions, the PHB yield was boosted to 92, 89, and 80 g/m2, respectively for the summer, rainy, and winter seasons. Extrapolation of the result showed that a hectare of A. fertilissima cultivation in fish pond discharge would give an annual harvest of 17 tons dry biomass, consisting of 14 tons of PHB with material properties comparable to those of the bacterial polymer, with simultaneous treatment of 32,640 m3 water discharge [59]. PHA producing bacteria from soil SPY-1, produced PHA better with the hydrolyzed seed thus the seed can be utilized as a cheap carbon source, for the screened bacteria to grow and accumulate PHA in the production medium. The strain had the capacity to accumulate 80% of the dry cell weight as PHA [60]. Isolates such as Bacillus cereus, Bacillus subtilis, and Bacillus megaterium were further explored for their potential to produce PHB using different low-cost agro-industrial materials. PHB production was studied using agro-industrial materials like jawar stem, neera, cashew apple pulp, sugar cane bagasse, coconut pulp and grapes pulp. Extraction of PHB was done by hot chloroform method. PHB production was quantified using crotonic acid assay. Highest cellular PHB content was obtained from Bacillus subtilis with Neera as a source of carbon which was found to be 0.284 g/L [61]. Bacillus megaterium was explored for a potential to synthesize polyhydroxyalkanoates by the using of different Carbon and nitrogen sources. The presence of biopolymer granule in cells of Bacillus megaterium strain L9 indicated that the accumulation was depended on the ratio of carbon and nitrogen sources in the used culture medium. The highest polyhydroxyalkanoates accumulation (0.25 g/L) was obtained by the using glucose and NH4Cl. Furthermore, regarding the utilization of beet molasses as sole carbon source in the culture medium at concentration of 3%, has induced considerably the polyhydroxyalkanoates yield accumulation after 48 h of growth (41% w/w), whereas the highest biomass (0.6 g/L) was obtained at concentration of 4% beet molasses supplemented with 0.05% ammonium chloride [62]. Inexpensive cardboard industry wastewater was tried as a carbon source to produce PHB. Different bacterial strains were isolated from soil and screened for polyhydroxybutyrate production using cardboard manufacturing industry wastewater as a carbon source. The bacterial isolate Bacillus sp. NA10 can be regarded as a potential strain for conversion of cardboard industry wastewater into PHB. The selected isolate efficiently utilized cardboard industry wastewater as sole carbon source for growth and PHB biosynthesis, accumulating PHB up to 66.6% of the cell dry mass with 0.072 g/L/h productivity [63]. The biosynthesis of polyhydroxybutyrate by Pseudomonas aeruginosa grown on reducing sugar hydrolysate obtained from raw cassava starch as the sole carbon source and di-ammonium sulphate as the limiting nutrient was investigated. The results obtained show that the hydrolysate supported the growth of the organism and when the fermentation was shut down after 84 h, a biomass yield on substrate (Yx/s) of 0.186 g/g was obtained with corresponding product yield on substrate (Yp/s) of 0.106 g/g. The results also show that the organism accumulated polyhydroxybutyrate in excess of 50% of the cell dry weight by giving a final polyhydroxybutyrate yield on biomass (Y p/x) of 0.577 g/g which agrees with the general trend in polyhydroxybutyrate production [64]. Table 3 presents the list of different renewable substrates utilized by several microorganisms for PHA production.
Recovery and Extraction of PHA
The recovery and purification of biopolymers are known to contribute significantly to the overall PHAs biomanufacturing costs. Intracellular accumulation of PHAs and a relatively low content of the product can result in a high recovery cost. Despite intensive investigations carried out in the area of PHA biosynthesis, research on Downstream processing (DSP) of PHA is limited. Several studies on isolation and purification of PHA were published recently, their final aim was to develop competitive processes for industrial implementation. Numerous factors should be taken into account when selecting the PHAs recovery method such as the PHAs producer, type and composition of biopolymers, product purity requirements, impact on the PHAs properties, cost and environmental considerations. Broadly, there are two schemes for recovering PHA from the reaction medium post fermentation, i.e., dissolving biomass to separate PHAs granules with strong oxidants such as acids, or surfactants, and extracting PHAs directly from the biomass using suitable solvents (Fig. 6). The role of solvent is to change the permeability of cell membrane and then to dissolve the polymer inside the cells. A pretreatment step could be used to achieve better recovery, and purification step to obtain higher purity of the biopolymer [92].
Characterization of PHA
Most of the techniques used for the characterization of polymers can be utilized for the characterization of biopolymers. Characterization of biopolymers has two purposes:
-
Development of parameters for processing;
-
Determination of end-use performance characteristics.
