Abstract
In this work, the synthesis of biopolyols derived from castor oil and different glycerols was performed by chemical glycerolysis with sodium hydroxide as catalyst at 225 °C. The biopolyol obtained from high purity glycerol led to predominantly MAG formation, whereas the biopolyol produced using crude glycerol, byproduct from biodiesel industry, resulted in higher DAG, TAG and FFA content due to the higher amount of water. Both biopolyols were employed for the synthesis of polyurethane (PU) foams by bulk polymerization using methylene diphenyl diisocyanate (MDI) at different NCO:OH ratios. The foams were evaluated by apparent density, insoluble fraction, Fourier transform infrared spectroscopy, scanning electron microscope and thermogravimetric analysis. The results showed that crude glycerol provided PU foams as much as commercial glycerol, however the characteristics may be different, mainly due to the presence of water in the reaction medium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the most commonly used polymers are polyurethanes foams (PU), a versatile polymer material which features a wide range of applications as thermal insulation of buildings and refrigerators, automotive manufacture and in chemical and food industries [1]. The formation of PU is carried out by the polymerization of a polyol and a diisocyanate molecule, where the hydroxyl group (OH) reacts with an isocyanate group (R–N=C=O) forming carbamate (urethane) linkages [2], consequently, the control of the NCO:OH molar ratio is one of the most important factors to control the polymer structure [3, 4]. To obtain PU foams a blowing agent should be employed. This agent can be either physical, like HCFCs, low boiling-point hydrocarbons or liquid CO2, even water. The chemical blowing agents (gaseous products) are formed by a chemical reaction during the polymerization, as the exothermic reaction between water and isocyanate group to yield a thermally unstable carbamic acid which decomposes to provide an amine functionality and carbon dioxide. The amine group that was formed in the first step reacts with another isocyanate group to give a disubstituted urea and additional heat is released. The exothermic reaction, releasing carbonic gas, is responsible for the polymeric mass expansion, forming cells connected in a three-dimensional structure [5, 6].
Usually, the PU foam industry uses polyols obtained from petrochemistry, however, alternatives routes to obtain bio-based polyols from renewable resources are being investigated [7,8,9,10]. Due to its chemical composition, castor oil is one of the most studied vegetable oil for PU synthesis [11,12,13]. The castor oil is composed by a triacylglycerol with six predominant fatty acids in its composition, being approximately 90% of ricinoleic acid that has a secondary hydroxyl group enabled to react with NCO functionality. Petrovic et al. [14] studied the transesterification reaction using castor oil and trimethylolpropane as monomers, the authors obtained flexible PU foams by bulk polymerization. Zhang et al. [15] produced flame-retardant PU foams using polyols from castor oil, glycerol, hydrogen peroxide and diethyl phosphate.
Other important green precursor that can be used for PU production is crude glycerol (CGL), a main byproduct of biodiesel production [16, 17]. Glycerol chemical structure has three hydroxyl groups (two primary and one secondary hydroxyl) in a three carbon chain, that commonly result in PU foams with low resistance and low brittleness [18]. Since CGL contains impurities, such as methanol, water, free fatty acids (FFAs), fatty acid methyl esters (FAMEs) [19], purification is, usually, the first step for the utilization. However, higher degrees of purity require several process steps increasing production costs. Therefore, utilizing CGL for the production of biopolyols has the potential to significantly impact the PU industry [20].
