Abstract
An iminodiacetate (IDA) group containing nonwoven polyethylene (PE) film was prepared by radiation induced graft polymerization of an epoxy group containing monomer, glycidyl methacrylate (GMA) onto nonwoven PE fabric and subsequent chemical modification to IDA group. The effect of solvents (water, methanol, ethanol, isopropyl alcohol and dimethyl sulfoxide) on modification of epoxy group of GMA grafted PE (GMA-g-PE) fabric to IDA group was investigated. Ethanol provided a higher density of IDA group on GMA-g-PE fabric. Fourier transform infrared spectroscopy, scanning electron microscopy, thermogravimetric analysis and dynamic mechanical analysis of IDA-GMA-PE adsorbent, GMA-g-PE fabric and pure PE fabric were studied. The ability of resultant IDA adsorbent to absorb chromium from aqueous medium was evaluated by batch method. The parameters such as feeding chromium concentration, pH of chromium solution and adsorption time of IDA adsorbent were discussed. The obtained results illustrate that the IDA adsorbent could be fruitful to absorb chromium from water. The equilibrium experimental data for the adsorption of chromium on IDA adsorbent fitted Langmuir isotherm model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Graft polymerization is well known reaction in radiation processing of polymers [1]. This reaction can be initialized by high-energy irradiation of gamma-ray or electron beam toward the polymer. The radiation-induced graft polymerization can be done to the conventional polymers having various shapes such as membrane, fabric, fiber etc [2–4]. Radiation-induced grafting of acrylic acid (AA) and methacrylic acid (MA) onto a polyethylene (PE) film has been studied [2]. Grafting of methacrylate monomer onto PE nonwoven fabric has been done by radiation technique [3]. Radiation induced graft co-polymerization of different functional monomers such as glycidyl methacrylate [5, 6], 4-hydroxybutyl acrylate glycidyl ether [7], dimethylaminoethyl methacrylate [8], acrylamide [9], 2-hydroxy ethyl methacrylate /glycidyl methacrylate [10] on polyethylene/polypropylene (PE/PP) nonwoven fabric has been reported. The radiation-induced graft polymer has been widely used as battery separators, heavy metal ion adsorbents etc. The radiation-induced graft polymerization and subsequent functionalization have certain advantages. It makes easy to control the site of the grafted polymer branches and the density of the functional group [11].
The fibrous adsorbents can be produced by introducing a functional chelate group such as amidoxime [12], phosphoric acid [3] and iminodiacetic acid [13] into nonwoven fabrics. Fabric adsorbent having amidoxime function was applied to the removal of Cd from the scallop waste [12]. Adsorbent having phosphoric acid was directly synthesized by grafting methacrylate monomer having phosphoric acid onto polyethylene nonwoven fabric and used as adsorbent for Pb(II) and Cd(II) [3]. An iminodiacetate (IDA) group as a chelate-forming group was introduced uniformly throughout a porous polyethylene membrane of a hollow-fiber form and the binary metal ions (Co2+ and Cu2+) were transported to the IDA group [13]. Glycidyl methacrylate grafted PE/PP nonwoven fabric was modified toward enhanced amidoximation to prepare a highly efficient uranyl adsorbent [5]. A CO2 adsorbent containing triethylamine (TEA) was prepared by radiation induced grafting of glycidyl methacrylate (GMA) onto PE-PP non-woven sheet followed by amination reaction [6]. Amine type adsorbent was synthesized from 4-hydroxybutyl acrylate glycidyl ether grafted PE/PP nonwoven fabric and used for adsorption of Cu(II), Pb(II), Zn(II), Ni(II) and Li(II) [7]. Dimethylaminoethyl methacrylate grafted PE/PP nonwoven fabric has been investigated for the removal of phosphate [8]. Acrylamide monomer grafted polyethylene-coated polypropylene nonwoven fabric was studied for the adsorption of Cu (II), Co(II) and Ni (II) [9]. The toxic metal adsorption rate by these fibrous adsorbents is found about 100 times higher than that of the commercial adsorbent resin in column mode experiments [14]. The removal of toxic metals from aqueous medium by using these high performance fibrous adsorbents is attracted.
