Abstract
In this study, a novel magnetic Cr(VI) ion imprinted polymer (Cr(VI)-MIIP) was successfully synthesized and used as a selective sorbent for the adsorption of Cr(VI) ions from aqueous solution. It can be synthesized through the combination of an imprinting polymer and magnetic nanoparticles. The high selectivity achieved using MIIP is due to the specific recognition cavities for Cr(VI) ions created in Cr(VI)-MIIP. Also, the magnetic properties that could be obtained using magnetic nanoparticles, helps to separate adsorbent with an external magnetic field without either additional centrifugation or filtration procedures. The magnetic Fe3O4 nanoparticles (MNPs) were synthesized using an improved co-precipitation method and modified with tetraethylorthosilicate (TEOS) before imprinting. The magnetic Cr(VI) ion imprinted polymer was prepared through precipitation copolymerization of 4-vinylpyridine as the complexing monomer, 2-hydroxyethyl methacrylate as a co-monomer, the Cr6+ anion as a template, and ethylene glycol dimethacrylate (EGDMA) as a cross-linker in the presence of modified magnetite nanoparticles. This novel synthesized sorbent was characterized using different techniques. Batch adsorption experiments were performed to evaluate the adsorption conditions, selectivity, and reusability. The results showed that the maximum adsorption capacity was 39.3 mg g−1, which was observed at pH 3 and at 25 °C. The equilibrium time was 20 min, and the amount of adsorbent which gave the maximum adsorption capacity was 1.7 g L−1. Isotherm studies showed that the adsorption equilibrium data were fitted well with the Langmuir adsorption isotherm model and the theoretical maximum adsorption capacity was 44.86 mg g−1. The selectivity studies indicated that the synthesized sorbent had a high single selectivity sorption for the Cr(VI) ions in the presence of competing ions. Thermodynamic studies revealed that the adsorption process was exothermic (\(\Delta H\) < 0) and spontaneous (\(\Delta G\) < 0). In addition, the spent MIIP can be regenerated up to five cycles without a significant decrease in adsorption capacity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, the rapid increase in levels of environmental contamination has resulted in increasing concern for both human health and global ecosystems [1]. Inorganic contaminants are heavy metals because of their toxicity towards aquatic-life, human beings, and the environment. One of these toxic heavy metals is chromium, which is considered a priority pollutant [3]. Chromium (Cr) is one of the most toxic pollutants generated through electroplating, mining, leather tanning, metal finishing, steel fabrication, textile industries, and chromate preparation. Therefore, developing an effective method to removal Cr from effluent has a great importance to the public health and ecological system [3, 4]. There exist three oxidation states for chromium in nature, Cr(II), Cr(III), and Cr(VI), however only the last two species are stable [5]. The hexavalent form is known to be highly mobile in soil and aquatic system, and it is also 500 times more toxic, mutagenic, and carcinogenic than Cr(III) [6]. The maximum permissible limit of Cr(VI) in wastewater has been recommended 0.005 mg L−1 via World Health Organization [7].
Hexavalent chromium exists in aqueous solution as oxyanions such as hydro chromate (\(\text{HCrO}_{4}^{-}\)), dichromate (\(\text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-}\)), and chromate (\(\text{C}{{\text{r}}_{\text{2}}}\text{O}_{\text{4}}^{\text{2-}}\)), whereas \(\text{HCrO}_{4}^{-}\) and \(\text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-}\) are predominant at pH values below 6.5 and change to \(\text{CrO}_{4}^{2-}\) at pH values greater than 6.5 [8]. Several methods, such as chemical precipitation, electrolysis methods, liquid–liquid extraction [9], liquid membrane separation [10], adsorption process [11], biological treatment [12], and chelating resins [13] have been described for the removal of Cr(VI) from aqueous solution.
Chemical precipitation may cause secondary pollution, and the electrolysis method is energy consuming and economically unfavorable. A large amount of high purity organic solvents are needed for liquid–liquid extraction and liquid membrane separation, most of which are harmful to the environment and health. Among them, the adsorption process is the most popular method to remove Cr(VI) from aqueous solution because of its high efficiency, low-cost, easy operation, and stable regeneration capability.
Many types of materials have been tested as adsorbents for the removal of Cr(VI), including activated carbon, and chitosan [14], fly ash [15], sawdust [16], magnetic nanocomposite [17], wool, and other low-cost adsorbents [18, 19]. These adsorbents have some disadvantages such as poor selectivity, low reuse and slow adsorption–desorption kinetics. Therefore, a new adsorbent for Cr(VI) with high selective adsorption capacity, fast adsorption–desorption kinetics, and high regeneration ability is in great demand.
Molecular imprinting technology (MIT) is a process that the monomer and the template molecule are compounded with covalent or non-covalent bond then they joined with the crosslinking agent for polymerization reaction. After leaching the template molecules with eluent, the resulting polymers will be furnished with specific cavities and binding sites that match exactly with the template molecules [20]. These polymers often have an affinity and selectivity towards molecular templates and are called molecular imprinted polymers (MIPs) [21, 22]. As a branch of MIP, ion imprinted polymer (IIP) has shown considerable promise as a method for preparing materials, which is capable of ion recognition [23]. Ionic imprinting technique is one of efficient methods for selectively adsorption of heavy metal ions. In an ion-imprinting process, the selectivity of a polymeric adsorbent depends on the specificity of the ligand, on the coordination geometry and coordination number of the ions as well as their charges and sizes.
The ion imprinted adsorbents have attracted great attention of so many researchers due to their high selectivity towards imprinted ion, and they are applicable in natural complex matrixes [24–28]. The high selectivity that could be obtained by IIP is due to these selective sites in these polymers. They have become so popular due to their mechanical and chemical stability, good mass transfer, easy preparation, easy to be eluted and regenerated, and fast binding kinetics [29]. However, it is still time-consuming and complicated to use the imprinted polymer process due to indispensable filtration, centrifugation, and other handling processes. In addition, these materials suffer from a lack of surface area [30].