The combination of a vegetable oil and glycerol (GL) can be used to produce monoacylglycerols (MAG) and diacylglycerol (DAG) by glycerolysis reaction. The glycerolysis can occur by two different paths. The first, usually named chemical glycerolysis, uses a basic or acid catalyst at high temperature (150–250 °C) to proceed the reaction. The second path, usually named enzymatic glycerolysis, uses low temperature (60 °C) and needs the presence of biocatalysts such as Lipases [21]. Chemical glycerolysis is used when low cost is required and presents a high conversion. Additionally, the amount of catalyst used is small (0.05–0.2% relative to the total reaction mass) [22,23,24]. Li et al. [20] evaluated the production of biopolyol derived from crude glycerol containing free glycerol, FAMEs, FFAs, Soaps, MAGs and DAGs. The crude glycerol was let to react at 150 °C for 2.5 and 5 h and a high vacuum (0.001 MPa) condition was applied to accelerate the removal of methanol and water. The biopolyols produced at different reaction times were used to produce polyurethane foams. Hu et al. [25] investigated the use of biodiesel-derived crude glycerol to produce polyols via thermochemical conversion to obtain waterborne polyurethane dispersions and the results indicated that crude glycerol-based polyols had a potential for waterborne PU coating applications. Another application of crude glycerol for the synthesis of polymeric resins was performed by Echeverri et al. [26] that conducted a study where the product of the glycerolysis of soybean oil and castor oil with crude glycerol was subjected to maleinization reaction, converted into resins and copolymerized with styrene.
Thereby, the purpose of this study was to investigate the characteristics of the effects of biopolyols composition on PU foam structure made from biopolyols obtained from the chemical glycerolysis between castor oil with two different glycerols (Fig. 1): a commercial glycerol and a crude glycerol obtained as a byproduct of biodiesel production.
Experimental
Materials
Castor oil (Quimidrol, hydroxyl value 135), crude glycerol (BSBios Indústria e Comércio de Biodiesel Sul Brazil S/A,) and glycerol P.A. (Vetec, 98%) and sodium hydroxide P.A. (Dinâmica, 99%) were used for biopolyol synthesis. Diphenylmethane diisocyanate (MDI) was kindly donated by Dow Chemical Company and was used as diisocyanate monomer. N-Methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) from Merk Millipore, n-heptanol (Sigma-Aldrich) and pyridine (Sigma-Aldrich) were used in gas chromatograph analyses. All chemicals were used as received.
Methods
Castor Oil Characterization
Castor oil composition was determined by gas chromatography. Samples were prepared according to ASTM D 6584-00 [27] methodology using MSTFA as derivatizing agent. The analyses were performed using a Thermo Trace 1310 chromatograph with flame ionization detector, using a Biodiesel Select (CP9080-30 m × 0.25 × 0.32 mm) as column, nitrogen (99.9%) as carrier gas. The water content was measured by Karl Fischer titration (Q349 QUIMIS). All samples were analyzed in triplicate.
Crude Glycerol (CGL) and Glycerol P.A. Characterization
For pH determination, glycerols (1.0 ± 0.1 g) were solubilized in 10 mL of DDI water (distilled and deionized). The pH of the solution was measured by a digital pH meter (pHmeterAN2000 Analion) at room temperature. The acid value analysis was performed according to NBR 11115 [28]. The viscosity of crude glycerol was measured at 25 ± 0.5 °C using a Brookfield DV III Ultra viscometer equipped with a small sample adapter, a temperature probe, and a temperature control unit. The water content of glycerols were determined by volumetric Karl Fischer titration using a Q349 QUIMIS. All analysis were performed in triplicate.
Biopolyol Preparation
Biopolyol was obtained by chemical glycerolysis based on the method presented by Sonntag [18] and Felizardo et al. [19]. In all reactions, it was employed castor oil:GL molar ratio of 1:5 (100 g of oil and 48.45 g of GL) and 0.2 wt.% of catalyst (0.29 g) in relation of the total mass of the reactants. The castor oil and glycerol (CGL or glycerol P.A.) mixture was added to a 250-mL three-neck flash immersed in an oil bath. The mixture was kept on N2 modified atmosphere and the temperature of the reaction medium was raised up to 225 °C. The catalyst was added when the reaction medium reached approximately 225 °C and the reaction proceeded for 2 h at constant temperature (225 °C) and stirring speed (300 rpm). Afterwards, the reaction products were transferred into a separation funnel, and the bottom phase contained unreacted glycerin and catalyst was discarded. When glycerol P.A. was used in the glycerolysis the polyol was named biopolyol 1 and when crude glycerol was used it was named biopolyol 2.