The industrial waste water containing chromium compounds are among the most dangerous inorganic water pollutants. Water containing chromium ion possesses a great threat to surface and groundwater quality, which is a threat to human health. Bangladesh has many leather tanning industries. There are undesirable amount of chromium ion in the waste water from leather tanning industries, which is released directly into the environment without any pre-treatment. In the environment, chromium exists mainly in the oxidation states, Cr (III) and Cr (VI). Cr (III) is relatively innocuous and Cr (VI) is toxic and carcinogenic. It is a strong oxidant capable of being absorbed by the skin [15]. The maximum contaminant level of chromium for drinking water is 0.05 mg/L as per the World Health Organization (WHO) set standard [16].
In the present investigation, glycidyl methacrylate (GMA) was grafted onto non-woven polyethylene (GMA-g-PE) fabric by pre-irradiation method [17]. The epoxy group of GMA-g-PE fabric was converted to iminodiacetate (IDA) adsorbent using disodium iminoacetate by chemical method. Thermo-gravimetric analysis (TGA) and Dynamic mechanical analysis (DMA) were used to investigate thermal stability of PE fabric, GMA-g-PE fabric and IDA adsorbent. The affinity of the resultant absorbent having IDA group toward chromium from its aqueous solution was evaluated using UV–Visible Spectrophotometer technique. Langmuir isotherm model was used to observe the adsorption process.
Experimental
Materials
A non-woven polyethylene (PE) fabric obtained from Kurashiki MFG Co. was used as a trunk polymer for grafting polymerization. Glycidyl methacrylate (GMA, CH2=CCH3COOCH2CHOCH2) was purchased from Tokyo Kasei Chemical Industry Co. Ltd., Japan and used without further purification. Polyoxyethylene sorbitan monolaurate (Tween-20) and nitric acid (HNO3) were supplied by Kanto Chemical Co. Inc., Japan. Disodium iminodiacetate (HN(CH2COONa)2 H2O) was purchased from Wake Pure Chemical industries Ltd., Japan. Methanol (MeOH), Ethanol (EtOH), Isopropyl alcohol (IPA) and Dimethyl sulfoxide (DMSO) were purchased from BDH England. Chromium Nitrate, Ammonium peroxodisulphide and 1, 5-Diphenylcarbazide were procured from E. Merck, (India) Ltd.
Preparation of Metal Adsorbent
Glycidyl methacrylate (GMA) was grafted onto non-woven polyethylene (PE) fabric in similar manner described previously [17]. The 3 cm × 3 cm size PE fabric was irradiated by gamma radiation from Co-60 source at 50 kGy radiation dose at ambient temperature. The irradiated PE fabric was stored in dry-ice temperature until use. The monomer emulsion of 5 wt% GMA was prepared by adding GMA to deionized water with 0.5 wt% Tween 20 as emulsifier by stirring with a mechanical stirrer at room temperature (27 °C) for 0.5 h. The emulsion of GMA was bubbled with nitrogen gas to remove dissolved oxygen. The de-aerated GMA-emulsion was poured into glass bottle containing irradiated PE fabric. After completely filling the glass bottle with GMA-emulsion, the bottle was tightly closed with a lid to prevent oxygen from air into the GMA-emulsion of bottle. After that the grafting reaction was carried out at 40 °C in a water bath upto 4 h. The GMA-g-PE fabric was washed with methanol to remove residual monomer and homopolymer of GMA. The degree of grafting was calculated as follows
where, W1 is the dry weight of grafted PE fabric and W0 is the dry weight of PE fabric.