Through characterization, the most important properties of interest are molecular mass, polydispersity, size, the degree of association, conformation, interaction, and so on. Some of the characterization techniques which are used for the analysis of biopolymers are listed in the following.
Quantification of PHA by Spectrophotometer
The crotonic acid assay is performed to determine the purity of polymer. The polymer is dissolved in chloroform and converted to crotonic acid by the addition of concentrated sulfuric acid and heating in a water bath. PHA can be quantified by spectrophotometer at 235 nm using sulfuric acid blank [93].
Nuclear Magnetic Resonance
NMR studies are carried out to determine the structural composition of PHA polymer. It is used to identify PHA double bonds. Two types of NMR techniques are available and they are 1H-NMR and 13C-NMR [35]. 13C-NMR can be performed at 75.4 MHz [94] or at 80 MHz [93]. 1H-NMR can be performed at 400 MHz [93] or at 10,330 Hz. Tetramethylsilane is used as the internal standard.
Differential Scanning Colorimetry
DSC is performed to determine the thermal properties of PHA like glass transition temperature (Tg), melting temperature (Tm), heating crystalline temperature (Thc), cooling crystalline temperature (Tcc), and degree of crystallization (Xc). For performing DSC, the samples are first heated up to a temperature and then cooled suddenly and then again heated. The temperature conditions applied for DSC is different for each. Samples are heated from 25 to 200 °C at a rate of 10 °C min−1. They are held at 200 °C for 2 min and then quenched to -50 °C. It is again heated to 200 °C at a rate of 10 °C min−1 [94].
Thermogravimetric Analysis
TGA is performed to study the thermal stability of PHA polymer [95]. This technique also involves consequent heating and cooling steps. The polymer is heated from 30 to 180 °C at a rate of 10 °C min−1, held at 180 °C for 5 min, cooled to − 100 °C at a rate of 50 °C min−1 and then reheated to 800 °C at 20 °C min−1 [93, 96].
Gas Chromatography
Analysis of PHA using GC provides information of the total amount and the composition of PHA. By combining GC with MS we can obtain information regarding the mass and monomers involved. GC can be performed only with volatile or semi-volatile compounds. So, in order to subject PHA to GC analysis, it has to be hydrolyzed and acylated. Sample volume is 1 µL and the carrier gas can be helium [94] or nitrogen [97]. The column used is 5% diphenyl–95% dimethyl polysiloxane with an inner diameter of 0.25 mm. The injection port is held at temperature 280 °C. Oven housing column is held at 100 °C for 3 min and then raised to 280 °C at a rate of 8 °C /min and held for 2 min. It is then raised to 310 °C at 20 °C /min and held for 10 min. Products are detected by flame ionization detector [66].
Fourier Transform Infrared Spectroscopy
FTIR is performed to determine the crystallinity index of the polymer. Knowledge of crystallinity index is important because degradation depends on the crystallinity of the polymer. FTIR is performed at a spectral range of 4000–400 cm−1 and the resolution is between 4 and 6 cm−1 [96, 98].
Gel Permeation Chromatography
GPC is performed to determine the molecular weight of PHA polymer [99], i.e weight average molecular weight (Mw), number average molecular weight (Mn) and size average molecular weight (Mz) [98] of the polymer. The polymer is dissolved in chloroform and introduced into the GPC system. Column has dimensions 30 cm × 10 µm. The eluted polymer can be detected using a differential refractometer [94].
High-Performance Liquid Chromatography
High-performance liquid chromatography (HPLC) is performed to determine the purity of PHA. PHB is converted to crotonic acid by treating with concentrated sulphuric acid and the obtained free acid is chromatographed on HPLC column. Sample concentration is in the range 0.2–560 µg/mL and sample injection volume is 10 µL [100].
Applications of Biopolymers
Biopolymers, due to its biocompatible and biodegradable nature, can be used to improve the performances of other biologically active molecules in a product. They can also be modified to suit various potential applications which includes synthesis of nanomaterials, biomedical applications, food industry and packaging [101]. Table 4 furnishes the application of PHA in various fields.
Synthesis of Nanomaterials
Nanotechnology is the science of nanomaterials which deals with its synthesis, characterization, and applications. Researchers are currently focusing on developing more eco-friendly shifted from physical and chemical processes towards “green” chemistry and bioprocesses. Metal nanoparticles, due to their quantum size effects, possess various novel properties. However, most of their synthesis protocol imposes a major threat to the environment. In common synthetic methods, the reducing agents used which include organic solvents and toxic-reducing agents like hydrazine, N-dimethylformamide, and sodium borohydride are considered to be highly toxic to the environment. All these chemicals are highly reactive and pose potential environmental and biological risks. With the increasing interest in minimization of waste and adoption of sustainable processes, the development of green chemistry approaches is desirable. Biopolymers have been extensively used as capping and reducing agent for the synthesis of various nanoparticles. Biopolymers can be used to replace various toxic regents in synthesizing different nanoparticles [101].