Biopolyols Characterization
The biopolyols were characterized by gas chromatography to determine the fractions of monoacylglycerols (MAG), diacylglycerol (DAG) and non-reacted castor oil (triacylglycerol, TAG). Samples were prepared as described previously (Sect. 2.2.1). Water content, pH and acid value analysis were performed according to the methods described in the Sect. 2.2.2. The hydroxyl value of biopolyols was measured following the procedure presented in ASTM D 4274-16 [29]. All analysis were performed in triplicate.
PU Foams Synthesis
To obtain PU foams, the biopolyol and MDI were added to a beaker at room temperature (~ 23 °C) and mixed vigorously (3000 rpm) for 1 min. After that, the mechanical stirrer was removed and the PU foam expansion started. To evaluate the effect of NCO:OH molar ratio on PU foam characteristics (cell size, cell morphology, foam density, insoluble fraction in toluene and thermogravimetric analysis), the NCO:OH molar ratio was varied from 0.7:1 to 1.1:1 for each biopolyol. 1.2:1 NCO:OH molar ratio was also evaluated for biopolyol 1 and this extrapolation was performed only with biopolyol 1 to verify whether there would be changes in the cell structure, density and insoluble fraction of the foam. The water content in the biopolyols was not considered in the calculations of NCO:OH molar ratio. The total mass of reactants was fixed in 40 g and the formulation is presented in Table 1.
PU Foams Characterization
PU foams were characterized by Fourier transform infrared spectroscopy (FTIR) in attenuated total reflectance mode (ATR) equipped with a ZnSe single crystal (Bruker Tensor 27). The samples were analyzed by transmittance, in the region between 4000 and 600 cm−1 with resolution of 4 cm−1 and 32 scans. The morphology of PU foams was evaluated by scanning electron microscopy with field emission (SEM-JEOL JSM-6390LV). The foams were cut and fixed with double-sided tape on a stub and coated with a thin layer of gold being observed with ×30 magnification. The average cell size was obtained from measuring 30–65 cells per sample using the software SizeMeter 1.1.
The apparent density analysis was performed according to the methodology described in ASTM D 1622/D 1622M-14 [30]. The samples were cut 40 × 40 × 10 mm with a vernier caliper (Starrett: 125MEB-6/150) aid. Then, it was weighed in a digital scale with a precision of 0.001 and 220 g capacity (model AUY220-Mars). The value calculated is expressed in kg m−3. The analyses were performed in duplicate.
The insoluble fraction of PU foams was evaluated according to the solvent-extraction methodology described in ASTM D 2765-01 [31] where 5 g of foam were soaked in toluene (Dinâmica, 99.5%) and refluxed with boiling toluene at atmospheric pressure for 24 h in a soxhlet apparatus. After that, the remaining insoluble sample was dried in a vacuum oven to remove the residual solvent. The insoluble fraction of the PU foam was calculated by the ratio of the weight of the dried insoluble sample to the weight of the original foam sample.
The samples with higher insoluble fraction were evaluated by thermogravimetric analysis (TGA). The analysis was performed with a STA 449 F3 Jupiter. Samples of approximately 10 mg were heated from 30 to 900 °C with a heating rate of 10 °C min−1 in a flowing nitrogen atmosphere.
Results and Discussion
Castor Oil and Glycerols Characterization
According to the results obtained by gas chromatography, the composition of the castor oil employed in this work was: 9.3 ± 0.2% of stearic acid (C18:0), 7.1 ± 0.4% of oleic acid (C18:1), 3.5 ± 0.1% of linoleic acid (C18:2), 8.4 ± 0.3% of linolenic acid (C18:3) and 57.7 ± 0.7% of ricinoleic acid (C18:1 12-OH). Although, the ricinoleic acid content is lower than the usual for castor oil, the amount of ricinoleic acid was high enough to guarantee that most of molecules formed after glycerolysis contained two or more hydroxyls groups, as can be observed in Fig. 1 that shows the scheme of chemical glycerolysis between triacylglycerol and glycerol.