GMA-g-PE fabric was immersed in 0.5 M disodium iminodiacetate in 50% EtOH and reaction was carried out at 80 °C in water bath up to 4 h to investigate the suitable reaction time for maximum modification of epoxy group to IDA group. To determine the effect of solvent concentration on modification reaction, GMA-g-PE fabric was immersed in 0.5 M disodium iminodiacetate in 0, 20, 30, 40 and 50% EtOH respectively and reaction was performed in water bath at 80 °C for 3 h. To study the effect of nature of solvent on modification reaction different solvents (water, 50% MeOH, 50% EtOH, 50% IPA and 50% DMSO) were used. After the introduction of IDA group onto the GMA-g-PE fabric, the remaining epoxide group was hydrolyzed into a diol group by treating IDA group containing GMA-g-PE fabric (IDA-GMA-PE adsorbent) in 1 N HNO3 at 80 °C for 4 h in a water bath. Subsequently IDA-GMA-PE adsorbent was washed repeatedly with deionized water until neutral and dried in a vacuum oven to constant weight. The conversion of epoxide group (X) and density of IDA group were calculated [18] as follows
where, W0, W1 and W2 are the dry weight of PE fabric, GMA-g-PE fabric and IDA-GMA-PE adsorbent respectively.
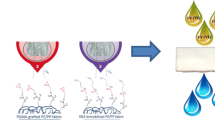
Figure 1 shows the preparation scheme of iminodiacetate (IDA) group containing metal adsorbent.
Thermo Gravimetric Analysis
The thermal gravimetric analysis (TGA) of PE fabric, GMA-g-PE fabric and IDA-GMA-PE adsorbent (2 mg) were determined on a Perkin-Elmer TGA 7-thermal analyzer from 25 to 480 °C with a heating rate of 10ºC/min, under nitrogen atmosphere with a flow rate of 20 ml/min.
Dynamic Mechanical Analysis
The thermal property of PE fabric, GMA-g-PE fabric and IDA-GMA-PE adsorbent were studied from 25 to 180 °C at a heating rate of 4 °C/min and an oscillating frequency of 1 Hz using dynamic mechanical analyzer (DMA), Triton technology TTDMA, UK.
FTIR (ATR) Spectroscopy
The PE, GMA-g-PE and IDA-GMA-PE films were characterized by FTIR (ATR) spectrophotometer (8400 S Shimadzu Japan) in the range 700–4000 cm−1 (resolution 4 cm−1, number of scans: 20 times).
Scanning Electron Microscopy
The morphological study of the PE, GMA-g-PE and IDA-GMA-PE films was done by using scanning electron microscopy (SEM) of Model JEOL 6400 at an accelerating voltage of 15 kV. The SEM specimens were sputter coated with platinum.
Determination of Chromium Adsorption
The chromium nitrate [Cr(NO3)2·9H2O] is used as source of hexavalent chromium. Equal amount of chromium nitrate and ammonium peroxodisulphide was added in distilled water and then boiled about one hour in water bath. The solution was cooled and preserved. pH adjustment were carried out using HCl and NaOH. The adsorbent was immersed in prepared aqueous solution of chromium (VI) at room temperature (27 °C) and periodically the residual concentration of chromium in the feed solution was determined spectrophotometrically (UV-2401PC, Shimadzu, Japan). The amount of chromium adsorption per unit mass of adsorbent was evaluated by using the following equation
where, qe is the amount of chromium adsorbed per unit dry mass of adsorbent (mg/g of adsorbent), C0 and Ce are the concentration of chromium (mg/L) in the initial solution and aqueous phase after treatment with adsorbent of certain period respectively, V is the volume of aqueous phase in liter (L) and W is the weight of dry adsorbent (g).
Results and Discussion
Radiation-induced graft polymerization is comparatively simple over other methods such as chemical initiator method, photo initiator method etc. The radicals on polymer matrix are obtained uniformly by the application of radiation such as electron beams and gamma rays due to high penetrating power. Radiation-induced graft polymerization method can be divided into two general categories: one is direct irradiation method, whereby a polymer substrate is irradiated in presence of monomer and other is pre-irradiation method, whereby a polymer substrate is activated by radiation and subsequently allow to grafting reaction with monomer. By pre-irradiation method, less amount of ungrafted homopolymer is obtained compared to direct grafting technique. Glycidyl methacrylate (GMA) bears a reactive epoxide group, which can be easily functionalized. Figure 2 shows the effect of reaction time on grafting of GMA onto PE fabric at pre-irradiation dose of 50 kGy. The degree of GMA-grafting onto PE fabric increases with increased reaction time. About 198% degree of grafting is obtained at 3 h reaction time. The GMA-g-PE with this degree of grafting value was used to modify the epoxy group of GMA to IDA group.