To increase the active surface area of the imprinted polymers and their mechanical stability, these polymers have been grafted on various supports, such as carbon nanotube (CNTs) [31], mesoporous silica [32], colloidal SiO2 particles [33], and Fe3O4 nanoparticles (NPs) [34]. For different purposes, Fe3O4 nanoparticles are excellent supports and attracted a great attention of many researchers in various research fields [35]. These magnetic nanoparticles (MNPs) possess significant advantages including small size, high surface-to-volume ratio, high magnetic susceptibility, and effective ability for binding. Furthermore, the MNPs have the desirable property that they can be easily separated with an external magnetic field without either additional centrifugation or filtration procedures [36]. When IIP particles are incorporated with Fe3O4 nanoparticles, they can be easily separated through the application of an external magnetic field. Therefore, the use of magnetic ion-imprinted polymers for the selective adsorption of heavy metal ions is rather common [37, 38].
Currently, many sorbents based on adsorption of chromium have been reported [39–43]. However, most of these studies have not reported the use of magnetic ion imprinted polymers for the adsorption of chromium. Also the selectivity studies of these sorbents have not been investigated exactly.
The aim of this work was to synthesize a new sorbent for selectively adsorbing the chromium ion in an aqueous solution which can be easily separated with an external magnetic field without either additional centrifugation or filtration procedures. The novel magnetic-ion imprinted polymer (MIIP) was synthesized using precipitation copolymerization. This sorbent was synthesized through the combination of an imprinting polymer and magnetic nanoparticles. Magnetic nanoparticles were synthesized using an improved co-precipitation method and modified with silica layer. The resultant magnetic ion imprinted polymers were systematically characterized, and the adsorption behaviors and selective recognition capacities of the adsorbent were investigated.
Experimental
Chemicals and Reagents
Ferric chloride (FeCl3·6H2O), ferrous chloride (FeCl2·4H2O), sodium hydroxide (NaOH), ammonium hydroxide (NH4OH) and tetraethylorthosilicate (TEOS) were obtained from Merck (Darmstadt, Germany) for the synthesis of Fe3O4@SiO2 nanoparticles. For the preparation of imprinted polymer, potassium dichromate (K2Cr2O7), 4-Vinyl pyridine (4-VP), 2-hydroxyethyl methacrylate (HEMA), ethylene glycol dimethacrylate (EGDMA), and α-αˊ-azoisobisbutyronitrile (AIBN) were obtained from Merck (Darmstadt, Germany). The monomers distilled under reduced pressure in the presence of hydroquinone and stored at −19 °C until use. Ethanol, isopropyl alcohol, and acetonitrile (HPLC grade) were purchased from Merck (Darmstadt, Germany). A stock solution (1000 mg L−1) of Cr (VI) was prepared through dissolving dried potassium dichromate, K2Cr2O7 (analytical reagent grade) in double distilled water. Working solutions were prepared daily from the stock solution through serial dilutions. The stock solution was stored at 4 °C when it was not being used. The initial pH of the solutions was adjusted adding hydrochloric acid (HCl) and sodium hydroxide (NaOH). All other chemicals were reagent grade and purchased from Merck (Darmstadt, Germany).
Instrumentation and Characterization
The concentration of Cr(VI) ions was determined using inductively coupled plasma–optical emission spectrometry (ICP–OES). The measurements were performed using a Perkin Elmer Optima 7300 DV (Kentucky, USA). Argon was used as a purge gas, and the measurements were made in triplicate for statistical purposes. All analyses were performed under the conditions suggested by the manufacturer. All pH measurements were recorded at 25 ± 1 °C with a digital pH meter (Sentix-Germany manufacture), which was equipped with a combined glass-calomel electrode. The FT-IR spectra of imprinted and non-imprinted polymer particles were recorded over the frequency range of 400–4000 cm−1 with an FT-IR spectrophotometer (Bruker, VEATOR22 model, USA). The crystal structures of the samples were characterized using X-ray diffraction (XRD). XRD analysis was performed on an XPERT-PRO (Panalytical, USA) diffractometer system with Cu Kα radiation (λ= 0.154 nm) at 40 kv and 40 mA. The data were collected over 2θ range of 10–80 with a step size 0.02° min−1. Scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) was used to study the surface morphology and element content of the materials. This was carried out by field emission scanning electron microscope (Sigma model, Zeiss, Germany). The accelerating voltage was 15 kv. The particle size was determined at 200 nm and 1 μm resolutions with a magnification of 50 kX. The BET surface area and pore size of the synthesized samples were obtained from the N2 adsorption/desorption isotherms at liquid N2 temperature on a BELSORP model (Microtrac Bel Corp, Japan) analyser. Thermal stability of the particles was carried out on a Bahr STA-503 (BAHR, Germany) instrument. Measurements were conducted by scanning from room temperature to 800 °C with a heating rate of 10 °C min−1. Magnetic behavior was analyzed using vibrating sample magnetometer (MDKFD-Meghnatis Daghigh Kavir. Co, Iran). The equipment including glass reactor, mechanical stirrer (RW20 model-IKA Germany manufacture) and vacuum drying oven (VOS-301SD, EYElA, Japan) were used during the synthesis process. Nitrogen was used as a purge gas, and magnetic separation was done by a super magnet with 1.4 T magnetic fields (10 × 5 × 4 cm).
Preparation of Adsorbent
Synthesis of Fe3O4 Magnetic Nanoparticles (MNPs)
Fe3O4 magnetic nanoparticles were synthesized through chemical co-precipitation method [44]. Briefly, 10.4 g of FeCl3.6H2O and 4.0 g of FeCl2.4H2O were dissolved in 50 mL of double distilled water followed by adding 1.7 mL of HCl (12 mol L−1) under magnetic stirring in order to prepare a stock solution of ferrous and ferric chloride. Then, the mixture solution was degassed with nitrogen for 20 min in order to remove oxygen. Simultaneously, 500 mL of the NaOH solution (1.5 M) was purged with nitrogen gas for 15 min, while the temperature increased to 80 °C in a glass reactor. Then, the stock solution was added fall in drop into this mixture under nitrogen gas and stirred (1000 rpm) using a glassware stirrer then the reaction was maintained for 30 min. During the whole process, the solution temperature was maintained at 80 °C, and the solution was purged with nitrogen gas to remove the dissolved oxygen. After the completion the reaction, the solution color changed to black and led to a black precipitate. Then, the synthesized Fe3O4 MNPs were separated by putting the vessel on a permanent magnet, and the supernatant was decanted from the reaction medium using the magnetic field. Finally, the obtained Fe3O4 MNPs were washed with doubly distilled water several times and re suspended in 500 mL of degassed double distilled water.