Biomedical Applications
In recent years, biopolymer materials have aroused great interest because of their biomedical applications, such as those in tissue engineering, pharmaceutical carriers, and medical devices. A common biopolymer, gelatin, was widely applied in medicine for dressing wounds, as an adhesive, and so on. PHAs have been applied extensively in biomedical engineering, such as tissue engineering and drug-delivery system. Biomaterials made from proteins, polysaccharides, and synthetic biopolymers are preferred but lack the mechanical properties and stability in aqueous environments necessary for medical applications. Cross-linking improves the properties of the biomaterials, but most cross-linkers either cause undesirable changes to the functionality of the biopolymers or result in cytotoxicity. Glutaraldehyde, the most widely used cross-linking agent, is difficult to handle and contradictory views have been presented on the cytotoxicity of glutaraldehyde-crosslinked materials [101].
Food Industry
Replacing the oil-based packaging materials with biobased films and containers might give not only a competitive advantage due to more sustainable and greener image but also some improved technical properties. Biopolymers are currently used in food coatings, food packaging materials, and encapsulation matrices for functional foods. They provide unique solutions to enhance product shelf life while also reducing the overall carbon footprint related to food packaging. Within food-related applications, these biobased materials are particularly useful in three main areas: food packaging, food coating, and edible films for food and encapsulation. The most commercially viable materials in food packaging are certain biodegradable polyesters and thermoplastics like starch, PLA, PHA, and so on, which can be processed by conventional equipment. These materials are already used in a number of monolayer and multilayer applications in the food-packaging field. The inherent high rigidity and the difficulty of processing them in conventional equipment are the main drawbacks of these types of materials. The hydrophilic nature of most of the biopolymers affects their use as high-end products. The absorption of moisture causes plasticization of these materials thereby deteriorating the barrier properties of these materials. Renewable polymers have also been used for encapsulation purposes. Encapsulation has previously been described as a technology to protect sensitive substances against the influences of adverse environments. The term “microencapsulation” refers to a defined method of wrapping solids, liquids, or gases in small capsules, which can release their contents under specific circumstances. Such technologies are of significant interest to the pharmaceutical sector. The increasing interest in edible films and coatings using biopolymers is due to their ability to incorporate a variety of functional ingredients. Plasticizers, such as glycerol, acetylated monoglycerides and polyethylene glycol, which are used to modify the mechanical properties of the film or coating, make significant changes to the barrier properties of the film. However, the major advantage of coatings is that they can be used as a vehicle for incorporating natural or chemical active ingredients, such as antioxidants and antimicrobial agents, enzymes, or functional ingredients, like probiotics, minerals, and vitamins. These ingredients can be consumed with the food, thus enhancing safety, nutritional, and sensory attributes. Edible films can be used as flavor or aroma carriers in addition to providing a barrier to aroma loss [101].
Packaging Applications
Currently, the most commercially viable materials in food packaging are certain biodegradable polyesters, which can be processed by conventional equipment. These materials are already used in a number of monolayer and multilayer applications in the food-packaging field. Among the most widely researched thermoplastics, the sustainable biopolymers used in monolayer packaging include starch, PHA, and PLA. For bio-based food-packaging applications, the most important parameter to be considered is its barrier properties. Hydrophilic polymers usually have poor moisture resistance which causes water vapor transmission through packaging and thus affects the quality of foods. This results in shorter shelf lives, increased costs, and eventually more waste. Another technique to improve the barrier properties of biopolymers is to add various nanofillers like nanoclays, metal oxide nanoparticles, and so on [101].
Conclusion
Research and development in the field of PHA production have been driven by the limited availability of fossil fuel resources, hiking of the petroleum price, and concerns over environmental issues. In the meantime, significant progress has been observed over the last few decades, with innovations of existing technologies and development of novel engineering approaches in the bioproduction of PHAs. The potential of various native PHA-producing bacterial and recombinant strains has been exploited further in order to increase PHAs’ yield and productivity. However, the major limitation for extensive application of PHAs is associated with their high production cost, expensive raw materials, and complicated downstream processes. In this regard, work is underway, looking for reliable processes utilizing cheap raw material from agricultural activities, so that the production cost can be lowered, thus enabling PHAs to compete with the plastics produced from fossil oil. The remarkable production of PHAs utilizing oil fats demonstrates them as a promising resource with greater advantages leading toward commercialization. Moreover, one major progress in PHA improvement allows for a greater use of these materials through the development of heteropolymers to overcome the limitations in properties that earlier restricted their use. This has increased the application of these biopolymers in a variety of fields, most notable being the packaging and biomedical applications [51, 52, 106, 107].