Glycerol physicochemical analyses are shown in Table 2. The pH of CGL was approximately 5.2 and the pH of PGL was 6.5. According to the literature this range of pH is suitable for glycerolysis and will not affect MAGs and DAGs formation [32]. Water content was obtained by Karl Fischer titration. The crude glycerol, CGL, presented a high water content (13.2 ± 0.5%), which enabled the formation of free fatty acids (FFA) in larger quantities during the glycerolysis reaction, while pure glycerol, PGL, presented a much smaller amount of water (1.7 ± 0.3%).
Biopolyols Characterization
The biopolyols physicochemical parameters are shown in Table 3. The water content of biopolyol 1 was 0.5 ± 0.2 and 1.4 ± 0.3% for the biopolyol 2. Gas chromatography of biopolyol 1 and biopolyol 2 are shown in Fig. 2a, b, where the products are separated according carbon numbers (ASTM D 6584-00) [27]. According to the results, MAG was detected with three different fatty acids: ricinoleic, palmitic and stearic acid. The total amount of MAG and DAG on biopolyol 1 was 77.8 ± 0.9 and 11.3 ± 0.1%, respectively. Small peaks were observed at 22.75 and 23.53 min of retention time which comprises 5.8% of the composition and indicated the presence of free fatty acid (FFA), as a result of hydrolysis reactions, which occurs concomitantly to the glycerolysis. The biopolyol 2 presented 21.7 ± 0.3% of MAG and 19.9 ± 0.4% of DAG. Furthermore, the results showed the presence of unreacted TAG and a high quantity of FFA (24.7 ± 0.2%) due to hydrolysis reactions. The formation of FFA was accentuated on biopolyol 2 because the crude glycerol contained a larger amount of water The higher amount of FFA in biopolyol 2 resulted in the lower hydroxyl value of biopolyol 2 compared to biopolyol 1 as FFA of ricinoleic acid presented just one hydroxyl per molecule instead of two to three hydroxyls per molecule of MAG and DAG.
PU Foams Characterization
For the PU foam synthesis, the molar ratio NCO:OH ranged from 0.7:1 to 1.2:1 when biopolyol 1 was used and from 0.7:1 to 1:1 when biopolyol 2 was used. When biopolyol 1 reacted with diisocyanate at a NCO:OH molar ratio of 0.7:1, it was not observed the formation of foam, probably due to the combination of low amount of water (0.5 wt.%) and diisocyanate, since water acts as a blowing agent for rigid polyurethane foams due to the formation of CO2 and urea bonds [5, 33]. The samples with higher NCO content (1.2:1) in the formulation showed a superior foam expansion. In general, the higher NCO:OH molar ratio led to major foam expansion, which occurs due the availability of functional groups present in the reaction medium.
The foams were analyzed by FTIR and the results are presented in the Figs. 3 and 4 and Table 4. FTIR spectra shows a peak at 3316 cm−1 characteristic for the vibrations of the N–H groups present in urethane groups [8] for both biopolyol 1 (Fig. 3) and biopolyol 2 (Fig. 4). The stretching observed in the region of 2924 and 2844 cm−1 indicates the presence of asymmetric and symmetric vibration of CH3, respectively. The peak at 1722 cm−1 is characteristic of carbonyl bonds from urethane. The stretching present in 1597 and 1220 cm−1 correspond to the presence of C–N bonds [34].
The PU foam morphology was analyzed by SEM. Figure 5 shows the SEM image of a PU foam obtained using biopolyol 1 with a NCO:OH molar ratio of 0.8:1. For this particular molar ratio the PU foam does not present well defined cells (Fig. 5a), possibly due to the lower concentrations of NCO groups leading to low CO2 formation. On the other hand, when the molar ratio was increased to1:1 the formation of closed cells with average size of 395 ± 72 µm could be observed (Fig. 5c). Increasing furthermore the NCO:OH molar ratio to 1.2:1 the average cells size increased to 600 ± 100 µm (Fig. 5d).