Synthesis of Fe3O4@SiO2
200 mL of synthesized Fe3O4 MNPs were dispersed in glass reactor followed by the addition of 600 mL ethanol with sonication for 10 min to prepare a steady suspension. Then, the mixture solution temperature was increased to 80 °C, and in this time 20 mL of ammonium hydroxide (25%) was added to the mixture under vigorous stirring from a glassware stirrer. Ultimately, 10.8 mL of TEOS dissolved in 100 mL of ethanol was added drop by drop to this mixture and the reaction was continued for 12 h at 80 °C under both reflux and stirring (1200 rpm) from a glassware stirrer. Finally, the silica-coated Fe3O4 MNPs were separated using a permanent magnet, washed with ethanol and doubly distilled water several times and dried in oven at 90 °C for 6 h.
Synthesis of the Magnetic Cr(VI) Ion Imprinted Polymer (Cr(VI)-MIIP) and Cr(VI) Non-Imprinted Polymer (Cr(VI)-MNIP)
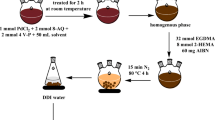
The magnetic ion imprinted polymer was synthesized via precipitation polymerization for Cr(VI) anions certain species. Initially, 4-Vinyl pyridine was treated with Cr(VI) anions solution in water and isopropyl alcohol (1.0:1.0 ratio, v/v) on the basis of a stoichiometry ratio and stirred for 1 h at room temperature to form a metal–monomer complex [40]. This contact time may allow Cr(VI) to be associated more effectively with the monomer. Then, 500 mg of Fe3O4 @SiO2 magnetic nanoparticles were added into the reaction mixture, and the mixture was stirred for 30 min. After this time, 2-HEMA, EGDMA, and AIBN were added into the resulting mixture, and the solution was purged with nitrogen gas for 15 min. The polymerization was performed at 60 °C under nitrogen gas for 48 h. The molar ratio of 4-VP to 2-HEMA to EGDMA was 0.1:0.3:2, and the amount of AIBN was 60 mg. After polymerization, the synthesized Cr(VI)-MIIPs were separated using an external magnetic field and eluted using a mixture of ethanol and double distilled water several times to remove all unreacted monomers and other ingredients. Then, the template ions were removed from polymers with acidified thiourea solution (0.5% thiourea in 0.5 M HCl). This cycle was repeated several times until template ion could not be detected. Finally, the particles were washed using double distilled water and dried in an oven at 70 °C. For comparison, magnetic non imprinted polymers were synthesized under the same procedure without template ion. Figure 1 exhibits a schematic diagram of the preparation procedure of Cr(VI)–MIIP.
Static Adsorption Experiments
The adsorption of Cr(VI) from aqueous solution was investigated in batch system. The effects of pH (2–6), the amount of adsorbent (0.5–2.5 g L−1), contact time (0–2 h), and temperature (25, 35, and 45 °C) on the efficiency of Cr(VI)-MIIP for Cr(VI) adsorption were studied. Only one of the parameters was changed at a time while others were kept constant during the experiments. In all experiments, 50 mL of chromium solution was poured into Erlenmeyer glass flasks, and the flasks were placed inside a mechanical shaker where the agitation speed was set to 300 rpm. The experimental conditions were modulated according to the requirement. After adsorption equilibrium, the adsorbent was separated via an external magnetic field, and the residual amount of Cr(VI) in the aqueous phase was determined using inductively coupled plasma–optical emission spectrometry (ICP–OES).
Each experiment was carried out three times and the average results are reported. The relative standard deviation values for the results are relatively low in the order of ±2.5%, which shows the good reproducibility of the experiments. The adsorption capacity and removal efficiency of the MIIP were calculated from the change in solution concentration using the following equations [45]:
where q (mg g−1) and E (%) represent the adsorption capacity and removal efficiency, respectively, C i (mg L−1) and C e (mg L−1) are the initial and equilibrium metal ion concentrations, respectively, V (L) is the volume of added solution, and m (g) is the mass of adsorbent (dry).
Selectivity Experiments
The selectivity of the prepared magnetic ion imprinted polymers for chromium (VI) was investigated in binary phase systems. The competitors examined were Ni2+, Cd2+, Cu2+, \(\text{NO}_{3}^{-}\), \(\text{SO}_{4}^{2-}\) and \(\text{SPO}_{4}^{3-}\). The experiments were performed in batch mode under optimum conditions with the concentration of 200 mg L−1 to reach adsorption balance. After adsorption equilibrium was reached, the adsorbent was separated via an external magnetic field, and the metals remaining in solution were quantified with ICP-OES. Also the respective magnetic NIP was used for the control experiment. The distribution coefficient (\({{K}_{d}}\)) and selectivity coefficient (\(K\)) of the competitive ions with respect to Cr(VI) were calculated using the followings equations:
where K d is the distribution coefficient (mL g−1), and the other variables are as already described.
where K is the selectivity coefficient, and B represents any other analyte that might have been used as a competitor to the template ion during the experiment. Furthermore, the imprinting effect (\({K}'\)) of the MIIP against the MNIP is calculated from the following equation:
where K MIP and K NIP are the selectivity coefficients of the MIP and NIP, respectively. The value of \({K}'\) represents the enhanced effect of imprinting on selectivity and adsorption affinity for the template compared to the non-imprinted polymer.