References
Hamieh A, Olama Z, Holail H (2013) Microbial production of polyhydroxybutyrate, a biodegradable plastic using agro-industrial waste products. Glob Adv Res J Microbiol 2:54–64
Rao MG, Bharathi P, Akila RM (2014) A comprehensive review on biopolymers. Sci Rev Chem Commun 4(2):61–68
Thompson RC, Moore CJ, Vom Saal FS, Swan SH (2009) Plastics, the environment and human health: current consensus and future trends. Philos Trans R Soc Lond Biol Sci 364(1526):2153–2166
Babu J, Nath SB, Kodali VP (2014) Isolation, Screening and Extraction of polyhydroxybutyrate (PHB) producing bacteria from sewage sample. Int J Pharm Tech Res 6(2):850–857
Zhu C, Chiu S, Nakas JP, Nomura CT (2013) Bioplastics from waste glycerol derived from biodiesel industry. J Appl Polym Sci 130(1):1–3
Nkwachukwu OI, Chima CH, Ikenna AO, Albert L (2013) Focus on potential environmental issues on plastic world towards a sustainable plastic recycling in developing countries. Int J Ind Chem 4(1):34
Rasheed R, Latha D, Ramachandran D, Gowri GR (2013) Characterization of biopolymer producing Streptomyces parvulus, optimization of process parameters and mass production using less expensive substrates. Int J Bioassay 2(4):649–654
Shivakumar S (2012) Polyhydroxybutyrate (PHB) production using agro-industrial residue as substrate by bacillus. Int J Chem Tech Res 4(3):1158–1162
Kolybaba M, Tabil LG, Panigrahi S, Crerar WJ, Powell T, Wang B (2006) Biodegradable polymers: past, present, and future. ASABE/CSBE North Central Intersectional Meeting. American Society of Agricultural and Biological Engineers
Azapagic A, Emsley A, Hamerton L (2003) Polymers in everyday use: principles, properties and environmental effects. In Polymers: the environment and sustainable development. Wiley, Hoboken, pp 17–46
Karak N (2012) Vegetable oil-based polymers: properties, processing and applications. Woodhead Publishing, Elsevier
Chandra R, Rustgi R (1998) Biodegradable polymers. Prog Polym Sci 23(7):1273–1335
Sivasubramanian V (2016) Biodegradable polymers and its recent perspectives. Environmental sustainability using green technologies. CRC Press, Boca Raton
Babu RP, O’connor K, Seeram R (2013) Current progress on bio-based polymers and their future trends. Prog Biomater 2(1):8
Habibi Y, Lucia LA (2012) Polysaccharide building blocks: a sustainable approach to the development of renewable biomaterials. Wiley, New York
Verma ML (2010) Microbial biosynthesis of biopolymers and applications in the biopharmaceutical, biomedical and food industries. In: Proceedings of the 2010 International Conference on Biomedical Engineering and Assistive Technologies (BEATS), Jalandhar, India, pp. 1–6
Benerji DSN, Ayyanna C, Rajini K, Rao BS, Banerjee DRN (2010) Studies on physico-chemical and nutritional parameters for the production of ethanol from mahua flower (Madhuca indica) using saccharomyces cerevisiae—3090 through submerged fermentation (smf). J Microb Biochem Technol 2:46–50
Santhanam A, Sasidharan S (2010) Microbial production of polyhydroxy alkanotes (PHA) from Alcaligens spp. and Pseudomonas oleovorans using different carbon sources. Afr J Biotech 9(21):3144–3150
Pandey P, Kumar B, Tiwari D (2010) Environmental considerations concerning the release of genetically modified organisms. ProEnvironment Promediu 3(6):381–384
Byrom D (1987) Polymer synthesis by microorganisms: technology and economics. Trends Biotechnol 5(9):246–250
Madison LL, Huisman GW (1999) Metabolic engineering of poly (3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63(1):21–53
Chen GQ, Wu Q (2005) Microbial production and applications of chiral hydroxyalkanoates. Appl Microbiol Biotechnol 67(5):592–599
Ren Q, Ruth K, Thöny-Meyer L, Zinn M (2010) Enatiomerically pure hydroxycarboxylic acids: current approaches and future perspectives. Appl Microbiol Biotechnol 87(1):41–52