Figure 3 shows the density of IDA group on IDA-GMA-PE fabric as a function of reaction time. The modification reaction is carried out in 50% EtOH with 0.5 M disodium iminodiacetate in a water bath at 80 °C up to 4 h reaction time. The density of IDA group on IDA-GMA-PE fabric increases with increased reaction time and attained a maximum value at 3 h reaction time. The GMA-g-PE fabric having 198% degree of grafting undergoes about 1.96 mmol/g (~56% conversion) density of IDA at 3 h reaction time. The conversion [18] of GMA-grafted hollow fiber (150% degree of grafting) in DMSO/water solution was obtained about 60% after 20 h modification reaction time. The conversion of epoxide group to IDA group depends on degree of GMA grafting. The maximum value of conversion is obtained at 120% degree of GMA grafting and beyond this degree of grafting the value of conversion is level off. It has also been reported [19] that the highest conversion of epoxide group to IDA group in IDA-2Na/Na2CO3 solution was obtained about 15%.
Figure 4 shows the effect of EtOH concentration in water as solvent on IDA density on IDA-GMA-PE fabric. The density of IDA group on IDA-GMA-PE fabric increases with increase concentration of EtOH at reaction time, 3 h. The density of IDA group on IDA-GMA-PE fabric is obtained about 0.06, 0.38, 0.98, 1.36 and 1.96 mmol/g of GMA-g-PE fabric for 0.0, 20, 30, 40 and 50% EtOH respectively. This result can be explained as increased ethanol in water may be increased diffusion of disodium iminodiacetate into grafted polymer branch due to increase swelling of GMA-g-PE fabric and resulted increase in density of IDA group.
Table 1 shows the effect of different solvents on modification of epoxy group to IDA group on GMA-g-PE fabric. In this study, water, 50% MeOH, 50% EtOH, 50% IPA and 50% DMSO are used to select a good solvent. The density of IDA and conversion of epoxy group to IDA group depends on nature of solvent. The maximum values of IDA density and conversion of epoxy group to IDA group are obtained from 50% EtOH as solvent. The lowest values of IDA density and conversion of epoxy group to IDA group is obtained from water as solvent. The solvent can arrange in their decreasing extents of modification of epoxy group to IDA group on GMA-g-PE as follows: 50%EtOH > 50%IPA > 50%MeOH > 50%DMSO > Water. The extents of modification in different solvents, 50% EtOH give better result than 50%IPA, 50%MeOH, 50%DMSO or water.
The TGA thermograms for PE fabric, GMA-g-PE fabric and IDA-GMA-PE adsorbent are presented in Fig. 5. The thermal stability of GMA-g-PE fabric and IDA-GMA-PE adsorbent is lower than that of original PE fabric. The original PE fabric shows high thermal stability up to 350 °C and above 350 °C, it starts to decompose. The TGA curve of the GMA-g-PE fabric shows two quite independent decomposition processes at the range of 200–350 °C and above 350 °C, due to the degradation of the grafted chain and that of the PE fabric base polymer. The GMA-g-PE fabric remains thermal stable up to 200 °C. There are four weight loss steps in the TGA curve for IDA-GMA-PE adsorbent. For IDA-GMA-PE adsorbent being exposed to air before subjecting to TGA, the first weight loss appears in the temperature range from 45 to 100 °C, is easily understood to be due to the loss of moisture absorbed from air. The weight loss stages at 100–200 °C, 200–350 °C and above 350 °C are originated from decarboxylation, degradation of grafted chain and degradation of PE fabric respectively.