Desorption and Regeneration Studies
The regeneration of sorbent is very important in economic study of the adsorption process. In this study, the stability and reusability of the magnetic imprinted polymers were evaluated through contacting adsorbent with chromium (VI) solution, which had an initial concentration of 5 mg L−1. The optimum conditions found in previous experiments were used. After adsorption and equilibrium, the aqueous phase was separated with an external magnetic field, and the magnetic polymers residue were stripped off the adsorbed chromium (VI) through stirring it in an acidified thiourea solution (0.5% thiourea in 0.5 M HCl). This leaching solution was found effective in desorbing Cr(VI) from the loaded adsorbent. This procedure was repeated for many times until Cr(VI) could not be detected in aqueous phase. Then, adsorbent was washed thoroughly with double distilled water to a neutral pH. The regenerated Cr(VI)-MIIP was reused in the following adsorption experiments, and the procedure was repeated for a number of times in order to investigate the stability and reusability of these magnetic polymers.
Result and Discussion
Characterization of Adsorbent
FT-IR Analysis
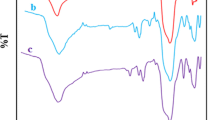
The FT-IR spectra of Fe3O4@SiO2, Cr(VI)-MNIP, and Cr(VI)-MIIP samples are shown in Fig. 2a. For Fe3O4@SiO2, the peak at 586 cm−1 can be assigned to the Fe–O bands from the magnetite phase. The strong peak at 1097 cm−1 can be attributed to Si–O–Si bond, which indicates the formation of a silica layer on the magnetite. Also the peaks at 468 and 802 cm−1 were attributed to Si–O and Si–O–H bonds respectively. For Cr(VI)-MIIP, the typical characteristic peak of Si–O–Si at 1097 cm−1 from Fe3O4@SiO2 spectra was shifted to a higher wave number of 1158 cm−1. The new strong peak at 1728 cm−1 indicated carbonyl groups, and corresponded to the C=O stretching vibrations of the polymer, and the peak at 2952 cm−1 indicated the methylene groups of the polymer, and corresponded to the C–H aromatic stretching vibrations, which can be identified as the characteristic bands of EGDMA. The appearances of these bands confirmed that polymerization was successful. Moreover, the peaks at 1608 and 1456 cm−1 assigned to the C=N and C–N groups were attribute to the characteristic stretching vibration of pyridine ring of 4-VP. Also the peak at 2368 cm−1 was represented vibration of aromatic ring (i.e., 4-VP) after Cr(VI) anions complexes. Ultimately, in all spectra, the broad peak at 3300–3500 cm−1 range indicated OH stretching vibrations in the structure of the adsorbent. These results suggested that the FT-IR features of MMIPs and MNIPs were almost the same because after removing the template ion, the chemical composition of the MMIPs was similar to that of the MNIPs.
XRD Analysis
Powder X-ray diffraction was used as a diagnostic tool to check the phase of iron oxide. Figure 2b compares the XRD patterns of Fe3O4@SiO2 and Cr(VI)-MIIP in 2θ region of 10°–80°. As can be seen in Fig. 2b, the XRD pattern of Fe3O4@SiO2 display diffraction peaks at 2θ = 29°, 35°, 42°, 53°, 57°, and 65°, which are attributed to d220, d311, d400, d422, d511, and d440 that confirm the presence of Fe3O4 nanoparticles in this composite. The characteristic diffraction peak was seen at 2θ = 35°. Other peaks indexed to 29°, 42°, 53°, 57°, and 65° are not clear, and the intensity of these peaks was decreased. The decrease in the intensity of the peaks of Cr(VI)-MIIP can be attributed to the further modification of Fe3O4 with imprinted polymer. The results reveal that the peaks position was unchanged during the synthesized process and the crystal structure of the Fe3O4 nanoparticles essentially remained stable in the polymerization process. This result showed that the Fe3O4 nanoparticles were indeed incorporated into the IIPs.
SEM and EDS Analysis
The morphology and size of the synthesized sorbent were studied by SEM and the results of the Fe3O4@SiO2 and Cr(VI)-MIIP samples are shown in Fig. 3. Figure 3a shows a homogeneous nanosized distribution for Fe3O4@SiO2 nanoparticles. The spherical nanoparticles with a uniform diameter of approximately 70 nm were obtained. These nanoparticles were fully coated in a silica layer. As shown in this figure, after the immobilization of SiO2 on the surface of Fe3O4, it was become very smooth. From the SEM micrograph of Cr(VI)-MIIP (Fig. 3b), it seems that the adsorbent was irregular and it was connected to one another. Also, the SEM micrograph shows some agglomerates that this matter could be attributed to the crosslinking and imprinting reaction, which were occurred on the Fe3O4@SiO2 surface. In comparison with Fe3O4@SiO2, the particles of Cr(VI)-MIIP were porous and rough, which was attributed to imprinted cavities were formed on the surface of Cr(VI)-MIIP. As a result, the rough surface structure is in favor of mass transfer and the formation of three-dimensional recognition sites.
EDS analysis of Fe3O4@SiO2 and Cr(VI)-MIIP was carried out and the related results of the elemental analysis are shown in Fig. 3. The signal for carbon, oxygen, iron, and silica appeared for the Fe3O4@SiO2 nanoparticles (Fig. 3c). As EDS spectra shows the intensity of iron decreased due to encapsulation of Fe3O4 nanoparticles with SiO2. In Fig. 3d, the main elements were Fe, Si, O, C, N, and Cr. The presence of an Fe band in the EDS spectra of Cr(VI)-MIIP confirms the incorporation of the magnetic core within the polymer matrix. Also, the appearance of N element indicated that HEMA were copolymerized on the surface of Fe3O4@SiO2 nanoparticles. Higher percentages of Si and C, and a lower percentage of Fe in the pattern of Fig. 3d confirmed the successfully synthesis of magnetic IIPs.