Khanna S, Srivastava AK (2005) Recent advances in microbial polyhydroxyalkanoates. Process Biochem 40(2):607–619
Okada M (2002) Chemical syntheses of biodegradable polymers. Prog Polym Sci 27(1):87–133
Braunegg G, Lefebvre G, Genser KF (1998) Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J Biotechnol 65(2):127–161
Avérous L, Pollet E (2012) Biodegradable polymers. In: Avérous L, Pollet E (eds) Environmental silicate nano-biocomposites. Green energy and technology. Springer, London, pp. 13–39
Lafferty RM, Krsatko B, Korsatko W (1988) Microbial production of poly- β-hydroxybutyric acid. In: .Rehm HJ, Reed G (eds) Biotechnology. Springer VCH Weinheim, pp 136–176
Okon Y, Itzigsohn R (1992) Poly-β-hydroxybutyrate metabolism in Azospirillum brasilense and the ecologiocal role of PHB in the rhizosphere. FEMS Microbiol Rev 9(2–4):131–140
Reusch RN, Sadoff HL (1983) D-(−)-poly-beta-hydroxybutyrate in membranes of genetically competent bacteria. J Bacteriol 156(2):778–788
Beijerinck MW (1888) Cultur des Bacillus radicicola aus den Knöllchen. Bot Ztg 46:740–750
Lemoigne M (1926) Produit de déshydratation et de polymérisation de l’acide β-oxybutyrique. Bull Soc Chim Biol 8:770–782
Lemos PC, Serafim LS, Reis MA (2006) Synthesis of polyhydroxyalkanoates from different short-chain fatty acids by mixed cultures submitted to aerobic dynamic feeding. J Biotechnol 122(2):226–238
Kunasundari B, Sudesh K (2011) Isolation and recovery of microbial polyhydroxyalkanoates. Expr Polym Lett 5(7):620–634
Tan GY, Chen CL, Li L, Ge L, Wang L, Razaad IM, Li Y, Zhao L, Mo Y, Wang JY (2014) Start a research on biopolymer polyhydroxyalkanoate (PHA): a review. Polymers 6(3):706–754
Giedraitytė G, Kalėdienė L (2015) Purification and characterization of polyhydroxybutyrate produced from thermophilic Geobacillus sp. AY 946034 strain. Chemija 26(1):38–45
Keshavarz T, Roy I (2010) Polyhydroxyalkanoates: bioplastics with a green agenda. Curr Opin Microbiol 13(3):321–326
Chaitanya K, Mahmood SK, Kausar R, Sunilkumar N (2014) Biotechnological production of polyhydroxyalkonates by various isolates: a review. Int J Pharm Sci Invent 3(9):1–11
Koller M, Salerno A, Dias M, Reiterer A, Braunegg G (2010) Modern biotechnological polymer synthesis: a review. Food Technol Biotechnol 48(3):255–269
Chen GQ (2010) Plastics completely synthesized by bacteria: polyhydroxyalkanoates. In: Chen GQ (ed) Plastics from bacteria, microbiology monographs. Springer, Berlin Heidelberg, pp 17–37
Chee JY, Yoga SS, Lau NS, Ling SC, Abed RM, Sudesh K (2010) Bacterially produced polyhydroxyalkanoate (PHA): converting renewable resources into bioplastics. Curr Res Technol Educ Top Appl Microbiol Microb Biotechnol 2:1395–1404
Reddy CS, Ghai R, Kalia V (2003) Polyhydroxyalkanoates: an overview. Biores Technol 87(2):137–146
Shah KR (2014) Optimization and production of polyhydroxybutyrate by Bacillus subtilis G1S1 from soil. Int J Curr Microbiology Applied Sci 3(5):377–387
Lakhawat SS, Pathak AN, Kulkarni M, Srikanth GV (2012) Mutagenesis of azotobacter vinelandii strain and production of polyβ-hydroxybutyrate from distillery spent wash. BioProcess J 11(3):45–51
Chaijamrus S, Udpuay N (2008) Production and characterization of polyhydroxybutyrate from molasses and corn steep liquor produced by Bacillus megaterium ATCC 6748. Agric Eng Int 1–12
Lakshmi RS, Hema TA, Divya TR, Starin ST (2012) Production and optimization of polyhydroxybutyrate from Rhizobium sp. present in root nodules. J Pharm Biol Sci 3(2):21–25
Ibrahim MH, Steinbüchel A (2009) Poly (3-hydroxybutyrate) production from glycerol by Zobellella denitrificans MW1 via high-cell-density fed-batch fermentation and simplified solvent extraction. Appl Environ Microbiol 75(19):6222–6231
Lee SY (1996) Bacterial polyhydroxyalkanoates. Biotechnol Bioeng 49(1):1–4
Padermshoke A, Katsumoto Y, Sato H, Ekgasit S, Noda I, Ozaki Y (2005) Melting behavior of poly (3-hydroxybutyrate) investigated by two-dimensional infrared correlation spectroscopy. Spectrochim Acta Part A 61(4):541–550