When biopolyol 2 was used to obtain PU foams with a NCO:OH molar ratio of 0.8:1, the PU foam cells showed a closed structure with an average size of 538 ± 159 µm (Fig. 6b). The different behavior observed when compared to biopolyol 1 could be explained due to the higher amount of water that reacted with diisocyanate generating more carbon dioxide that acted as blowing agent [5]. Using a higher NCO:OH molar ratio (1:1), there was an expansion of PU cells with an average size of 580 ± 132 µm (Fig. 6d). The size and morphology of the cells of this foam were similar to of that obtained with biopolyol 1 with higher NCO:OH molar ratio.
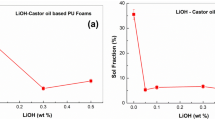
MAGs and DAGs of both biopolyols presented molecules with three hydroxyl groups that could lead to polymer crosslinking that is usually determined as the insoluble fraction of the polymer network. Results show that the insoluble fraction depended on the NCO:OH molar ratio. Lower values of NCO:OH molar ratio (0.7:1) resulted in PUs with lower insoluble fraction for both biopolyols. Increasing NCO:OH molar ratio increased the insoluble fraction of PU foams as more hydroxyl groups were converted to urethane linkage. It was also observed that foams obtained from biopolyol 2 presented an insoluble fraction higher than the foams obtained from biopolyol 1. The difference in insoluble fraction for foams obtained from biopolyol 1 and 2 with the same NCO:OH ratio could be credited to the higher amount of water in biopolyol 2 that led to the formation of more urea linkages and consequently to higher physical crosslinking. When water is present in the reaction medium there is the formation of urea linkages and, eventually, depending on the water content, segments of polyurea, that are not soluble in toluene, could be formed. In polyurea intermolecular hydrogen bonds are formed between the active hydrogen atoms of one urea donor group (–NH–) and the acceptor carbonyl group from another urea motif (–CO–) forming strong physical bonds between the polymer chains [35]. Therefore, the insoluble fraction that was estimated by soxhlet extraction was formed by chemical crosslinking provided by polyols containing three hydroxyl groups and physical crosslinking between the urea moieties.
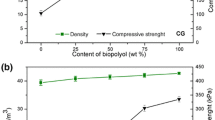
The density of PU foam decreased when the insoluble fraction increased, as reported in Table 5. In fact, the higher degree of crosslinking given by the insoluble fraction could increase the resistance for cell expansion. Nonetheless, the higher concentration of diisocyanate led not only to an increased conversion of pending OH groups of MAGs and DAGs but also to faster reaction with residual water that generated CO2 blowing agent resulting in higher foaming despite the increased resistance.
DTG curves of rigid polyurethane foams prepared using both biopolyols are shown in Fig. 7 and reveal that the thermal decomposition of rigid PU foams in nitrogen was a multistage process. In the range of 200–430 °C the events were attributed to the decomposition of urethane and urea linkages, which involves dissociations to isocyanate and the alcohol, amines and olefins or to secondary amines [20, 36]. It is observed that all foams were thermally stable up to 200 °C. The first stage of weight loss, around 229 °C, referred to the degradation of –C–H linkage and PU segments. The second stage, observed in 350 °C was due to the degradation of C=O and N–H linkages. In the last stage, around 460 °C, occurred the complete degradation of the polymer, being the temperature range of 440–490 °C associated with clearance of carbon–carbon bonds [37].
Conclusions
The synthesis of biopolyols derived from castor oil and different glycerols was performed by chemical glycerolysis with sodium hydroxide as catalyst at 225 °C. The biopolyol obtained from high purity glycerol led to predominantly MAG formation, whereas the biopolyol produced using crude glycerol, resulted in higher DAG, TAG and FFA content due to the higher amount of water. Both biopolyols were employed for the synthesis of polyurethane (PU) foams by bulk polymerization using methylene diphenyl diisocyanate (MDI) at different NCO:OH ratios.