BET Surface Area and Average Pore Diameter Analysis
The BET surface area measurements for the unleached magnetic Cr(VI) ion imprinted polymer and leached magnetic Cr(VI) ion imprinted polymer are summarized in Table 1. According to the IUPAC recommendations, total porosity can be classified into three groups, according to diameter (d). The three groups are macroporous (d > 50 nm), mesoporous (2 < d < 50 nm), and microporous (d < 2 nm). Based on Table 2, it can be concluded that the synthesized sorbents are mesopores. Also, it can be seen that there were slightly increase in the specific surface area and average pore diameter of the magnetic polymers as a result of leaching action. This may be due to the formation of larger pores during the Cr(IV)-imprinting process.
Thermo-gravimetric Analysis (TGA)
Thermo-gravimetric analysis of Fe3O4@SiO2 and Cr(VI)-MIIP was investigated using TGA analysis, and the results are shown in Fig. 4. The TGA spectra in Fig. 4a show that the Fe3O4@SiO2 has good thermal stability. From room temperature to 800 °C, there was only a little lost for about 8%. In details, the rate of weight loss below 150 °C was related to remnant ethanol dehydration in the SiO2 layer, and when the temperature was changed from 400 to 600 °C, the decreased weight might be due to the decomposition of the organic matter. In Fig. 4b, it is obvious that MIIP was stable up to 200 °C, a rapid weight loss was occurred from 300 to 500 °C, and the decreased weight of in M-MIP was approximately 82%. The high rate of weight loss was originated from the decomposition of imprinted polymer on the surface of Fe3O4@SiO2. Hence, the results completely demonstrated the formation of an imprinted polymer.
Magnetism Analysis (VSM)
The magnetic properties of the Fe3O4@SiO2 and Cr(VI)-MIIP were characterized by VSM at room temperature and the results are shown in Fig. 5. The saturation magnetization value of Fe3O4@SiO2 and Cr(VI)-MIIP was about 10 and 1 emu/g, respectively. The value of saturation magnetization of Cr(VI)-MIIP was decreased in comparison with Fe3O4@SiO2. The decrease in magnetization value can be attributed to the existence of IIPs on the surface of the Fe3O4@SiO2 nanoparticles. However, the Cr(VI)-MIIP with less magnetite encapsulation possesses enough magnetic response to meet the need of magnetic separation. Also, it was apparent that the magnetic hysteresis loops, which consist of coercive forces and remanent magnetization, were relatively low. This was indicated that the two samples were superparamagnetic and could be easily and quickly separated from a suspension. In the absence of an external magnetic field, a brown homogeneous dispersion was existed. When an external magnetic field (0.8 T) was applied, the brown particles were attracted to the wall of vial in a short time (about 100 s).
Optimization for Maximum Adsorption
Effect of Sample pH
The pH of the aqueous solution is one of the most important parameters on the adsorption of metal ions. Hence, the effect of pH on the efficiency of Cr(VI)-MIIP for Cr(VI) adsorption was studied in the pH range of 1–7 and the results are illustrated in Fig. 6a. The obtained results indicate that the adsorption capacity of Cr(VI) increased significantly with the increase of pH from 1.0 to 3.0, then decreased at pH 3.0 to 6.0. The maximum adsorption capacity was observed around pH 3.0. This is attributed to the influence of solution pH on the solution chemistry of metals and the activity of the functional groups on the adsorbent. When pH was below 2, the binding affinity of Cr(VI) to adsorbent was low because there were an excess of hydrogen ions in solution at low pH values, the active sites became protonated, and their ability for interaction with Cr(VI) ions decreased. This condition may be utilized for desorption of Cr(VI) ions from the adsorbent. At higher pH value s of 2–4, the amount of H+ were reduced, and the adsorption of chromium was mainly caused by the electrostatic interaction between the protonated adsorbent and the chromium anionic specie. However, at pH values greater than 6, the Cr(VI) removal efficiency decreased because of strong competition between hydroxyl ions and dichromate ions in solution for the fabricated active adsorption sites on the magnetic polymers. Therefore, maintaining the solution pH at 3.0 can help attain good adsorption results and all further experiments were carried out at pH 3.
a The effect of pH on the adsorption of chromium (VI) on Cr(VI)-MIIP (initial concentration = 5 mg L−1, mass of adsorbent = 1.7 g L−1, time = 120 min, and T = 25 °C), b The effect of adsorbent dose on the adsorption of chromium (VI) on Cr(VI)-MIIP (initial concentration = 5 mg L−1, solution pH 3.0, time = 120 min, and T = 25 °C)
Effect of the Amount of Adsorbent
The effect of the amount of adsorbent on Cr(VI) adsorption was investigated in the range of 0.5–2.5 g L− 1 adsorbent in a series of solution containing 5 mg L−1 chromium while other parameters were kept constant. The results are shown in Fig. 6b. The obtained results indicate that by increasing the amount of the adsorbent dose from 0.5 to 2.5 g L−1, the adsorption percentage of chromium was increased and the adsorption capacity was decreased. The increased in adsorption percentage could be attributed to an increase in surface area and the availability of active sites for Cr(VI) ions. Besides at a constant concentration, increasing the adsorbent led to more unsaturated sites, which can decrease the adsorption capacity per unit weight of adsorbent. Hence, the optimum amount of magnetic polymer was chosen 1.7 g L−1.