Ewa Rudnik (2008) Properties and applications. Compost Polym Mater 1:(38–69)
Bugnicourt E, Cinelli P, Lazzeri A, Alvarez VA (2014) Polyhydroxyalkanoate (PHA): review of synthesis, characteristics, processing and potential applications in packaging. Expr Polym Lett 8(11):791–808
Ojumu TV, Yu J, Solomon BO (2004) Production of polyhydroxyalkanoates, a bacterial biodegradable polymers. Afr J Biotechnol 3(1):18–24
Raza ZA, Abid S, Banat IM (2018) Polyhydroxyalkanoates: characteristics, production, recent developments and applications. Int Biodeterior Biodegrad 126:45–56
Prieto MA, de Eugenio LI, Galán B, Luengo JM, Witholt B (2007) Synthesis and degradation of polyhydroxyalkanoates. In: Ramos JL, Filloux A (eds) Pseudomonas. Springer, Dordrecht, pp 397–428
Koller M, Atlić A, Dias M, Reiterer A, Braunegg G (2010) Microbial PHA production from waste raw materials. In: Chen GQ (ed) In Plastics from Bacteria. Microbiology monographs. Springer, Berlin, pp 85–119
Chen GQ, Zhang J, Wang Y (2015) White biotechnology for biopolymers: hydroxyalkanoates and polyhydroxyalkanoates: production and applications. In: Industrial biorefineries & white biotechnology. Elsevier, Amsterdam, pp 555–574
Kulkarni SO, Kanekar PP, Jog JP, Sarnaik SS, Nilegaonkar SS (2015) Production of copolymer, poly (hydroxybutyrate-co-hydroxyvalerate) by Halomonas campisalis MCM B-1027 using agro-wastes. Int J Biol Macromol 72:784–789
Fernández D, Rodríguez E, Bassas M, Viñas M, Solanas AM, Llorens J, Marqués AM, Manresa A (2005) Agro-industrial oily wastes as substrates for PHA production by the new strain Pseudomonas aeruginosa NCIB 40045: Effect of culture conditions. Biochem Eng J 26(2–3):159–167
Samantaray S, Nayak JK, Mallick N (2011) Wastewater utilization for poly-β-hydroxybutyrate production by the cyanobacterium Aulosira fertilissima in a recirculatory aquaculture system. Appl Environ Microbiol 77(24):8735–8743
Preethi R, Sasikala P, Aravind J (2012) Microbial production of polyhydroxyalkanoate (PHA) utilizing fruit waste as a substrate. Res Biotechnol 3(1):61–69
Ghate B, Pandit P, Kulkarni C, Deepti DM, Patel TS (2011) PHB production using novel agro-industrial sources from different bacillus species. Int J Pharm Biosci 2(3):242–249
Medjeber N, Abbouni B, Menasria T, Beddal A, Cherif N (2015) Screening and production of polyhydroxyalcanoates by Bacillus megaterium by using cane and beet molasses as carbon sources. Der Pharm Lett 7(6):102–109
Bhuwal AK, Singh G, Aggarwal NK, Goyal V, Yadav A (2014) Poly-β-hydroxybutyrate production and management of cardboard industry effluent by new Bacillus sp. NA10. Bioresour Bioprocess 1(9):1–11
Aremu MO, Layokun SK, Solomon BO (2010) Production of Poly (3-hydroxybutyrate) from cassava starch hydrolysate by Pseudomonas aeruginosa NCIB 950. Am J Sci Ind Res 1(3):421–426
Budde CF, Riedel SL, Hübner F, Risch S, Popović MK, Rha CK, Sinskey AJ (2011) Growth and polyhydroxybutyrate production by Ralstonia eutropha in emulsified plant oil medium. Appl Microbiol Biotechnol 89(5):1611–1619
Zhu C, Nomura CT, Perrotta JA, Stipanovic AJ, Nakas JP (2010) Production and characterization of poly-3-hydroxybutyrate from biodiesel-glycerol by Burkholderia cepacia ATCC 17759. Biotechnol Progr 26(2):424–230
Palmeri R, Pappalardo F, Fragalà M, Tomasello M, Damigella A, Catara AF (2012) Polyhydroxyalkanoates (PHAs) production through conversion of glycerol by selected strains of Pseudomonas mediterranea and Pseudomonas corrugata. Chem Eng Trans 27
Rasheed R, Latha D, Ramachandran D, Gowri GR (2013) Characterization of biopolymer producing Streptomyces parvulus, optimization of process parameters and mass production using less expensive substrates. Int J Bioassays 2(4):649–654
Aravind J, Sasikala P, Preethi R (2012) Production of polyhydroxyalkanoate (PHA) using hydrolyzed grass and Syzygium cumini seed as low cost substrates. J Microbiol Biotechnol Food Sci 2(3):970–982