Figure 6 reveals the storage modulus of PE fabric, GMA-g-PE fabric and IDA-GMA-PE adsorbent with varying temperature. The initial value of storage modulus for PE fabric is higher than that of GMA-g-PE fabric and IDA-GMA-PE adsorbent and modulus decreases with increase in temperature. It is observed that the glassy transition region for GMA-g-PE fabric obtains at the temperature range from 25 to 70 °C and the leathery state plateau region occurs at low temperature. On the other hand the glassy transition region for PE fabric extends from 25 to 110 °C and the leathery state plateau region occurs at high temperature. It is also found that there is a sharp decrease of modulus in glassy transition region for both PE and GMA-g-PE but for IDA-GMA-PE adsorbent curve shows a slow decrease of modulus in glassy region (25–140 °C).
Figure 7a–c shows the IR spectra of PE, GMA-g-PE and IDA-GMA-PE fabrics respectively. PE may be considered as an infinite chain of CH2 groups, therefore the characteristic features of the IR spectrum of PE are it’s C–H stretching and C–H deformation vibrations. C–H asymmetric and symmetric stretching vibrations are observed at 2916 and 2848 cm−1 respectively. C–H bending, wagging and rocking deformation of CH2 group are observed at 1465, 1369 and 721 cm−1 respectively. For GMA-g-PE, the additional bands, which are characteristic of GMA structure, appeared: the C=O stretching vibration occur at 1737 cm−1 and peaks for epoxy rings observed at 3020 and 869 cm−1 [5]. Again the IDA-GMA-PE fabric showed decrease of the epoxy group’s peaks and emergence of new –OH peak at 3441 cm−1 [6].
Characterization of the nonwoven fibers by SEM was made to compare the difference in the physical appearance of PE, GMA-g-PE and IDA-GMA-PE fabrics. The SEM image of PE, GMA-g-PE and IDA-GMA-PE fabrics are shown in Fig. 8a–c respectively. The change in the physical appearance and also slight increase in the average diameter of fibers after grafting with GMA are the additional physical evidences for the grafting. Further change in physical appearance and large increase in average diameter after conversion to IDA group provide evidences for the modification.
SEM images give another evidence for grafting and conversion to IDA group as represented by the difference in average fibre diameters [5, 6].
Figure 9 shows the amount of chromium adsorbed by IDA-GMA-PE adsorbent from aqueous solution. The pH value of chromium solution is kept 1.73 and concentrations of chromium in solutions are kept 50, 75 and 100 mg/L respectively. Adsorption of chromium increases with increased concentration of chromium ions in adsorption medium. It is observed that the maximum values of chromium adsorption are ~0.88, ~1.44 and ~1.62 mmol/g of adsorbent at 48 h absorption time respectively. The all adsorbed chromium is eluted by 1 M HNO3 solution and desorption ratio was 99%. After elution of chromium, the adsorbent is used repeatedly upto 5 cycles. No loss of activity was observed.
The pseudo-second order kinetic model was applied to fit the Cr(VI) adsorption by the adsorbent. The pseudo-second-order equations [20], is expressed as:
where Q t and Qe are the amount of ions adsorbed (mmol g−1) at any time and equilibrium time, respectively k 2 (g min−1 mmol−1) is the rate constant of second-order adsorption. Pseudo second-order rate constants could be determined experimentally by plotting t/Qt against t as shown in Fig. 10. All the second order kinetic parameters for Cr(VI) adsorption are given in Table 2. It can be seen that the experimental Qe values are slightly lower than the Qe values calculated from second order kinetic model. It may be attributed to the incomplete contact of metal ion and IDA-GMA-PE adsorbent.