Adsorption Kinetics
The adsorption kinetics experiments for Cr(VI) on Cr(VI)-MIIP were investigated under optimum conditions at two different initial concentrations of chromium (5 and 50 mg L−1), and the results are shown in Fig. 7a. It is appear that Cr(VI)-MIIP showed good performance in adsorption during the first 2 min and reached equilibrium after 10 min. There was no obvious change from 10 to 60 min. It is reasonable to assume that the fast adsorption rate of chromium is probably due to both the abundant ion imprinted cavities on the surface of the adsorbent and small diffusion resistance in the thin imprinted polymer layer. The small diffusion resistance causes the templates to enter the cavities easily and bind up to the recognition sites. In other words, the short time in reaching the adsorption equilibrium indicates that the MIIP possesses high absorbability, and the mass-transfer resistance on the surface of the adsorbent is small. The kinetics of adsorption is important because it controls the process efficiency. In order to investigate the controlling mechanism of the adsorption processes, such as mass transfer and chemical reactions, the kinetic data obtained from batch experiments were analyzed using two common semi-empirical kinetic models: the pseudo-first-order equation (Eq. 6) proposed by Lagergren [46], and the pseudo-second-order equation (Eq. 7) proposed by Ho and Mckay [47]:
The effect of contact time on the adsorption of chromium (VI) on Cr(VI)-MIIP (initial concentrations = 5 and 50 mg L−1, solution pH 3.0, mass of adsorbent = 1.7 g L− 1, and T = 25 °C) (a), kinetic models for adsorption of chromium (VI) on Cr(VI)-MIIP at two initial concentrations: pseudo-first-order model (b) and pseudo-second-order model (c)
In the above equations, \({{q}_{e}}\) and \({{q}_{t}}\) (mg g−1) represent the adsorbed amount of chromium at equilibrium and at time (t), respectively. Also, \({{k}_{1}}\) (min−1) and \({{k}_{2}}\) (g mg−1 min−1) are the pseudo-first-order and pseudo-second-order adsorption rate constant, respectively. The kinetics modeling for the adsorption of chromium onto Cr(VI)-MIIP are shown in Fig. 7b, c. In addition, the adsorption kinetics constants, determination coefficient values (R2), and root mean square errors (RMSE) from the two rate equations are summarized in Table 2. Adsorption kinetics provides exquisite information regarding the mechanisms of process. It can be seen that the kinetics of Cr(VI) adsorption on Cr(VI)-MIIP were fitted to a pseudo-first-order kinetic model. The similarity between experimental data and the model prediction was reflected using the determination correlation coefficients (R2). A relatively high R2 value demonstrated the fact that the model can successfully describe the kinetics of Cr(VI) adsorption.
Adsorption Isotherms
The equilibrium adsorption isotherm is fundamental to describe the interactive behavior between the solution and adsorbent, and it is important in designing an adsorption system. In order to optimize the design of an adsorption system, it is significant to establish the appropriate correlation for the equilibrium curve. For this purpose, the equilibrium experiments were done at three temperatures (25, 35, and 45 °C). The results of the tests are shown in Fig. 8a. It is clearly demonstrated that the adsorption capacities of the MIIP increased when the initial concentrations of Cr(VI) increased. Finally, the adsorption became gradual and reached to saturation. Also the temperature had a significant effect on the sorption behavior. It is also seen that the adsorption decreases as the temperature increases. This might be due to the fact that the interaction between the Cr(VI) ions and the active groups of Cr(VI)-MIIPs was weaker at higher temperatures. As the initial concentration increased to 400 mg L−1, the maximum adsorption capacity was obtained. It was observed that the maximum adsorption capacity was 39.3 mg g−1 for Cr(VI)-MIIP. This can be explained that there are many specific binding sites on the surface of adsorbent. In order to analyze the equilibrium adsorptive behaviors of Cr(VI) in MIIP, the Langmuir (Eq. 8) and the Freundlich (Eq. 9) isothermal models were applied. It is well known that the Langmuir equation has been applied in many monolayer sorptions. In other words, Langmuir adsorption isotherm assumes that adsorption takes place on surfaces that are energetically homogeneous and distant to each other [48] while the Freundlich model assumes that adsorption occurs on a heterogeneous surface [49]. The Langmuir and Freundlich isotherms are described using the following equations:
where \({{C}_{e}}\)(mg L−1) and \({{q}_{e}}\)(mg g−1) are the concentration and adsorbed amount of Cr(VI) at adsorption equilibrium, respectively, b(L mg−1) is the Langmuir constant, \({{K}_{f}}\)((mg g−1) (L mg−1)1/n) is the Freundlich constant, n is Freundlich exponent (1/n represents the intensity of the sorption process), and q max (mg g−1) is the maximum adsorption capacity. The isotherm parameters obtained from the Eqs. (8) and (9) are given in Table 3. Also, the equilibrium isotherms for the adsorption of Cr(VI) onto Cr(VI)-MIIP at various temperatures (25, 35, and 45 °C) and optimum pH of adsorption are depicted in Fig. 8b, c. The results show that at all tested temperatures, the experimental data were better described with Langmuir isotherm than Freundlich isotherm in accordance with the determination coefficients (R2). It was revealed that the adsorption was mainly monolayer adsorption mechanism. Also, the value of q max, which was obtained from Langmuir curve, was mainly consistent with the experimental value. The error might be due to the some occurrence through bulk polymerization in the process of experiment. It also assumes that all the binding sites on the sorbent are free sites and ready to accept the sorbent from solution. The Langmuir parameter b can be used to predict the affinity between the Cr(VI) ions and Cr(VI)-MIIPs using the dimensionless separation factor, \({{R}_{L}}\), defined as:
where \({{C}_{i}}\) is the initial chromium concentration (mg L−1) and b is the Langmuir constant (L mg−1). R L is the essential characteristic parameter of the Langmuir isotherm that indicates the favorability and the capacity of the adsorbent/adsorbate system. The favourable adsorption will occur when R L value is in the range of 0–1 [50]. The values of R L are shown in Table 3. In our study, at all temperatures R L were found to be in the range of 0–1, which indicated the favourable adsorption of chromium on the Cr(VI)-MIIP adsorbent.
The maximum chromium (VI) adsorption capacity of the magnetic Cr(VI) ion imprinted polymer was 39.3 mg g−1. Comparisons between maximum uptake capacities (q max) and equilibrium time of Cr(VI)-MIIP and other adsorbents for chromium (VI) reported in the literature are presented in Table 4. The result shows that Cr(VI)-MIIP exhibits a reasonable capacity for chromium adsorption from aqueous solutions.