The higher NCO:OH molar ratio led to higher polymer insoluble fraction and lower foam density due to increased OH conversion. The polymerizations performed with the biopolyol obtained from crude glycerol presented higher foam expansion, in relation to the foam obtained with biopolyol synthetized from commercial glycerol, due to the higher amount of water that reacted with isocyanate generating more carbon dioxide that acted as blowing agent. Thermogravimetric analyzes showed that foams obtained from both biopolyols presented similar thermal degradation behavior and great thermal stability. Results showed that low-cost crude glycerol provided PU rigid foams with high thermal stability as much as commercial glycerol, however the characteristics as apparent density may be different, mainly due to the presence of water in the reaction medium.
References
Ionescu M (2005) Chemistry and technology of polyols for polyurethanes. Rapra Technology, United Kingdom
Mandal BM (2013) Fundamentals of polymerization. World Scientific Publishing Company, Tuke Link
Tay G-S, Nanbo T, Hatakeyama H, Hatakeyama T (2011) Polyurethane composites derived from glycerol and molasses polyols filled with oil palm empty fruit bunches studied by TG and DMA. Thermochim Acta 525:190–196
Braun D, Cherdron H, Rehahn M, Ritter H, Volt B (2005) Polymer synthesis: theory and practice: fundamentals, methods, experiments. Springer, New York
Al-Moameri H, Zhao Y, Ghoreishi R, Suppes GJ (2016) Simulation blowing agent performance, cell morphology, and cell pressure in rigid polyurethane foams. Ind Eng Chem Res 55:2336–2344
Choe KH, Lee DS, Seo WJ, Kim WN (2004) Properties of rigid polyurethane foams with blowing agents and catalysts. Polym J 36:368–373
Simón D, Borreguero AM, de Lucas A, Rodríguez JF (2015) Valorization of crude glycerol as a novel transesterification agent in the glycolysis of polyurethane foam waste. Polym Degrad Stab 121:126–136
Zieleniewska M, Leszczyński MK, Kurańska M, Prociak A, Szczepkowski L, Krzyżowska M, Ryszkowska J (2015) Preparation and characterisation of rigid polyurethane foams using a rapeseed oil-based polyol. Ind Crops Prod 74:887–897
Güner FS, Yağcı Y, Erciyes AT (2006) Polymers from triglyceride oils. Prog Polym Sci 31:633–670
Kosmela P, Hejna A, Formela K, Haponiuk JT, Piszczyk Ł (2016) Biopolyols obtained via crude glycerol-based liquefaction of cellulose: their structural, rheological and thermal characterization. Cellulose 23:2929–2942. https://doi.org/10.1007/s10570-016-1034-7
Miao S, Wang P, Su Z, Zhang S (2014) Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater 10:1692–1704
Hejna A, Kirpluks M, Kosmela P, Cabulis U, Haponiuk J, Piszczyk Ł (2017) The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind Crops Prod 95:113–125
Carriço CS, Fraga T, Pasa VMD (2016) Production and characterization of polyurethane foams from a simple mixture of castor oil, crude glycerol and untreated lignin as bio-based polyols. Eur Polym J 85:53–61
Petrovic ZS, Cvetkovic I, Hong D, Wan X, Zhang W, Abraham T, Malsam J (2008) Polyester polyols and polyurethanes from ricinoleic acid. J Appl Polym Sci 108:1185–1190
Zhang L, Zhang M, Hu L, Zhou Y (2014) Synthesis of rigid polyurethane foams with castor oil-based flame retardant polyols. Ind Crops Prod 52:380–388
Hejna A, Kosmela P, Formela K, Formela K, Piszczyk Ł (2016) Potential applications of crude glycerol in polymer technology-current state and perspectives. Renew Sustain Energy Rev 66:449–475
Gama NV, Silva R, Costa M, Barros-Timmons A, Ferreira A (2016) Statistical evaluation of the effect of formulation on the properties of crude glycerol polyurethane foams. Polym Test 56:200–206