Altaee N, Yousif AFE, Sudesh K (2016) Recovery and subsequent characterization of polyhydroxybutyrate from Rhodococcus equi cells grown on crude palm kernel oil. J Taibah Univ Sci 10:543–550
Mayeli N, Motamedi H, Heidarizadeh F (2015) Production of polyhydroxybutyrate by Bacillus axaraqunsis BIPC01 using petrochemical wastewater as carbon source. Braz Arch Biol Technol 58(4):643–650
Lee WH, Loo CY, Nomura CT, Sudesh K (2008) Biosynthesis of polyhydroxyalkanoate copolymers from mixtures of plant oils and 3-hydroxyvalerate precursors Bioresource technology 99(15):pp 6844–6851
Kumar BS, Prabakaran G (2006) Production of PHB (bioplastics) using bio-effluent as substrate by Alcaligens eutrophus. Indian J Biotechnol 5(1):76–79
Singh G, Kumari A, Mittal A, Yadav A, Aggarwal NK (2013) Poly β-hydroxybutyrate production by Bacillus subtilis NG220 using sugar industry waste water. Biomed Res Int. https://doi.org/10.1155/2013/952641
Wang B, Sharma-Shivappa RR, Olson JW, Khan SA (2013) Production of polyhydroxybutyrate (PHB) by Alcaligenes latus using sugarbeet juice. Ind Crops Prod 43:802–811
Gouda MK, Swellam AE, Omar SH (2001) Production of PHB by a Bacillus megaterium strain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen sources. Microbiol Res 156(3):201–207
Bhattacharyya A, Pramanik A, Maji SK, Haldar S, Mukhopadhyay UK, Mukherjee J (2012) Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei. AMB Expr 2(1):34
Verlinden RA, Hill DJ, Kenward MA, Williams CD, Piotrowska-Seget Z, Radecka IK (2011) Production of polyhydroxyalkanoates from waste frying oil by Cupriavidus necator. AMB Expr 1(1):11
Peter HY, Chua H, Huang AL, Ho KP (1999) Conversion of industrial food wastes by Alcaligenes latus into polyhydroxyalkanoates. Appl Biochem Biotechnol 78(1–3):445–454
Omar S, Rayes A, Eqaab A, Voß I, Steinbüchel A (2001) Optimization of cell growth and poly (3-hydroxybutyrate) accumulation on date syrup by a Bacillus megaterium strain. Biotechnol Lett 23(14):1119–1123
Chee JY, Tan Y, Samian MR, Sudesh K (2010) Isolation and characterization of a Burkholderia sp. USM (JCM15050) capable of producing polyhydroxyalkanoate (PHA) from triglycerides, fatty acids and glycerols. J Polym Environ 18(4):584–592
Thakor N, Trivedi U, Patel KC (2005) Biosynthesis of medium chain length poly (3-hydroxyalkanoates)(mcl-PHAs) by Comamonas testosteroni during cultivation on vegetable oils. Bioresour Technol 96(17):1843–1850
Yu J, Stahl H (2008) Microbial utilization and biopolyester synthesis of bagasse hydrolysates. Bioresour Technol 99(17):8042–8048
Lee WH, Loo CY, Nomura CT, Sudesh K (2008) Biosynthesis of polyhydroxyalkanoate copolymers from mixtures of plant oils and 3-hydroxyvalerate precursors. Bioresour Technol 99(15):6844–6851
Cavalheiro JM, de Almeida MC, Grandfils C, Da Fonseca MM (2009) Poly (3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem 44(5):509–515
Loo CY, Lee WH, Tsuge T, Doi Y, Sudesh K (2005) Biosynthesis and characterization of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) from palm oil products in a Wautersia eutropha mutant. Biotechnol Lett 27(18):1405–1410
Graciano Fonseca G, Vasconcellos Antonio R (2006) Polyhydroxyalkanoates production by recombinant Escherichia coli harboring the structural genes of the polyhydroxyalkanoate synthases of Ralstonia eutropha and Pseudomonas aeruginosa using low cost substrate. J Appl Sci 6(8):1745–1750
Marsudi S, Unno H, Hori K (2008) Palm oil utilization for the simultaneous production of polyhydroxyalkanoates and rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol 78(6):955–961
Fernández D, Rodríguez E, Bassas M, Viñas M, Solanas AM, Llorens J, Marqués AM, Manresa A (2005) Agro-industrial oily wastes as substrates for PHA production by the new strain Pseudomonas aeruginosa NCIB 40045: effect of culture conditions. Biochem Eng J 26(2–3):159 – 67