The pH value of the metal ion solution is a very important parameter in investigating the adsorption process. It may not only affect the electronic status of the pendant functional groups i.e. protonation/deprotonation of the basic group or dissociation/association of the acidic group but it may also alter the oxidation form of the metal ion present in the medium. Figure 11 shows the effect of pH values in chromium solution on adsorption of chromium by IDA-GMA-PE adsorbent. The pH of chromium solution is varied from 1.73 to 5.63 and concentration of chromium in solution is kept 100 mg/L. The chromium adsorption is found to depend on pH values of chromium solutions. The maximum values of chromium adsorption are ~0.38, ~0.50, ~0.93 and ~1.62 mmol/g of adsorbent with pH values 5.63, 3.55, 2.25 and 1.73 respectively at 48 h adsorption time.
The interaction of metal ion with IDA-GMA-PE adsorbent can be investigated by using adsorption isotherm. In this study the Langmuir isotherm models was used to investigate the relationship between metal uptake and equilibrium concentration of metal ion in the medium. The Langmuir isotherm model is effective for homogeneous and monolayer adsorption onto a surface with a finite number of identical sites. The linear form of Langmuir isotherm is presented by
where Ce (mg L−1) is the equilibrium concentration of metal in solution, qm (mg g−1) is the theoretical maximum adsorption capacity, qe (mg g−1) is the amount of metal adsorbed by per gram adsorbent, KL is Langmuir constant related to the affinity of binding sites, which is a measure of the energy of adsorption. qm and KL were calculated from slope and intercept of the linear plot of Ce/qe versus Ce respectively.
The Langmuir plot of metal ion adsorption onto IDA-GMA-PE adsorbent is shown in Fig. 12. The linear correlation coefficient (r) is 0.995, which reveals that the adsorption data is well fitted the Langmuir model. The isotherm constants qm and KL are calculated as 83.75 mg g−1 (~1.61 mmol/g) and 0.302 respectively. Adsorption capacity of IDA-GMA-PE adsorbent compared with some other adsorbents is shown in Table 3.
Conclusion
A metal ion adsorbent containing IDA group is prepared from GMA-g-PE fabric. The maximum value of modification of epoxy group to IDA group onto GMA-g-PE fabric is obtained at 3 h reaction time. Among the different solvents, 50%EtOH is found to be a good solvent for modification of epoxy group to IDA group on GMA-g-PE fabric. It was observed that thermal stability of IDA-GMA-PE adsorbent and GMA-g-PE fabric is lower than that of pure PE fabric. The chromium ion adsorption by IDA-GMA-PE adsorbent depends on concentration of chromium and pH of chromium solution. The chromium adsorption of IDA-GMA-PE adsorbent reached to 83.75 mg/g. The experimental data fitted Langmuir isotherm model. Primarily the result indicates that IDA adsorbent can be used to adsorb chromium from aqueous medium.
References
Kabanov VY, Aliev RE, Kudryavtsev V (1991) Present status and development trends of radiation-induced graft polymerization. Int J Radiat Appl Instrum C 37:175
S-H. Choi, S-Y. Park, Y. C. Nho (2000) Electrochemical properties of polyethylene membrane modified with carboxylic acid group. Radiat Phys Chem 57:179
Basuki F, Seko N, Tamada M, Sugo T, Kume T (2003) Direct synthesis of adsorbent having phosphoric acid with radiation-induced graft polymerization. J Ion Exchange 14:209
Kuraga J, Trobradovic H, Jyo A, Sugo T, Tamada M, Katakai A, Kume T (2003) Behavior of iminodiacetate fiber in column-mode adsorption of Lead(II). J Ion Exchange 14:77
Kavaklı PA, Seko N, Tamada M (2007) O. Güven, Radiation-induced graft polymerization of glycidyl methacrylate onto PE/PP nonwoven fabric and its modification toward enhanced amidoximation. J Appl Polym Sci 105:1551
M. M. Nasef, A. Abbasi, T.M. Ting (2014) New CO2 adsorbent containing aminated poly(glycidyl methacrylate) grafted onto irradiated PE-PP nonwoven sheet. Radiat Phys Chem 103:72
H. Ma, K. Morita, H. Hoshina, N. Seko (2011) Synthesis of amine-type adsorbents with emulsion graft polymerization of 4-hydroxybutyl acrylate glycidylether. Mater Sci Appl 2:777
P. A. Kavaklı, C. Kavaklı, N. Seko, M. Tamada, O. Güven (2007) Radiation-induced grafting of dimethylaminoethylmethacrylate onto PE/PP nonwoven fabric. Nucl Instr Meth Phys Res B 265:204.