Mamiński M, Parzuchowski PG, Trojanowska A, Dziewulski S (2011) Fast-curing polyurethane adhesives derived from environmentally friendly hyperbranched polyglycerols: the effect of macromonomer structure. Biomass Bioenerg 35:4461–4468
Hu S, Luo X, Wan C, Li Y (2012) Characterization of crude glycerol from biodiesel plants. J Agric Food Chem 60:5915–5921
Li C, Luo X, Li T, Tong X, Li Y (2014) Polyurethane foams based on crude glycerol-derived biopolyols: one-pot preparation of biopolyols with branched fatty acid ester chains and its effects on foam formation and properties. Polymer 55:6529–6538
Zhong N, Li L, Xu X, Cheong LZ, Xu Z, Li B (2013) High yield of monoacylglycerols production through low-temperature chemical and enzymatic glycerolysis. Eur J Lipid Sci Technol 115:684–690
Felizardo P, Machado J, Vergueiro D, Correia MJN, Gomes JP, Bordado JM (2011) Study on the glycerolysis reaction of high free fatty acid oils for use as biodiesel feedstock. Fuel Process Technol 92:1225–1229
Sonntag NOV (1982) Glycerolysis of fats and methyl esters: status, review and critique. J Am Oil Chem Soc 59:795–802
Noureddini H, Medikonduru V (1997) Glycerolysis of fats and methyl esters. J Am Oil Chem Soc 74:419–425
Hu S, Luo X, Li Y (2015) Production of polyols and waterborne polyurethane dispersions from biodiesel-derived crude glycerol. J Appl Polym Sci 132:1–8
Echeverri DA, Rios LA, Rivas BL (2015) Synthesis and copolymerization of thermosetting resins obtained from vegetable oils and biodiesel-derived crude glycerol. Eur Polym J 67:423–438
ASTM D 6584 (2014) Test method for determination of free and total glycerin in B-100 biodiesel methyl esters by gas chromatography1. West Conshohocken, PA
Associação Brasileira De Normas Técnicas (2014) NBR 11115: Insumos—Substâncias graxas: Determinação do índice de acidez
ASTM D 4274 (2016) Standard test methods for testing polyurethane raw materials: determination of hydroxyl numbers of polyols. West Conshohocken, PA
ASTM D 1622/D 1622M-14 (2012) Standard test method for apparent density of rigid cellular plastics1. West Conshohocken, PA
ASTM D 2765-01 (2001) Standard test methods for determination of gel content and swell ratio of of crosslinked ethylene plastics1. West Conshohocken, PA
Monte Blanco SF, Santos JS, Feltes MM, Dors G, Licodiedoff S, Lerin LA, Olivera D, Ninow JL, Furigo A Jr (2015) Optimization of diacylglycerol production by glycerolysis of fish oil catalyzed by Lipozyme TL IM with Tween 65. Bioprocess Biosyst Eng 38:2379–2388
Ionescu M, Radojčić D, Wan X, Shrestha ML, Petrović ZS, Upshaw TA (2016) Highly functional polyols from castor oil for rigid polyurethanes. Eur Polym J 84:736–749
Stuart BH (2004) Organic molecules. Wiley, Sydney
Sánchez-Ferrer A, Rogez D, Martinoty P (2010) Synthesis and characterization of new polyurea elastomers by sol/gel chemistry. Macromol Chem Phys 211:1712–1721
Stirna U, Fridrihsone A, Lazdiņa B, Misāne M, Vilsone D (2013) Biobased polyurethanes from rapeseed oil polyols: structure, mechanical and thermal properties. J Polym Environ 21:952–962
Li S, Bouzidi L, Narine SS (2017) Polyols from self-metathesis-generated oligomers of soybean oil and their polyurethane foams. Eur Polym J 93:232–245
Acknowledgements
The authors would like to thank: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support (Grant No. 552251/2011-9); Laboratório Central de Microscopia Eletrônica at the Federal University of Santa Catarina (LCME-UFSC) for SEM images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bresolin, D., Valério, A., de Oliveira, D. et al. Polyurethane Foams Based on Biopolyols from Castor Oil and Glycerol. J Polym Environ 26, 2467–2475 (2018). https://doi.org/10.1007/s10924-017-1138-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-1138-7