Simon-Colin C, Raguénès G, Crassous P, Moppert X, Guezennec J (2008) A novel mcl-PHA produced on coprah oil by Pseudomonas guezennei biovar. tikehau, isolated from a “kopara” mat of French Polynesia. Int J Biol Macromol 43(2):176–181
Pantazaki AA, Papaneophytou CP, Pritsa AG, Liakopoulou-Kyriakides M, Kyriakidis DA (2009) Production of polyhydroxyalkanoates from whey by Thermus thermophilus HB8. Process Biochem 44(8):847–853
Kosseva MR, Rusbandi E (2017) Trends in the biomanufacture of polyhydroxyalkanoates with focus on downstream processing. Int J Biol Macromol 107A:762–778
Salgaonkar BB, Mani K, Braganca JM (2013) Characterization of polyhydroxyalkanoates accumulated by a moderately halophilic salt pan isolate Bacillus megaterium strain H16. J Appl Microbiol 114(5):1347–1356
Valappil SP, Misra SK, Boccaccini AR, Keshavarz T, Bucke C, Roy I (2007) Large-scale production and efficient recovery of PHB with desirable material properties, from the newly characterised Bacillus cereus SPV. J Biotechnol 132(3):251–258
Nair AM, Annamalai K, Kamala Kannan S, Kuppusamy S (2014) Characterization of polyhydroxyalkanoates produced by Bacillus Subtilis isolated from soil samples. Malaya J Biosci 1(1):8–12
Shrivastav A, Mishra SK, Shethia B, Pancha I, Jain D, Mishra S (2010) Isolation of promising bacterial strains from soil and marine environment for polyhydroxyalkanoates (PHAs) production utilizing Jatropha biodiesel byproduct. Int J Biol Macromol 47(2):283–287
Munir S, Jamil N (2015) Characterization of polyhydroxyalkanoates produced by contaminated soil bacteria using wastewater and glucose as carbon sources. Trop J Pharm Res 14(9):1605–1611
Teeka J, Imai T, Reungsang A, Cheng X, Yuliani E, Thiantanankul J, Poomipuk N, Yamaguchi J, Jeenanong A, Higuchi T, Yamamoto K (2012) Characterization of polyhydroxyalkanoates (PHAs) biosynthesis by isolated Novosphingobium sp. THA_AIK7 using crude glycerol. J Ind Microbiol Biotechnol 39(5):749–758
Mizuno K, Ohta A, Hyakutake M, Ichinomiya Y, Tsuge T (2010) Isolation of polyhydroxyalkanoate-producing bacteria from a polluted soil and characterization of the isolated strain Bacillus cereus YB-4. Polym Degrad Stab 95(8):1335–1339
Masood F, Yasin T, Hameed A (2015) Production and characterization of tailor-made polyhydroxyalkanoates by Bacillus cereus FC11. Pak J Zool 47:491–503
Farzana Khan Perveen FK (2015) Recent advances in biopolymers. Intech Publishers, London
Philip S, Keshavarz T, Roy I (2007) Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J Chem Technol Biotechnol 82(3):233–247
Wang JH, Qin J, Chakravarty J, Tsai FJ, Smith RC Jr, Fenwick CD, Wallajapet PR, Osteen DK, Evans EA, Englebert SS (2007) Kimberly-Clark Worldwide Inc, assignee. Nonabsorbent surge layer having discrete regions of superabsorbent and method for making, United States patent US 7,189,888
Zinn M, Witholt B, Egli T (2001) Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv Drug Deliv Rev 53(1):5–21
Hiraishi A, Khan ST (2003) Application of polyhydroxyalkanoates for denitrification in water and wastewater treatment. Appl Microbiol Biotechnol 61(2):103–109
Sabbir A, Fatma T (2014) Polyhydroxybutyrate—a biodegradable plastic and its various formulations. Int J Innov Res Sci Eng Technol 3(2):9494–9499
Ghate B, Pandi P, Kulkarni C, Mungi DD, Patel TS (2011) PHB production using novel agro-industrial sources from different Bacillus species. Int J Pharm Biol Sci 2(3):242–249
Acknowledgements
The authors are thankful to National Institute of Technology Calicut, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sathya, A.B., Sivasubramanian, V., Santhiagu, A. et al. Production of Polyhydroxyalkanoates from Renewable Sources Using Bacteria. J Polym Environ 26, 3995–4012 (2018). https://doi.org/10.1007/s10924-018-1259-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1259-7