Adsorption Thermodynamics
In environmental engineering, Gibbs free energy and entropy parameters are always used to determine which process will occur spontaneously. The adsorption experiments were carried out at an initial concentration of 100 mg L− 1 chromium and at three temperatures (25, 35, and 45 °C). Based on the results obtained in adsorption isotherms, the thermodynamics of the adsorption processes was studied and the Gibbs free energy (\(\Delta G\)), enthalpy (\(\Delta H\)), and entropy (\(\Delta S\)) parameters were calculated using the following equations [62]:
where C e (mg L−1) is the equilibrium concentration in solution, K c (mL g−1) is the distribution coefficient at each temperature, R is the universal gas constant (8.314 J mol K−1), and T is the adsorption absolute temperature (K). From the slope and intercept of the Van’t Hoff’s equation, which is shown in Eq. (13), \(\Delta H\) and \(\Delta S\) can be calculated. Plotting \(\ln \,{{K}_{c}}\) against \({}^{1}\!\!\diagup\!\!{}_{T}\;\) gave a straight line with slope and intercept equal to 3666.4 and −6.3746, respectively. The value of determination coefficient (R2) was 0.9986. Thermodynamic constants of chromium adsorption using Cr(VI)-MIIP adsorbent at an initial concentration of 100 mg L−1 are listed in Table 5. The negative values of \(\Delta G\) indicated that Cr(VI) adsorption was spontaneous and thermodynamically favorable in the corresponding temperature range. The reduction of Gibbs free energy with increasing the temperature shows that the adsorption becomes less favorable at higher temperatures. The negative values of enthalpy prove that adsorption was exothermic. Also the negative value of entropy can be explained through the decreased degree of randomness during the adsorption process, which might be ascribed to the stable structure formed by the combination of chromium ions and binding sites from the magnetic polymer.
Evaluation of Selective Adsorption
In order to investigate the selective properties of the MIIP toward the Cr(VI) ion, Ni2+, Cd2+, Cu2+, \(\text{NO}_{3}^{-}\), \(\text{SO}_{4}^{2-}\) and \(\text{SPO}_{4}^{3-}\) were chosen as the competitor ions individually because of their similar properties. The selective adsorption studies were carried out under the optimum conditions. The distribution coefficient (K d ), the selectivity coefficient (K), and the relative selectivity coefficient (\({K}'\)) are listed in Table 6. It can be seen that MIIP had the highest adsorption capacity for Cr(VI) among all six adsorbates. The results demonstrated that \({{K}_{d}}\) of Cr(VI) was larger than others, which prove Cr(VI)-MIIP possessed specific binding to Cr(VI), but \({{K}_{d}}\) of the six substances were almost the same in the adsorption using MNIPs. Moreover, the selectivity coefficients of imprinted polymer showed a more significant increase than the values of non-imprinted polymer, which depended on the imprinting effect of the MIIP. These observations are attributed to the specific recognition cavities for Cr(VI) ions created in Cr(VI)-MIIP unlike Cr(VI)-MNIP, which were developed using ion-imprinting. Therefore, the results indicated that MIIP had a much higher selectivity for Cr(VI) in the presence of competitors. The reason is that the surface layer of Cr(VI)-MIIP microspheres distribute a large number of imprinting cavities, and these imprinting cavities are complementary to the Cr(VI) ions in size, shape, and coordination geometry. It mean that the Cr(VI)-MIIP possess strong binding affinity to selectively adsorb Cr(VI) ions, and it can recognize \(\text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-}\) very well even in the presence of interfering ions to a quite high extent.
Desorption and Repeated Use
For economic reasons, the regeneration of the spent adsorbent is likely to be a key evaluation factor. In order to investigate the reusability and stability of Cr(VI)-MIIP, several adsorption–desorption cycles were performed using the same magnetic imprinted adsorbent. Desorption of Cr(VI) ions from the adsorbent was performed in a batch experimental set-up, and the desorption time was found to be 30 min. The Cr(VI)-MIIP was regenerated with the acidified thiourea solution (0.5% thiourea in 0.5 M HCl) and double distilled water then the regenerated Cr(VI)-MIIP was reused to adsorb Cr(VI). The adsorption results for the reutilization experiments are shown in Fig. 9. The results show that Cr(VI)-MIIP can be used repeatedly at least five times without significant loss of adsorption capacity for chromium. In other words, it can be concluded that the Cr(VI)-MIIP has excellent reuse/regeneration ability, and the adsorption efficiency remained almost constant.
Conclusions
In conclusion, a new sensitive and highly selective magnetic sorbent was prepared by precipitation polymerization, and was successfully applied to selectively adsorption of chromium in aqueous solution. The synthesized adsorbent exhibited a strong response to an external magnetic field with fast adsorption efficiency and displayed high selectivity towards the template. All adsorption experiments were carried out in batch sorption mode. Isotherm study showed that the experimental data fitted well with the Langmuir isotherm model, which means that the adsorption process involved monolayer adsorption on the surface of the imprinted polymer. The maximum sorption calculated from the Langmuir isotherm was 44.86 mg g−1 at 25 °C and pH 3. For chromium adsorbed onto MIIP, the pseudo-first-order kinetics model provides the best description of the adsorption process. The competitive sorption studies showed that Cr(VI)-MIIP had high single selectivity sorption for Cr(VI) in the presence of other metal ions. It showed that the selectivity coefficient of the Cr(VI)-MIIP is significantly higher than corresponding MNIP. Thermodynamic calculations indicated that the adsorption process was spontaneous and exothermic. They also revealed that Cr(VI)-MIIP could be reused for five times without decreasing its adsorption capacity significantly. From the results obtained in this study, it can be concluded that this new magnetic ion-imprinted polymer provided an excellent platform for the adsorption of chromium ion in aqueous solution, and it can be a promising candidate for matrices purification and pollution abatement.