Ibrahim SM, El-Salmawi KM, El-Naggar AA (2006) Use of radiation grafting of polyethylene-coated polypropylene nonwoven fabric by acrylamide for the removal of heavy metal ions from wastewaters. J Appl Polym Sci 102:3240–3245
El Salmawi KM, Ibrahim SM (2005) Radiation grafting of 2-hydroxy ethyl methacrylate (HEMA)/glycidyl methacrylate (GMA) onto polyethylene-coated polypropylene non-woven fabric. Polym Plast Technol Eng 44:1671
J. Okamoto (1987) Radiation synthesis of functional polymer. Radiat Phys Chem 29:469
T. Shiraishi, M. Tamada, K. Saito, T. Sugo (2003) Recovery of cadmium from waste of scallop processing with amidoxime adsorbent. Radiat Phys Chem 66:43
Konishi S, Saito K, Furusaki S, Sugo T (1996) Binary metal-ion sorption during permeation through chelating porous membranes. J Membrane Sci 111:1
A. Jyo, S. Aoki, T. Kishita, K. Yamabe, M. Tamada, T. Sugo (2001) Phosphonic acid fiber for selective and extremely rapid elimination of Lead(II). Anal Sci 17:201
I. B. Singh, D. R. Singh (2002) Cr(VI) removal in acidic aqueous solution using iron bearing industrial solid wastes and their stabilization with cement. Environ Technol 23:85
WHO (2006) Guidelines for drinking water quality. Vol 54 World Health Organization, Geneva
Y. Ueki, N. C. Dafader, H. Hoshina, N. Seko, M. Tamada (2012) Study and optimization on graft polymerization under normal pressure and air atmospheric conditions, and its application to metal adsorbent. Radiat Phys Chem 81:889
Yamagishi H, Saito K, Furusaki S, Sugo T, Ishigaki I (1991) Introduction of a high-density chelating group into porous membrane without lowering the flux. Ind Eng Chem Res 30:2234
Hemdan ES, Porath J (1985) Development of immobilized metal affinity chromatography I. Comparison of two iminodiacetate gels. J Chromatogr 323:247
Namasivayam C, D.J.S.E. Arası (1997) Removal of congo red from wastewater by adsorption onto waste red mud. Chemosphere 34:401
H. Heydari, H. Sharififard, M. Nabavinia, H. Kiani, M. Parvizi (2013) Adsorption of chromium ions from aqueous solution by carbon adsorbent. Int J Environ Earth Sci Eng 7:106
Tahir SS, Naseem R (2007) Removal of Cr(III) from tannery wastewater by adsorption onto betonite clay. Sep Purif Technol 53:312
S. K. Yadav, A. K. Dixit (2016) Efficient removal of Cr(VI) from aqueous solution onto palm trunk charcoal: kinetic and equilibrium studies. Chem Sci J 7:1
Duranoğlu D, Trochimczuk AW, Bekera U (2012) Kinetics and thermodynamics of hexavalent chromium adsorption onto activated carbon derived from acrylonitrile-divinylbenzene copolymer. Chem Eng J 187:193
A. Ali, K. Saeed, F. Mabood (2016) Removal of chromium (VI) from aqueous medium using chemically modified banana peels as efficient low-cost adsorbent. Alexandria Eng J 55:2933.
Acknowledgements
We would like to thank International Atomic Energy Commission for technical support to carry out this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dafader, N.C., Rahman, N., Majumdar, S.K. et al. Preparation and Characterization of Iminodiacetate Group Containing Nonwoven Polyethylene Fabrics and Its Application in Chromium Adsorption. J Polym Environ 26, 740–748 (2018). https://doi.org/10.1007/s10924-017-0991-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-0991-8