References
Kot A, Namiesnèik J (2000) Trends Anal Chem 19:69
Owlad M, Aroua MK, Daud WAW, Baroutian S (2009) Water Air Soil Pollut 200:59
Testa JJ, Grela MA, Litter MI (2004) Environ Sci Technol 38:1589
Xing YQ, Chen XM, Wang DH (2007) Environ Sci Technol 41:1439
Mohan D, Pittman Jr. CU (2006) J Hazard Mater B 137:762
Dubey SP, Gopal K (2007) J Hazard Mater 145:465
Kozlowski CA, Walkowiak W (2002) Water Res 36:4870
Sun JM, Li F, Huang JC (2006) Ind Eng Chem Res 45:1557
Clevenger T, Novak JT (1983) J Water Pollut Control Fed 55:984
Fraser BG, Pritzker MD (1994) Sep Sci Technol 29:2097
Ouk SK, Neufeld RD (1997) J Chem Technol Biotechnol 70:3
Mohanty K, Jha M, Meikap BC, Biswas MN (2006) Chem Eng J 117:71
Yang J, Yu M, Qiu T (2014) J Ind Eng Chem 20:480
Lu Y, Yan CL, Gao SY (2009) Appl Surf Sci 255:6061
Araki K, Maruyama T, Kamiya N, Goto M (2005) J Chromatogr B 818:141
Singh DK, Mishra S (2009) J Hazard Mater 164:1547
Hoai NT, Yoo DK, Kim D (2010) J Hazard Mater 173:462
Srividya K, Mohanty K (2009) Chem Eng J 155:666
Arica MY, Bayramoglu G (2005) Colloids Surf A 253:203
Chen L, Wang X, Lu W, Wu X, Li J (2016) Chem Soc Rev 45:2137
Özcan AA, Demirli Ş (2014) Sep Sci Technol 49:74
Chen L, Xu S, Li J (2011) Chem Soc Rev 40:2922
Ahmadi SJ, Noori KO, Shirvani AS (2010) J Hazard Mater 175:193
Andac M, Özyapı E, Senel S, Say R, Denizli A (2006) Ind Eng Chem Res 45:1780
Cai X, Li J, Zhang Z, Yang F, Dong R, Chen L (2014) ACS Appl Mater Interfaces 6:305
Xu S, Chen L, Li L, Guan Y, Lu H (2012) J Hazard Mater 237–238:347
Fu J, Chen L, Li J, Zhang Z (2015) J Mater Chem A 3:13598
Lee SC, Patil UM, Kim SJ, Ahn S, Kang SW, Jun SC (2016) RSC Adv 6:44087
Birlik E, Buyuktiryaki S, Ersoz A, Say R, Denizli A (2006) Sep Sci Technol 41:3109
Gao BJ, Wang J, An FQ, Liu Q (2008) Polymer 49:1230
Ebrahimzadeh H, Moazzen E, Amini MM, Sadeghi O (2013) Chem Eng J 315:215
Liu Y, Liu Z, Gao J, Dai J, Han J, Wang Y, Xie J, Yan Y (2011) J Hazard Mater 186:197
An FQ, Gao BJ, Feng XQ (2008) J Hazard Mater 157:286
Ren YM, Wei XZ, Zhang ML (2008) J Hazard Mater 158:14
Zhao YG, Shen H, Pan SH, Hu M (2010) J Hazard Mater 182:295
Chen L, Liu J, Zeng Q, Wang H, Yu A, Zhang H, Ding L (2009) J Chromatogr A 1216:3710
Zhang ML, Zhang ZH, Liu YN, Yang X, Luo LJ, Chen JT, Yao SZ (2011) Chem Eng J 178:443
Li J, Dong R, Wang X, Xiong H, Xu S, Shen D, Song X, Chen L (2015) RSC Adv 5:10611
Pakade V, Cukrowska E, Darkwa J, Torto N, Chimuka L (2011) Water SA 37:529
Bayramoglu G, Arica MY (2011) J Hazard Mater 187:213
Marjanović V, Lazarević S, Janković-Častvan I, Potkonjak B, Janaćković Ð, Petrović R (2011) Chem Eng J 166:198
Duranoğlu D, Kaya IGB, Beker U, Filiz SB (2012) Chem Eng J 181–182:103
Tavengwa NT, Cukrowska E, Chimuka L (2013) Talanta 116:670
Faraji M, Yamini Y, Tahmasbi E, Saleh A, Nourmohammadian F (2010) J Iran Chem Soc 7:130
Prasanna Kumar Y, King P, Prasad VSRK (2007) Chem Eng J 129:161
Azizian S (2004) J Colloid Interface Sci 276:47
Ho YS, McKay G (1998) Chem Eng J 70:115
Langmuir I (1918) J Am Chem Soc 40:1361
Freundlich HMF (1906) Z Phys Chem 57:385
Webi TW, Chakravort RK (1974) AIChE J 20:228
Wang XS, Chen LF, Li FY, Chen KL, Wan WY (2010) J Hazard Mater 175:816
Xing GX, Zhang SF, Ju BZ, Yang JZ (2006) Carbohydr Polym 66:246
Cheng RM, Ou SJ, Xiang B, Li YJ, Liao QQ (2009) J Polym Res 16:703
Bayramoglu G, Arica MY (2005) Sep Purif Technol 45:192
Huang GL, Shi JX, Langrish TAG (2009) Chem Eng J 152:434
Liu W, Zhang J, Zhang C, Wang Y, Li Y (2010) Chem Eng J 162:677
Janos P, Hula V, Bradnova P, Pilarova V, Sedlbauer J (2009) Chemosphere 75:732
Hu J, Chen GH, Lo IMC (2005) Water Res 39:4528
Li H, Li Z, Liu T, Xiao X, Peng Z, Deng L (2008) Bioresour Technol 99:6271
Lv X, Xu J, Jiang G, Tang J, Xu X (2012) J Colloid Interface Sci 369:460
Tan T, He X, Du W (2001) J Chem Technol Biotechnol 76:191
Azouaou N, Sadaoui Z, Djaafri A, Mokaddem H (2010) J Hazard Mater 184:126
Acknowledgements
This work was financially supported by the Iranian Nanotechnology Initiative Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassanpour, S., Taghizadeh, M. & Yamini, Y. Magnetic Cr(VI) Ion Imprinted Polymer for the Fast Selective Adsorption of Cr(VI) from Aqueous Solution. J Polym Environ 26, 101–115 (2018). https://doi.org/10.1007/s10924-016-0929-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-016-0929-6